Abstract
OBJECTIVE
In type 1 diabetes, allogeneic pancreatic islet transplant restores insulin production, but life-threatening immunosuppression is required to avoid graft rejection. Induction of antigen (Ag)–specific tolerance by cell therapy with regulatory T-cells (Tregs) represents an attractive alternative approach but its therapeutic efficacy in islet transplant remains to be determined. Among the different subsets of CD4+ Tregs, the T inducible regulatory type 1 (Tr1) cells can be generated from naive T-cells in the presence of interleukin-10 (IL-10) and represent one promising therapeutic choice. This study was designed to define the efficacy of Tr1-cell therapy in preclinical models of islet transplant.
RESEARCH DESIGN AND METHODS
Non–Ag-specific polyclonal Tr1 cells and donor Ag-specific Tr1 cells were transferred, in the absence of any pharmacological treatment, in two distinct mouse models of islet transplant. The two models differed in their therapeutic stringency, based on the mean rejection time of untreated mice that underwent a transplant.
RESULTS
Transfer of polyclonal Tr1 cells engendered graft tolerance only in the nonstringent mouse model. Conversely, cell therapy with Ag-specific Tr1 cells induced an IL-10–dependent tolerance in the stringent mouse model of islet transplant. The therapeutic advantage of Ag-specific Tr1 cells over polyclonal Tr1 cells was due to their donor Ag specificity.
CONCLUSIONS
These results demonstrate that Tr1-cell therapy leads to tolerance in settings of islet transplant and that its therapeutic efficacy is highly dependent on the antigen specificity of these cells.
Pancreatic islet transplant remains the only therapeutic option currently available for individuals with established type 1 diabetes and, when used, it is largely restricted to individuals in whom conventional insulin therapy fails to maintain adequate metabolic control. In 2000, Shapiro et al. from Edmonton demonstrated that a steroid-free immunosuppressive treatment results in successful transplant of pancreatic islets (1). A larger multicenter trial confirmed these initial findings, but also unfortunately revealed that insulin independence was not sustainable and graft function was lost in nearly all patients 5 years after transplant (2). Many pharmacological-based attempts to prevent rejection of transplanted islets have been tried, with some recent reports suggesting significant progress toward that goal (reviewed in 3). However, each of these approaches is based on continuous administration of immunosuppressants with well-described and deleterious side effects. One attractive alternative would involve the induction of graft-specific tolerance, allowing for immunosuppression to be withdrawn without the risk of graft rejection.
To this end, interest has continued to grow for the use of T regulatory cells (Tregs) as a therapeutic means to modulate undesired immune responses and to achieve antigen (Ag)–specific tolerance (4). Tregs are a specific subset of T-cells that keep the immune system under control and thereby preserve homeostasis and tolerance to self-antigens (5). The CD4+ Tregs have been categorized into two major subgroups based on their ontogeny. These include the naturally occurring forkhead box P3 (FoxP3)+CD4+CD25+ Tregs (nTregs), which develop in the thymus and are present in normal naive mice and healthy individuals from birth, as well as the inducible Tregs, which are generated in the periphery under various tolerogenic conditions (reviewed in 6). Many different subsets of inducible Tregs have been described. Among these, the T regulatory type 1 (Tr1) cells, which constitutively produce high levels of interleukin-10 (IL-10) in the absence of IL-4 (IL-10+IL-4−), are among the most extensively characterized subsets (reviewed in 7).
In terms of relationship between Tregs and therapeutic effectiveness, it has been reported that one exceptional type 1 diabetic patient who remained insulin-free 11 years after islet transplant had a significantly higher frequency of circulating nTregs, compared with healthy age-matched control subjects (8). Similarly, IL-10 production by peripheral blood mononuclear cells isolated from insulin-independent patients who underwent islet transplant was significantly higher compared with that produced by insulin-dependent subjects who underwent a transplant (9). These findings suggest an active role of Tregs in maintaining long-term tolerance after allogeneic islet transplant in autoimmune type 1 diabetic patients and support efforts to develop a Treg-based therapeutic approach.
A growing body of evidence from animal models of transplant suggests that cell therapy with nTregs promotes tolerance but their efficacy is strictly dependent on their Ag specificity. Importantly, to date, the ex vivo generation/expansion of Ag-specific nTregs remains one of the major challenges of the field, seeking to devise a means for inducing long-term tolerance (reviewed in 10). On the contrary, Tr1 cells are inducible Tregs and therefore cells of the desired Ag specificity can be easily generated. Tr1 cells can be induced in vitro and in vivo in the presence of high levels of IL-10 and T-cell receptor–mediated stimulation (7) and thus can be envisaged as a therapeutic tool to transfer immunological tolerance. That said, the efficacy of adoptive Tr1-cell therapy as well as the Ag-specificity requirement in preclinical models of islet transplant have thus far not been reported.
We demonstrate that Ag-specific Tr1 cells transfer stable long-term, graft-specific, and IL-10–dependent tolerance in a stringent model of allogeneic islet transplant, whereas, at the same time, non–Ag-specific polyclonal Tr1 cells fail to provide this same activity. These data set the basis for future clinical trials with Tr1-cell therapy in type 1 diabetic patients who underwent an islet transplant.
RESEARCH DESIGN AND METHODS
Mice and islet transplant.
BALB/c, C57BL/6, and C3H female mice were purchased from Charles River (Calco, Italy). All mice were maintained under specific pathogen-free conditions. Diabetes was induced by intravenous injection of 170 mg/kg streptozotocin (Sigma-Aldrich, St. Louis, MO). Glucose level in the tail venous blood was quantified using the Glucometer Elite system (Bayer, Wuppertal, Germany) and always measured in the morning.
Pancreatic islets were separated by density gradient centrifugation after in situ digestion with collagenase P (Roche Applied Science, Indianapolis, IN). After being cultured overnight at 37°C, handpicked pancreatic islets were transplanted (300 islets/mouse) under the kidney capsule of recipient diabetic mice, as previously described (11). A diagnosis of graft rejection was made after two sequential glucose measurements higher than 300 mg/dl. All animal care procedures were performed according to protocols approved by the San Raffaele Hospital Institutional Animal Care and Use Committee (IACUC no. 350).
Two million T-cells (described below) were resuspended in 200 μl PBS and injected intravenously in diabetic recipient mice 1 day before undergoing islet transplant.
The transplant recipient mice that did not reject the allograft 100 days after transplant were boosted in vivo with donor-origin splenocytes. A total of 30 × 106 splenocytes isolated from the original islet donors were injected intraperitoneally, and the blood glucose level was monitored daily thereafter. Long-term tolerant C57BL/6 mice that underwent a transplant were treated with αIL-10 receptor (αIL-10R) monoclonal antibody (mAb; 1B1.2 clone from ATCC, Manassas, VA) diluted in saline solution and administered intraperitoneally at 145, 146, and 147 days after transplant to reach a dose of 1 mg/mouse.
In vitro Tr1-cell induction.
Naive splenic CD4+ T-cells were isolated from BALB/c or C57BL/6 mice with the CD4+ T Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and the αCD62L mAb-coated microbeads (Miltenyi Biotec) following the manufacturer's instructions. The naive T-cells were plated at 1 × 106 cells/ml and cultured in Iscoves modified Dulbecco's medium (Invitrogen, Carlsbad, CA) plus 10% FBS (Logan, Basel, Switzerland). The cells were stimulated with 10 μg/ml plate-bound αCD3 and 1 μg/ml soluble αCD28 mAbs (BD Biosciences, San Diego, CA) for three rounds of stimulation (for a total of 3 weeks) with or without 1,000 units/ml of human (hu)–IL-10 (BD Pharmingen, San Diego, CA). Starting from the second round of stimulation, 50 units/ml of IL-2 (BD Pharmingen) were added to the cultures. All cells were cultured in humidified incubators at 37°C with 5% CO2.
In vivo Tr1-cell induction.
Diabetic C57BL/6 mice received a transplant of BALB/c islets and were treated with αCD45RB mAb (MB23G2 clone from ATCC), rapamycin (Rapamune; Wyeth Europe, Taplow, U.K.), and hu-IL-10 (BD Pharmingen). αCD45RB mAb was injected intravenously at days 0, 1, and 5 after transplant at 100 μg per dose. Rapamycin was diluted in water and administered by gavage for 30 consecutive days once a day at 1 mg/kg. hu-IL-10 (BD Pharmingen) was diluted in PBS and administered intraperitoneally twice daily for 30 consecutive days at 0.05 μg/kg. Thirty days after transplant, the spleens were collected from mice that underwent transplant and were manually smashed through screens. Splenic CD4+ T-cells were magnetically purified using the CD4+ T Cell Isolation Kit (Miltenyi Biotec) to test for the presence of Tr1 cells. Alternatively, CD4+CD25− splenic T-cells were purified by the CD4+ T Cell Isolation Kit and by the αCD25 mAb-coated microbeads (Miltenyi Biotec) to perform adoptive transfer experiments.
Enzyme-linked immunosorbent assay.
Purified CD4+ T-cells were cultured in 96-well plates at 0.3 × 106 cell/well in the presence of 0.5 × 106/well of irradiated antigen-presenting cells (APCs), which consisted of spleens magnetically depleted of CD90+ T-cells by the use of αCD90 mAb-coated microbeads (Miltenyi Biotec) from the original islet donors or a third-party donor (i.e., C3H mice). Supernatants were collected 7 days after culture for IL-4 and IL-10 detection. Cytokines present in the supernatants were quantified by a sandwich enzyme-linked immunosorbent assay using standard commercially available kits (Pharmingen OptEIA mouse; BD Pharmingen).
Intracellular staining.
CD4+ T-cells were stimulated with 500 ng/ml 12-O-tetradecanoylphorbol-13-acetate (TPA; Calbiochem, Merck KGaA, Darmstadt, Germany) and 1 μg/ml ionomycin (Calbiochem) for 6 h in the presence of Golgi Stop (BD Bioscience). Cells were then collected, washed, and stained for 30 min at 4°C with αCD4 fluorescein mAb (RM4-5; BD Biosciences). The stained cells were washed, fixed, and permeabilized with saponin 20% (Sigma-Aldrich) for 20 min at room temperature. Permeabilized cells were stained with αIL-10 phycoerythrin (JES5-16E3; eBioscience) and αIL-4 APC (11B11; BD Biosciences) mAbs. Data were acquired on FACSCanto (BD Biosciences) and analyzed with FCS express V3 (De Novo Software, Los Angeles, CA).
Statistics.
Differences between groups were assessed using Student t test. P values were two tailed with a confidence of 95%. In all cases, two-tailed P < 0.05 was considered significant. Islet allograft survivals were determined using Kaplan-Meier survival curves and were compared by the log-rank test. Analyses were performed using the Prism V4.03 software (Graph-Pad, San Diego, CA).
RESULTS
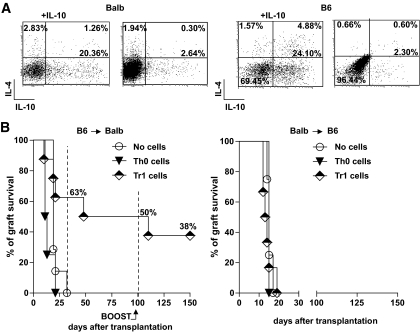
Non–Ag-specific Tr1 cells were generated in vitro from splenic naive CD4+CD62L+ T-cells isolated from either BALB/c (BALB) or C57BL/6 (B6) mice. Repetitive polyclonal activation of naive T-cells in the presence of high doses of IL-10 led to the generation of 20–25% of Tr1 cells (i.e., IL-10+IL-4−), as previously demonstrated (12). The same cells cultured in the absence of IL-10 did not differentiate into Tr1 cells and were defined as T helper 0 (Th0) cells (Fig. 1A). CD4+CD25+FoxP3+ nTregs were not expanded in these culture conditions (data not shown).
FIG. 1.
A: Naive CD4+CD62L+ T-cells isolated from the spleens of BALB (left) or B6 (right) mice were activated with αCD3 and αCD28 mAbs in the presence or absence of exogenous IL-10 for 3 weeks. The ability to produce IL-10 and IL-4 was tested by intracellular staining at the end of the culture and upon TPA/ionomycin stimulation. The frequency of Tr1 cells (i.e., IL-10+IL-4− cells) differentiated in the presence (+IL-10) or absence of IL-10 is reported in bold. One representative experiment of five (for BALB) and three (for B6) mice is shown. B: Chemically induced diabetic BALB mice received a transplant of islets from B6 mice. The day before transplant, recipient mice were injected with PBS (no cells, n = 7), 2 × 106 of BALB CD4+ T-cells enriched in Tr1 cells upon culture in the presence of IL-10 (Tr1 cells, n = 8), or 2 × 106 BALB CD4+ T-cells cultured for 3 weeks in the absence of IL-10 (Th0 cells, n = 4). Graft survival was monitored by glycemia levels. A graft was considered rejected when glycemia was >300 mg/dl. At 100 days after transplant, 30 × 106 splenocytes isolated from B6 mice were injected in tolerant mice to boost their immune system. The percentage of graft survival at various time points after transplant is shown (left). Chemically induced diabetic B6 mice received a transplant of islets from BALB mice. The day before transplant, recipient mice were injected with PBS (no cells, n = 8), 2 × 106 B6 CD4+ T-cells enriched in Tr1 cells upon culture in the presence of IL-10 (Tr1 cells, n = 6), or 2 × 106 B6 CD4+ T-cells cultured for 3 weeks in the absence of IL-10 (Th0 cells, n = 2). Graft survival was monitored by glycemia levels. A graft was considered rejected when glycemia was >300 mg/dl (right).
The ability of in vitro–generated polyclonal Tr1 cells to transfer immunological tolerance in mice undergoing allogeneic islet transplant was tested. A bulk population of host-origin Tr1-enriched cells was transferred in chemically induced diabetic BALB mice the day before receiving B6 islets. Untreated mice and mice receiving cultured Th0 cells were used as controls. Five of eight mice that underwent a transplant receiving Tr1 cells did not reject the graft 25 days after transplant, whereas none of the control mice had a functional graft at the same time point (Fig. 1B, left panel). Long-term graft survival 100 days after transplant was observed in 50% of mice receiving Tr1 cells. To further test the strength of Tr1-cell–mediated tolerance, mice that underwent transplant were rechallenged in vivo with splenocytes isolated from the original donors (i.e., B6 mice). Upon injection of allogeneic splenocytes, only one of four mice rejected the graft (Fig. 1B, left panel). Notably, long-term engrafted mice not receiving the in vivo rechallenge with allogeneic splenocytes remained normoglycemic for at least an additional 50 days (latest time point analyzed) (data not shown). Overall, the proportion of mice achieving long-term tolerance (i.e., accepting the primary graft and retaining the graft after Ag rechallenge) upon Tr1-cell transfer in the absence of any pharmacological treatment was 38%. On the contrary, all untreated mice or mice receiving cultured Th0 cells rejected the graft by day 25 after transplant.
The stringency of an animal model of islet transplant is influenced by the major histocompatibility complex mismatch between donors and recipients and can be assessed by the mean graft rejection time. The shorter the mean rejection time of untreated mice, the higher the stringency of the model. In our hands, untreated BALB mice that received a transplant of B6 islets (B6→BALB) have a mean rejection time of 25 ± 4 days. Conversely, untreated B6 mice that receive a transplant of BALB islets (BALB→B6) reject the graft in a mean of 15 ± 3 days. The ability of in vitro–generated polyclonal Tr1 cells to transfer immunological tolerance was therefore tested also in this latter more stringent islet transplant model. A bulk population of host-origin Tr1-enriched cells was transferred in chemically induced diabetic B6 mice the day before receiving BALB islets. Untreated mice and mice receiving cultured Th0 cells were used as controls. All mice promptly rejected the graft irrespective of the cells transferred (Fig. 1B, right panel). In conclusion, transfer of polyclonal Tr1 cells promotes engraftment and induces long-term tolerance after islet transplant, but its efficacy is highly dependent on the stringency of the animal model used.
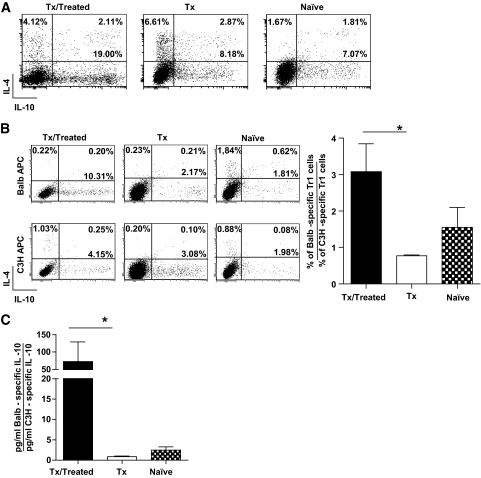
We previously demonstrated that tolerance is induced in vivo by rapamycin + IL-10 treatment via the induction of Tr1 cells in the nonstringent model of islet transplant (B6→BALB) (11). In contrast, in the stringent islet transplant model (BALB→B6), we observed that the addition of a depleting agent (i.e., αCD45RB mAb) was fundamental for the generation of Tr1 cells in vivo (see Research Design and Methods for treatment details) (N.G. et al., unpublished observations). Accordingly, 15–20% of Tr1 cells were found in the spleen of B6 mice that received a transplant of BALB islets and were treated with αCD45RB mAb + rapamycin + IL-10 (Fig. 2A). We hypothesized that, because these Tr1 cells were generated in vivo after islet transplant, they may carry a donor Ag specificity. To test this, splenic CD4+ T-cells isolated from treated mice that underwent a transplant were stimulated in vitro with APCs of the original donors (i.e., BALB mice) or third-party donors (i.e., C3H mice). The frequency of donor-specific Tr1 cells was significantly higher than that of cells specific for third-party Ag. In contrast, the frequency of splenic Tr1 cells was low in both untreated mice that underwent a transplant and naive mice, and there was no suggestion of any Ag specificity (Fig. 2B). The amount of IL-10 released in culture supernatants by splenic CD4+ T-cells isolated from treated mice that underwent a transplant upon Ag-specific stimulation was significantly higher in response to the original donor Ag compared with third-party Ag (Fig. 2C), although no differences were found in the production of IL-4 (data not shown). These data demonstrate the presence of Ag-specific Tr1 cells in the spleen of B6 mice that received a transplant of BALB islets and were treated with an IL-10–based protocol.
FIG. 2.
A: Splenic CD4+ T-cells isolated from treated B6 mice that underwent a transplant (Tx/treated, n = 4), untreated B6 mice that underwent a transplant (Tx, n = 4), and naive B6 mice (naive, n = 2) were cultured for 1 week in the presence of αCD3 and αCD28 mAbs. The ability to produce IL-10 and IL-4 was tested by intracellular staining at the end of the culture and upon TPA/ionomycin stimulation. The frequency of Tr1 cells (i.e., IL-10+IL-4− cells) is reported in bold. One representative plot for each group tested is shown. B: Splenic CD4+ T-cells isolated from treated B6 mice that underwent a transplant (Tx/treated, n = 4), untreated B6 mice that underwent a transplant (Tx, n = 4), and naive B6 mice (naive, n = 2) were cultured for 1 week with irradiated APCs isolated from the original donor of the islets (BALB) (upper panels) or from unrelated third-party APCs (C3H) (lower panels). The ability to produce IL-10 and IL-4 was tested by intracellular staining at the end of the culture and upon TPA/ionomycin stimulation. The frequency of Tr1 cells (i.e., IL-10+IL-4− cells) is reported in bold. One representative plot for each group tested is shown (left). The ratio between the percentage of Tr1 cells specific for the original donor and the percentage of Tr1 cells specific for a third-party donor are shown (right). C: At the end of 1 week of in vitro Ag-specific stimulation, IL-10 secretion was measured by enzyme-linked immunosorbent assay. The ratio between IL-10 produced specifically in response to APCs from the original donor and IL-10 produced in response to third-party APCs is shown. *P < 0.05.
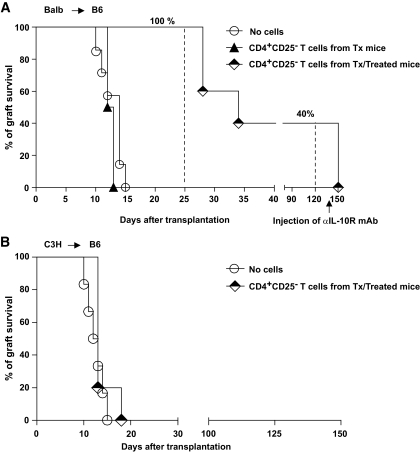
We tested whether, in contrast to polyclonal Tr1 cells, the in vivo–generated Ag-specific Tr1 cells were able to transfer immunological tolerance in a stringent model of allogeneic islet transplant. To exclude any possible contamination with CD4+CD25+ Tregs naturally present in the spleen of mice that underwent a transplant, CD4+CD25− T-cells were purified and adoptively transferred in chemically induced diabetic B6 mice the day before receiving BALB islets. Untreated mice and mice receiving CD4+CD25− T-cells isolated from the spleens of untreated mice that underwent a transplant were used as controls. All mice receiving T-cells enriched in donor Ag-specific Tr1 cells had a functioning graft 25 days after transplant, whereas none of the control mice had a functional graft at the same time point. Long-term graft survival 100 days after transplant was observed in 40% of the mice receiving Ag-specific Tr1 cells. To confirm that long-term tolerance was mediated by IL-10, αIL-10R mAb was given to long-term tolerant mice previously injected with Ag-specific Tr1 cells. All mice promptly rejected the graft upon αIL-10R mAb treatment, strongly suggesting that Tr1-cell therapy maintains tolerance in vivo via IL-10 (Fig. 3A). To further prove that Tr1 cells transfer tolerance in a stringent model of islet transplant due to their Ag specificity, BALB-specific CD4+CD25− T-cells were transferred in B6 mice receiving islets from C3H donors. Transfer of BALB-specific Tr1 cells did not prevent rejection of C3H islets (Fig. 3B), further proving that their functional advantage over polyclonal Tr1 cells was due to their Ag specificity. Taken collectively, these data support the concept that Tr1 cells, to be of a therapeutic value in the context of islet transplant, must be donor specific.
FIG. 3.
A: Chemically induced diabetic B6 mice received a transplant of islets from BALB mice. The day before transplant, recipient mice were injected with PBS (no cells, n = 7), 2 × 106 CD4+CD25− T-cells isolated from the spleen of B6 treated mice that underwent a transplant (Tx/treated, n = 5), or 2 × 106 CD4+CD25− T-cells isolated from the spleen of B6 untreated mice that underwent a transplant (Tx mice, n = 2). At 150 days after transplant, tolerant mice were injected with αIL-10R mAb. Graft survival was monitored by glycemia levels. A graft was considered rejected when glycemia was >300 mg/dl. The percentage of graft survival at various time points after transplant is shown. B: Chemically induced diabetic B6 mice received a transplant of islets from C3H mice. The day before transplant, recipient mice were injected with PBS (no cells, n = 6), or 2 × 106 CD4+CD25− T-cells isolated from the spleen of B6 mice that received a transplant of BALB islets and were treated (Tx/treated, n = 5). Graft survival was monitored by glycemia levels. A graft was considered rejected when glycemia was >300 mg/dl.
DISCUSSION
The field of islet transplantation would without question benefit from the introduction of new treatments that engender stable long-term tolerance. Here we show that cell therapy with alloAg-specific Tr1 cells promotes an IL-10–dependent graft-specific tolerance in a stringent mouse model of islet transplant, in the absence of any pharmacological treatment. Importantly, the efficacy of this cell therapy is strictly dependent on the Tr1-cell Ag specificity.
Several preclinical studies have established that transfer of Tregs restrains T-cell–mediated diseases and promotes tolerance (4). Cotransfer of freshly isolated nTregs together with a bone marrow allograft ameliorates graft-versus-host disease (GvHD) and facilitates engraftment in mouse models of bone marrow transplant (BMT) (reviewed in 13). In these models, transfer of nTregs enriched for alloAg specificity shows only moderately improved efficacy compared with the transfer of polyclonal nTregs (4). In contrast, in experimental models of autoimmune diabetes, only autoAg-specific Tregs have demonstrated therapeutic effectiveness (4). These different results might be ascribed to the presence of a lymphopenic environment after BMT that supports the homeostatic expansion of transferred Tregs and may be different in normal immunocompetent mice. Accordingly, transfer of polyclonal nTregs induces tolerance to skin and heart grafts only in lymphopenic hosts reconstituted with effector T-cells (14,15). In contrast, transfer of polyclonal nTregs in immunocompetent mice that received a transplant of allogeneic pancreatic islets (16) or skin (17) fails to block graft rejection. To improve the efficacy of cell therapy in immunocompetent hosts, alloAg-specific nTregs (17,18) or high numbers of nTregs (19) are required, although both approaches need the addition of supplementary treatments such as the administration of depleting agents, immunosuppression, and/or low doses of irradiation. Our data show that the mere transfer in immunocompetent mice of Tr1 cells promotes long-term tolerance in the absence of any exogenous pharmacological treatment. To achieve high efficacy in a stringent islet transplant model, Tr1 cells need to be donor specific.
We previously demonstrated, in a nonstringent mouse model, that rapamycin + IL-10 treatment induces tolerance in BALB mice that received a transplant of B6 islets (11). However, rapamycin + IL-10 treatment does not promote tolerance in the more stringent model of B6 mice that received a transplant of BALB islets (N.G. et al., unpublished observations). Interestingly, the transfer of alloAg-specific Tr1 cells promotes long-term tolerance in this latter stringent model, proving that cell therapy with Tr1 cells has a therapeutic advantage over rapamycin + IL-10 treatment. The superiority of a Tr1-cell–based therapy over a pharmacological-based approach may be due to the fact that Tr1 cells, in addition to IL-10, produce other still unknown immunomodulatory factors that can powerfully control undesired immune responses. Alternatively, the IL-10 physiologically produced upon Ag-specific Tr1-cell activation may exclusively exert an immunomodulatory activity, whereas the exogenous administration of a fixed dose of recombinant IL-10 exerts an additional immunostimulatory action, as previously demonstrated (20).
IL-10 is a soluble factor that plays a central role in controlling inflammatory processes, suppressing T-cell responses, and maintaining immunological tolerance after transplant (20). Bacchetta et al. showed that severe combined immunodeficient patients who successfully received a transplant of HLA-mismatched hematopoietic stem cells have circulating host-reactive T-cell clones producing high levels of IL-10 in the absence of IL-4. The presence of these cells correlates with the absence of GvHD as well as long-term graft tolerance without the need of immunosuppression (21). Similarly, increased frequencies of IL-10–producing Tr1 cells in thalassemic patients with a persistent state of mixed chimerism after a successful BMT have recently been noted (22). Other groups have reported that high spontaneous IL-10 production by peripheral blood before BMT is associated with a low incidence of GvHD and transplant-related mortality (23,24). Patients who spontaneously develop tolerance to kidney or liver allografts have circulating Tr1 cells, which suppress naive T-cell responses via production of IL-10 and transforming growth factor-β (25). A new consensus on the immunomodulatory role of IL-10 also in patients who underwent islet transplant has begun to emerge (9) and our preclinical data corroborate this new notion. Stable long-term tolerance to allogeneic transplanted islets is indeed strictly IL-10 dependent, regardless of the approach used, either pharmacologically or cellularly based. Long-term tolerance mediated by the administration of rapamycin + IL-10 (11), or by the transfer of alloAg-specific Tr1 cells, is indeed rapidly broken upon αIL-10R mAb administration.
Taken together, our results set the basis for the investigation of a Tr1-cell therapy in patients undergoing islet transplant. Importantly, type 1 diabetic subjects who receive a transplant of allogeneic islets not only develop new alloreactive immune responses but also have preexisting autoreactive immunity. We previously demonstrated that Tr1 cells generated in vivo in NOD mice control diabetes development by blocking the migration of diabetogenic T-cells in the pancreas (26), thus supporting the Tr1 cell's ability to restrain autoimmune reactions. However, it remains to be determined whether alloreactive specific Tr1 cells can contain also autoimmune responses by bystander suppression, as demonstrated in other settings (27).
A clinical trial of adoptive therapy with ex vivo–generated host-specific Tr1 cells to prevent the occurrence of GvHD in leukemia patients who received a transplant of haploidentical hematopoietic stem cells is already ongoing in our institute. Data from this effort, to date, demonstrate the feasibility and safety of this approach (7) providing strong rationale for its consideration as an application to islet transplant for patients with type 1 diabetes (3). We recently identified a population of tolerogenic dendritic cells (DCs), termed DC-10, that is present in the peripheral blood and secondary lymphoid organs of humans and can be differentiated in vitro from peripheral blood monocytes in the presence of exogenous IL-10. DC-10 cells are potent inducers of alloAg-specific Tr1 cells (S. Gregori et al., unpublished data). We are currently developing this protocol to evaluate whether DC-10 isolated from the spleen of pancreas donors can induce donor-specific Tr1 cells.
One should not forget that a Tr1-cell–based therapy may not by itself account for islet engraftment, long-term function, and immunological tolerance, in contrast to what we observed in preclinical animal models. Cell therapy with Tregs in type 1 diabetic patients might therefore need to be associated, albeit temporarily, with a finely tuned immunosuppressive treatment. Importantly, the influence of the commonly used immunosuppressive drugs on Tr1-cell induction, function, and survival is largely unknown. Much effort has been recently devoted to the definition of a Treg-permissive immunosuppressive therapy to be administered with Ag-specific Tr1 cells and then slowly tapered down to achieve drug-free long-term graft tolerance (3).
ACKNOWLEDGMENTS
This work was supported by the Juvenile Diabetes Research Foundation Grant 7-2006-328.
No potential conflicts of interest relevant to this article were reported.
We are grateful to Dr. Ezio Bonifacio (DFG Center for Regenerative Therapies, Dresden, Germany) for critical discussion of the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV: Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230–238 [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR: International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 3.Gagliani N, Ferraro A, Roncarolo MG, Battaglia M: Autoimmune diabetic patients undergoing allogeneic islet transplantation: are we ready for a regulatory T-cell therapy? Immunol Lett 2009;127:1–7 [DOI] [PubMed] [Google Scholar]
- 4.Roncarolo MG, Battaglia M: Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol 2007;7:585–598 [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Yamaguchi T, Nomura T, Ono M: Regulatory T cells and immune tolerance. Cell 2008;133:775–787 [DOI] [PubMed] [Google Scholar]
- 6.Curotto de Lafaille MA, Lafaille JJ: Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 2009;30:626–635 [DOI] [PubMed] [Google Scholar]
- 7.Allan SE, Broady R, Gregori S, Himmel ME, Locke N, Roncarolo MG, Bacchetta R, Levings MK: CD4+ T-regulatory cells: toward therapy for human diseases. Immunol Rev 2008;223:391–421 [DOI] [PubMed] [Google Scholar]
- 8.Berney T, Ferrari-Lacraz S, Bühler L, Oberholzer J, Marangon N, Philippe J, Villard J, Morel P: Long-term insulin-independence after allogeneic islet transplantation for type 1 diabetes: over the 10-year mark. Am J Transplant 2009;9:419–423 [DOI] [PubMed] [Google Scholar]
- 9.Huurman VA, Velthuis JH, Hilbrands R, Tree TI, Gillard P, van der Meer-Prins PM, Duinkerken G, Pinkse GG, Keymeulen B, Roelen DL, Claas FH, Pipeleers DG, Roep BO: Allograft-specific cytokine profiles associate with clinical outcome after islet cell transplantation. Am J Transplant 2009;9:382–388 [DOI] [PubMed] [Google Scholar]
- 10.Sagoo P, Lombardi G, Lechler RI: Regulatory T cells as therapeutic cells. Curr Opin Organ Transplant 2008;13:645–653 [DOI] [PubMed] [Google Scholar]
- 11.Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E, Roncarolo MG: Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes 2006;55:40–49 [PubMed] [Google Scholar]
- 12.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG: A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997;389:737–742 [DOI] [PubMed] [Google Scholar]
- 13.Coghill JM, Carlson MJ, Moran TP, Serody JS: The biology and therapeutic potential of natural regulatory T-cells in the bone marrow transplant setting. Leuk Lymphoma 2008;49:1860–1869 [DOI] [PubMed] [Google Scholar]
- 14.Dai Z, Li Q, Wang Y, Gao G, Diggs LS, Tellides G, Lakkis FG: CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J Clin Invest 2004;113:310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karim M, Feng G, Wood KJ, Bushell AR: CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood 2005;105:4871–4877 [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Schröppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y, Bromberg JS: Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity 2009;30:458–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsang JY, Tanriver Y, Jiang S, Xue SA, Ratnasothy K, Chen D, Stauss HJ, Bucy RP, Lombardi G, Lechler R: Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest 2008;118:3619–3628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, van Meerwijk JP: Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med 2008;14:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma A, Qi S, Wang Z, Massicotte E, Dupuis M, Daloze P, Chen H: Combined therapy of CD4(+)CD25(+) regulatory T cells with low-dose sirolimus, but not calcineurin inhibitors, preserves suppressive function of regulatory T cells and prolongs allograft survival in mice. Int Immunopharmacol 2009;9:553–563 [DOI] [PubMed] [Google Scholar]
- 20.Roncarolo MG, Battaglia M, Gregori S: The role of interleukin 10 in the control of autoimmunity. J Autoimmun 2003;20:269–272 [DOI] [PubMed] [Google Scholar]
- 21.Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J, de Waal Malefyt R, de Vries JE, Roncarolo MG: High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med 1994;179:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serafini G, Andreani M, Testi M, Battarra M, Bontadini A, Biral E, Fleischhauer K, Marktel S, Lucarelli G, Roncarolo MG, Bacchetta R: Type 1 regulatory T cells are associated with persistent split erythroid/lymphoid chimerism after allogeneic hematopoietic stem cell transplantation for thalassemia. Haematologica 2009;94:1415–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker KS, Roncarolo MG, Peters C, Bigler M, DeFor T, Blazar BR: High spontaneous IL-10 production in unrelated bone marrow transplant recipients is associated with fewer transplant-related complications and early deaths. Bone Marrow Transplant 1999;23:1123–1129 [DOI] [PubMed] [Google Scholar]
- 24.Holler E, Roncarolo MG, Hintermeier-Knabe R, Eissner G, Ertl B, Schulz U, Knabe H, Kolb HJ, Andreesen R, Wilmanns W: Prognostic significance of increased IL-10 production in patients prior to allogeneic bone marrow transplantation. Bone Marrow Transplant 2000;25:237–241 [DOI] [PubMed] [Google Scholar]
- 25.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, Pelletier RP, Orosz CG: Human allograft acceptance is associated with immune regulation. J Clin Invest 2000;106:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battaglia M, Stabilini A, Draghici E, Migliavacca B, Gregori S, Bonifacio E, Roncarolo MG: Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes 2006;55:1571–1580 [DOI] [PubMed] [Google Scholar]
- 27.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG: Tr1 cells: from discovery to their clinical application. Semin Immunol 2006;18:120–127 [DOI] [PubMed] [Google Scholar]