Abstract
OBJECTIVE
Common variants in the gene TCF7L2 confer the largest effect on the risk of type 2 diabetes. The present study was undertaken to increase our understanding of the mechanisms by which this gene affects type 2 diabetes risk.
RESEARCH DESIGN AND METHODS
Eight subjects with risk-conferring TCF7L2 genotypes (TT or TC at rs7903146) and 10 matched subjects with wild-type genotype (CC) underwent 5-h oral glucose tolerance test (OGTT), isoglycemic intravenous glucose infusion, and graded glucose infusion (GGI). Mathematical modeling was used to quantify insulin-secretory profiles during OGTT and glucose infusion protocols. The incretin effect was assessed from ratios of the insulin secretory rates (ISR) during oral and isoglycemic glucose infusions. Dose-response curves relating insulin secretion to glucose concentrations were derived from the GGI.
RESULTS
β-cell responsivity to oral glucose was 50% lower (47 ± 4 vs. 95 ± 15 × 109 min−1; P = 0.01) in the group of subjects with risk-conferring TCF7L2 genotypes compared with control subjects. The incretin effect was also reduced by 30% (32 ± 4 vs. 46 ± 4%; P = 0.02) in the at-risk group. The lower incretin effect occurred despite similar glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) responses to oral glucose. The ISR response to intravenous glucose over a physiologic glucose concentration range (5–9 mmol/l) was similar between groups.
CONCLUSIONS
The TCF7L2 variant rs7903146 appears to affect risk of type 2 diabetes, at least in part, by modifying the effect of incretins on insulin secretion. This is not due to reduced secretion of GLP-1 and GIP but rather due to the effect of TCF7L2 on the sensitivity of the β-cell to incretins. Treatments that increase incretin sensitivity may decrease the risk of type 2 diabetes.
Recently, polymorphisms in the transcription factor 7-like 2 gene (TCF7L2) have been associated with type 2 diabetes (1), increasing risk ∼1.46-fold (2). Current evidence suggests that TCF7L2 increases the risk for type 2 diabetes by reducing glucose-induced insulin secretion. In the Diabetes Prevention Program, carriers of the T allele (rs79012146 or rs12255372) had significantly lower values for insulin/glucose ratio and insulin response than CC homozygotes (3). The mechanisms for reduced insulin secretion are uncertain. TCF7L2 is a transcription factor involved in the Wnt signaling pathway and is ubiquitously expressed (4). Thus, one possibility is it has direct effects on the pancreatic β-cell. Another possibility is that there is an effect on proglucagon gene expression in intestinal endocrine cells, thereby reducing the incretin response to oral nutrients (5). In turn, this would be expected to lead to reduced insulin secretion.
Indeed, several studies have reported reduced insulin secretion after an oral glucose tolerance test (OGTT) in subjects with TCF7L2 variants using ratios between insulin and glucose levels such as insulinogenic index (3,6,7). Reduced insulin secretion in response to oral compared with intravenous glucose has also been reported (8,9), consistent with alterations in the incretin system. Studies that have used intravenous glucose to assess the direct effects on the pancreatic β-cell have yielded mixed results. Some studies have shown diminished insulin responses to standard intravenous glucose tolerance tests (10–13), whereas others have shown no effect of TCF7L2 variants on the response to hyperglycemic clamp or Arg (9,14,15). The effect of TCF7L2 polymorphisms on insulin action is also unclear. Several studies have shown no change in insulin sensitivity (6,8), whereas other studies have shown increased (3,14) or decreased (9,11) insulin sensitivity.
The present study was undertaken in an attempt to clarify the effect of TCF7L2 on insulin secretion. Certain features of the study design set this study apart from previous studies. All subjects had normal or only mildly impaired glucose tolerance, thus avoiding confounding effects of hyperglycemia on insulin secretion. Insulin secretory responses to oral glucose derived by mathematical modeling of peripheral C-peptide were compared at the same plasma glucose concentrations achieved by intravenous infusion of glucose. Insulin sensitivity was also quantified during the OGTT. Finally, the effects of the at-risk variant in TCF7L2 on the dose-response relationships between intravenous glucose and insulin secretion were assessed across a physiologic range of glucose concentrations.
RESEARCH DESIGN AND METHODS
Eight subjects with the risk-conferring genotype (TT [seven subjects] or TC [one subject] at rs7903146) and ten age-, sex-, and BMI-matched subjects with the wild-type genotype (CC at rs7903146) participated. All subjects were nondiabetic, younger than 65 years, in good general health and with stable weight for 6 months, and were recruited using advertisements. They were unrelated and had no family history of type 2 diabetes. The studies were approved by the Human Research Protective Office Committee at Washington University School of Medicine and written informed consent was obtained from each participant.
Study protocols.
The subjects underwent three separate protocols designed to test pancreatic β-cell secretion and the incretin effect. Insulin secretion was assessed during the 5-h OGTT and in response to graded glucose infusion (GGI). The incretin effect was assessed by comparing insulin secretion in response to oral glucose with the response to an isoglycemic intravenous glucose infusion designed to match the plasma glucose concentrations achieved after oral glucose.
The subjects were instructed to adhere to their regular diet and to refrain from exercise for 3 days before the studies. All studies were performed in the Intensive Research Unit of the Washington University Institute for Clinical and Translational Sciences after an overnight fast with the subjects in the recumbent position. Intravenous cannulae were placed in a forearm vein for blood withdrawal, and the forearm was warmed to arterialize the venous sample. When necessary, a second catheter was placed for administration of glucose or insulin as described below.
Five-hour oral OGTT.
At time 0, participants ingested a 75-g glucose load. Blood samples were collected at −10, 0, 10, 20, 30, 60, 90, 120, 150, 180, 240, and 300 min after glucose ingestion to determine plasma glucose, insulin, and C-peptide concentrations.
Isoglycemic intravenous glucose infusion.
Intravenous infusions of glucose (20% dextrose) were designed to reproduce the plasma glucose profile after oral glucose ingestion. Accuracy was assured by repeatedly measuring plasma glucose concentrations, which allowed frequent adjustments of the infusion rate according to deviations from the profile that was to be mimicked. Blood samples were drawn and treated as described for OGTT.
Graded glucose infusion.
GGI involves the intravenous administration of glucose at progressively increasing rates (1, 2, 3, 4, 6, and 8 mg/kg per min) for 40 min each, as we have previously described (16,17). This protocol raises the plasma glucose concentration from basal to mildly hyperglycemic levels and is used to define the dose-response relationships between glucose and insulin secretion. To compare each subject's plasma insulin response at the same plasma glucose level, the best-fit quadratic curve (using SigmaPlot Version 11; SigmaPlot, San Jose, CA) was drawn through the data. The insulin concentration at molar increments of plasma glucose beginning at 5 mmol/l was then obtained by interpolation. The same technique applied to the insulin secretion rate (ISR) provides a plot of the ISR at molar increments of plasma glucose (18).
Body composition.
Fat mass was measured using dual energy X-ray absorptiometry (Delphi 4500-W; Hologic, Waltham, MA).
Analyses.
The areas under the curve (AUCs) were calculated using the trapezoid method (19).
Indexes of β-cell function were estimated from plasma glucose and C-peptide concentrations using the oral minimal model of C-peptide secretion and kinetics (20,21) and incorporating parameters for C-peptide kinetics and volume of distribution as measured by Van Cauter et al. (22). This model calculates the ISR (pmol/min) as a function of time and several indexes of β-cell responsivity: 1) dynamic responsivity index (Φd [109]), which is an index of insulin secretion in response to the rate of change in glucose concentration; 2) static responsivity index (Φs [109 min−1]), which is an index of insulin secretion in response to a given glucose concentration; and 3) overall responsivity index (Φo [109 min−1]), which is a global sensitivity-to-glucose index of postprandial insulin secretion.
The calculation of incretin effects from estimates of β-cell secretory responses is based on the assumption that after glucose ingestion, the pancreas is stimulated by the increase in plasma glucose and the added effect of incretin factors secreted by the intestine. Thus, intravenous glucose infusion (IGI) provides a measure of the β-cell secretory response to the glucose stimulus in the absence of superimposed incretin effects. The incretin effect is calculated from the insulin secretion response (SR) to oral and intravenous glucose according to the following formula (23):
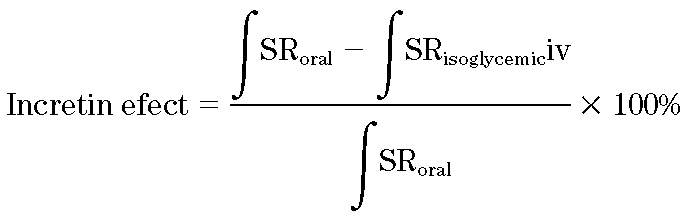 |
The dose-response relationship between ISR and glucose over the physiological range was determined from the GGI. ISRs and glucose concentrations used in the analysis represented the average of the values between 10 and 40 min at each glucose infusion rate. Mean ISR for each glucose infusion rate was then plotted against the corresponding mean glucose concentration to define the dose-response relationship. The slope of the line relating these two variables provided a measure of the sensitivity of the β-cell to glucose.
Whole-body insulin sensitivity was estimated using the oral glucose minimal model (24,25), which has been shown previously to be well correlated with the M value from the euglycemic hyperinsulinemic clamp (26).
Insulin clearance rate was calculated by dividing the ISR AUC by the insulin AUC (16).
Biochemical measurements.
Plasma glucose was measured immediately using an automated glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin, C-peptide, and glucagon were measured by double-antibody radioimmunoassays (Linco Research, St. Louis, MO). Human glucose-dependent insulinotropic polypeptide (GIP; total) ELISA kit was used for the quantification of human GIP in plasma samples (Linco Research). This kit has 100% cross-reactivity to human GIP (1–42) and GIP (3–42). Human (total) electrochemiluminescent GLP-1 assay kit was used for the quantification of human glucagon-like peptide 1 (GLP-1; Meso Scale Discovery, Gaithersburg, MD). This kit has 100% cross-reactivity to human GLP-1 (7–36) amide, human GLP-1 (9–36) amide, GLP-1 (1–36) amide, GLP-1 (7–37), and GLP-1 (1–37).
Genotyping.
DNA was prepared from peripheral blood lymphocytes and the TCF7L2 polymorphisms (dbSNP rs7903146) typed using a TaqMan SNP Genotyping Assay (Applied Biosystems, Foster City, CA).
Statistical analyses.
Group differences in demographics and parameters of glucose homeostasis were compared using the two-tailed unpaired Student t tests for continuous variables and the Fisher exact test for categorical variables. The AUCs for glucose, insulin, ISR, and incretin effects were compared using two-tailed unpaired Student t tests. SPSS Version 15.0 (SPSS, Chicago, IL) was used for all statistical analyses. A P value < 0.05 was considered statistically significant. Results are reported as mean ± SE.
RESULTS
The group with the at-risk genotype (TT and TC) at rs7903146 and the control group (CC genotype) were well matched for age, sex, and body weight and composition (Table 1). Both groups had normal fasting glucose concentrations and hemoglobin A1C. Two-h glucose and glucose AUC tended to be greater in the at-risk group (Table 1, Fig. 1) but the differences did not reach statistical significance.
TABLE 1.
Anthropometrics and metabolic data of study participants
| Control, CC genotype | TCF7L2 genotypes TT and TC | P | |
|---|---|---|---|
| n | 10 | 8 | |
| Demographics | |||
| Age (years) | 44.8 ± 2.5 | 43.3 ± 4.5 | 0.80 |
| Women (%) | 80 | 90 | 0.64 |
| Caucasian/African American (n) | 7/3 | 6/2 | 0.64 |
| Body weight (kg) | 92.3 ± 4.1 | 93.8 ± 5.4 | 0.80 |
| BMI (kg/m2) | 31.9 ± 1.3 | 34.2 ± 2.1 | 0.32 |
| Body fat (%) | 38.9 ± 2.1 | 38.2 ± 3.1 | 0.85 |
| Glucose homeostasis | |||
| Fasting glucose (mmol/l) | 5.3 ± 0.1 | 5.3 ± 0.2 | 0.70 |
| 2-hour glucose (mmol/l) | 7.7 ± 0.3 | 8.6 ± 0.5 | 0.09 |
| Hemoglobin A1C (%) | 5.7 ± 0.5 | 5.7 ± 0.5 | 0.92 |
| Insulin secretion indices | |||
| Static β-cell responsivity Φs (109 min−1) | 67.4 ± 8.8 | 40.7 ± 3.7 | 0.02 |
| Dynamic β-cell responsivity Φd (109) | 1,119 ± 265 | 662 ± 147 | 0.18 |
| Overall β-cell responsivity Φo (109 min−1) | 94.4 ± 15.4 | 46.6 ± 4.2 | 0.01 |
| Insulin sensitivity SI (103 min−1 per pmol/l) | 14.2 ± 2.4 | 15.3 ± 2.5 | 0.41 |
| Insulin clearance (ml/min) | 1,941 ± 163 | 2,157 ± 38,122 | 0.49 |
Data are means ± SE. Statistically significant P values are shown in bold.
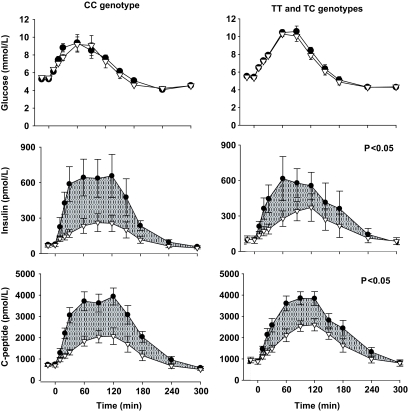
FIG. 1.
Plasma glucose, insulin, and C-peptide concentrations during the 5-h OGTT (●) and isoglycemic intravenous glucose infusion (△) in control subjects and carriers of the at-risk TCF7L2 variant. Shaded areas indicate the incretin effects. P values indicate the significance of the differences in incretin effects between groups. Values are means ± SE.
The static β-cell responsivity (Φs) and dynamic β-cell responsivity (Φd) to oral glucose tended to be lower in the at-risk group than in the control group, with the difference in the former reaching significance (P = 0.02). Consistent with these findings, the overall β-cell responsivity (Φo) was significantly lower (P = 0.01), by ∼50% in the at-risk group (Table 1). Insulin sensitivity and insulin clearance rates were similar between the groups (P > 0.05).
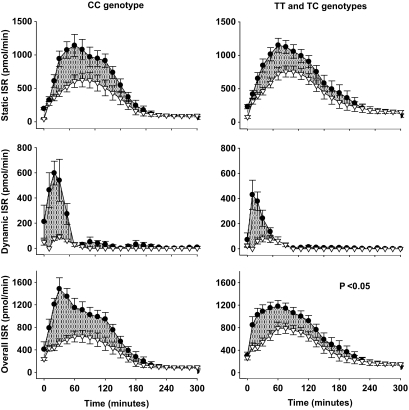
The plasma glucose profile in the OGTT and IGI were well matched in both groups (Table 2, Fig. 1). Consistent with incretin effects in both groups, the AUCs for insulin and C-peptide (Table 2, Fig. 1) and the AUCs for static, dynamic, and overall ISRs (Table 2, Fig. 2) were lower in the IGI than in the OGTT. However, subjects with the at-risk TCF7L2 genotypes had significantly reduced incretin responses for insulin AUC (41 vs. 59%; P = 0.004), C-peptide AUC (32 vs. 45%; P = 0.03), and overall insulin secretory response to glucose AUC (32 vs. 46%; P = 0.02).
TABLE 2.
AUCs for glucose, hormones, and the incretin effect
| Study | Control, CC genotype | TCF7L2 genotypes TT and TC | P |
|---|---|---|---|
| Glucose AUC (103 mmol/l · min) | |||
| OGTT | 1.9 ± 0.1 | 2.1 ± 0.5 | 0.08 |
| IGI | 1.9 ± 0.9 | 2.0 ± 0.5 | |
| P | 0.65 | 0.43 | 0.17 |
| Insulin AUC (103 pmol/l · min) | |||
| OGTT | 107.0 ± 23.3 | 107.2 ± 30.4 | 0.99 |
| IGI | 45.2 ± 11.1 | 64.2 ± 18.8 | 0.35 |
| P | 0.02 | 0.25 | |
| Incretin effect (%) | 58.5 ± 3.7 | 40.6 ± 4.1 | 0.004 |
| C-peptide AUC (103 pmol/l · min) | |||
| OGTT | 703.7 ± 63.9 | 729.4 ± 61.8 | 0.77 |
| IGI | 392.0 ± 54.3 | 498.5 ± 56.4 | 0.17 |
| P | 0.001 | 0.02 | |
| Incretin effect (%) | 45.1 ± 4.1 | 32.2 ± 3.4 | 0.03 |
| Static ISR AUC (103 pmol · min) | |||
| OGTT | 152.2 ± 15.3 | 170.2 ± 17.6 | 0.44 |
| IGI | 93.9 ± 13.8 | 123.4 ± 18.4 | 0.20 |
| P | 0.008 | 0.09 | |
| Incretin effect (%) | 39.2 ± 4.9 | 29.2 ± 3.5 | 0.12 |
| Dynamic ISR AUC (103 pmol · min) | |||
| OGTT | 26.7 ± 3.2 | 15.5 ± 3.4 | 0.19 |
| IGI | 5.3 ± 0.8 | 5.8 ± 1.9 | 0.77 |
| P | 0.006 | 0.03 | |
| Incretin effect (%) | 71.3 ± 6.5 | 42.8 ± 22.3 | 0.12 |
| Overall ISR AUC (103 pmol · min) | |||
| OGTT | 179.9 ± 16.9 | 185.7 ± 19.5 | 0.79 |
| IGI | 99.1 ± 13.6 | 129.4 ± 18.2 | 0.19 |
| P | 0.001 | 0.05 | |
| Incretin effect (%) | 45.5 ± 4.1 | 31.6 ± 3.6 | 0.02 |
Data are means ± SE. IGI, isoglycemic intravenous glucose infusion. Statistically significant P values are shown in bold.
FIG. 2.
Insulin secretory indexes during the 5-h OGTT (●) and isoglycemic intravenous glucose infusion (△) in control subjects and carriers of the at-risk TCF7L2 variant. Shaded areas indicate the incretin effects. P values indicate the significance of the differences in incretin effects between groups. Values are means ± SE.
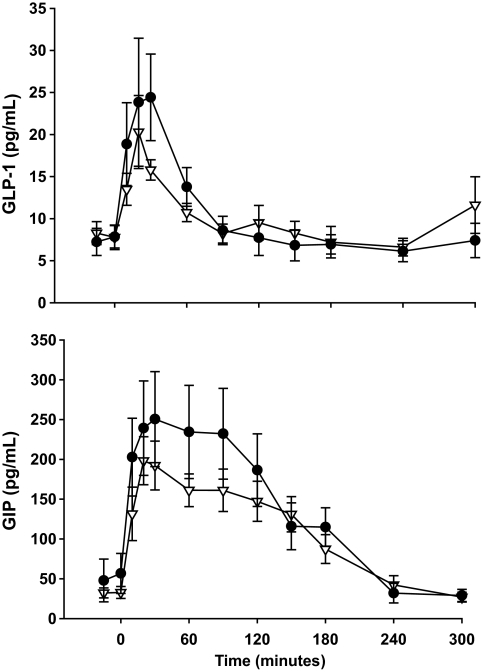
By contrast, both the GLP-1 (AUC: 3.2 ± 0.6 vs. 2.9 ± 0.4 × 103 pg/ml · min; P = 0.67) and GIP (AUC: 4.4 ± 1.0 vs. 3.3 ± 0.4 × 103; P = 0.31) responses to oral glucose were similar between the two groups (Fig. 3).
FIG. 3.
Plasma concentrations of GLP-1 and GIP during the 5-h OGTT in control subjects (△) and carriers of the at-risk TCF7L2 variant (●). There were no significant between group differences in AUCs (P < 0.05). Values are means ± SE.
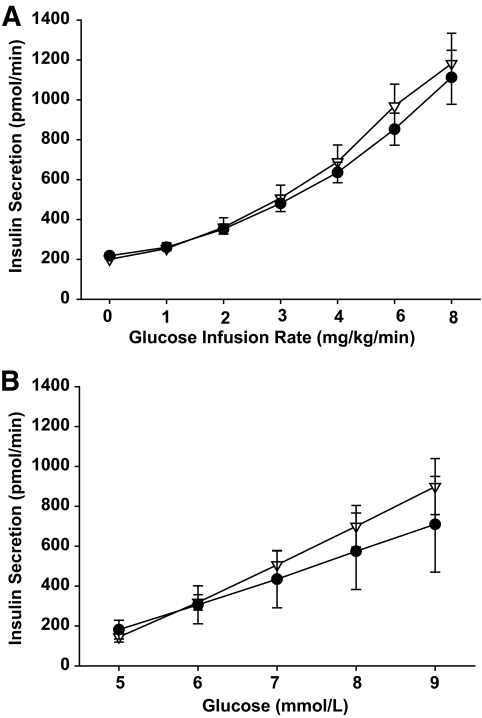
The GGI demonstrated that the ISR-glucose dose-response relationships were not different (AUC: 1.7 ± 0.2 vs. 2.0 ± 0.3 ×103 pmol/min · mmol/l; P = 0.41) between groups (Fig. 4) over a physiologic range of glucose concentrations (5–9 mmol/l).
FIG. 4.
Insulin secretory rates plotted against glucose infusion rate (A) and plasma glucose (B) during the graded-glucose infusion in control subjects (△) and carriers of the at-risk TCF7L2 variant (●). There were no significant between group differences in AUCs (P < 0.05). Values are means ± SE.
The statistical results remained the same when the one subject with TC genotype was excluded from the analyses (Φs, 43.3 ± 3.4 vs. 67.5 ± 8.8 109 min−1, P = 0.04; Φo, 49.5 ± 3.8 vs. 94.5 ± 15.4, P = 0.03), although they were borderline significant for difference in incretin effect (34.3 ± −4.3 vs. 45.5 ± 4.1%; P = 0.08).
DISCUSSION
The present study used the isoglycemic intravenous glucose infusion (IGI), the definitive test of the incretin effect, to examine the incretin effect in subjects with a TCF7L2 variant that enhances type 2 diabetes risk. Using measurements of insulin secretion in response to oral and matched intravenous infusions of glucose, the incretin effect was reduced by ∼30% and this was consistent with the ∼50% reduction in overall β-cell responsivity to glucose demonstrated during the OGTT. The reduced incretin effect was accompanied by normal levels of GIP and GLP-1, suggesting a reduced ability of the pancreatic β-cell to respond to incretins. Another new finding is that the defect in insulin secretion appears to be selective for oral glucose and is not present in response to intravenous glucose. Thus, the graded glucose infusion study demonstrated that insulin secretory response to intravenous glucose over a physiologic range of glucose concentrations is well preserved in subjects with type 2 diabetes–associated genotypes. The diminished β-cell response to oral relative to intravenous glucose is consistent with a key abnormality in the enteroinsular axis in subjects with diabetes-associated TCF7L2 genotype.
Although the incretin effect was reduced in subjects with the diabetes-risk genotypes, the concentrations of GLP-1 and GIP after oral glucose ingestion were not different from those in control subjects. We measured total rather than intact levels of GLP-1 and GIP. However, a previous study showed that the pattern of GLP-1 and GIP release appears similar when total or active levels are measured (27). Our results are consistent with the results of previous studies that have reported similar total GLP-1 and GIP levels independent of TCF7L2 genotype (8,9). Our study assessed the entire secretory profile of both GIP and GLP-1 during the course of a 5-h OGTT, something not consistently done in previous studies. Our results expand and strengthen the evidence that the incretin secretory capacity is normal in subjects with at-risk and non–at-risk TCF7L2 genotypes, suggesting an inability of the pancreatic β-cell in subjects with an at-risk genotype to appropriately respond to normal concentrations of GLP-1 and GIP. This conclusion is consistent with data from two previous studies. Schäfer et al. (14) and Pilgaard et al. (9) reported that the GLP-1– and/or GIP-induced insulin secretion during a hyperglycemic clamp is reduced in subjects with the at-risk TCF7L2 genotype. In the latter study, the reduced insulin response occurred at GIP and GLP-1 concentrations administered to closely mimic physiologic concentrations (∼120 and 1,000 pmol/l, respectively) and at glucose concentrations close to physiologic postprandial levels of glucose (∼10 mmol/l), conditions that approximate those in the IGI in the present study. Collectively, these findings point to a reduction in incretin signaling at the level of the pancreatic β-cell in subjects with type 2 diabetes–associated TCF7L2 genotypes.
The molecular mechanisms responsible for the reduced β-cell response to GLP-1 and GIP as a function of TCF7L2 genotype have not been defined and warrant further investigation. TCF7L2 is a Wnt signaling-associated transcription factor that plays an important role in the synthesis of GLP-1 in intestinal L-cells (4). It was thus anticipated that genetic variation in TCF7L2 would lead to reduced secretion of GLP-1 and GIP. Clearly this is not the case. In addition, the diabetes-associated variants in TCF7L2 have been shown to have increased, rather than decreased, islet levels of TCF7L2 mRNA (8). Interestingly, Shu et al. (28) recently reported that increased levels of TCF7L2 mRNA in β-cells are actually associated with decreased TCF7L2 proteins, indicating altered regulation at a posttranscriptional level. The reduction in TCF7L2 protein was found to correlate with downregulation of GIP and GLP receptors, thus resulting in decreased Akt phosphorylation and Akt-mediated Foxo-1 phosphorylation, and, therefore, impaired β-cell function.
Based on the results of a graded intravenous infusion of glucose, our study is the first to demonstrate that the dose-response relationships between glucose and insulin secretion over the physiologic range of glucose concentrations (5–9 mmol/l) are relatively well preserved in subjects with at-risk and non–at-risk TCF7L2 genotypes. These findings may not be surprising, given that the response to intravenous glucose is not influenced by intestinal incretin peptides. Previous studies that have examined the direct effect of intravenous glucose on the pancreatic β-cell using various protocols have yielded inconsistent results. For example, intravenous glucose tolerance tests showed that the acute insulin response (AIR) was unchanged (9,14,15) or reduced (12,13) as a function of TCF7L2 genotype. Moreover, hyperglycemic clamp with Arg showed that the AIR was unchanged (at 10 mmol/l glucose) (14), whereas the same test showed that AIR was reduced (at 14 and 24 mmol/l glucose) in another study (8). Although the latter might suggest that the maximal insulin secretory response is impaired in subjects with a diabetes-associated TCF7L2 genotype, the physiologic significance of this finding has yet to be determined. On the other hand, the results from our GGI protocol show that within the physiologic range of glucose concentrations (5–9 mmol/l), TCF7L2 genotype does not significantly affect the insulin response to intravenous glucose, as evidenced by the normal β-cell dose response.
Finally, we also demonstrated that the TCF7L2 genotype does not significantly affect overall insulin sensitivity. We used the oral glucose minimal model to derive insulin sensitivity index, which has the advantage of being simultaneously assessed with β-cell responsivity during the OGTT. The sensitivity index measures the overall effect of insulin to stimulate glucose disposal and inhibit glucose production, and has been validated against the euglycemic-hyperinsulinemic clamp (26). Indeed, our results are consistent with previous studies using euglycemic-hyperinsulinemic clamp that have also reported normal whole-body insulin sensitivity in subjects with diabetes-associated genotypes (8,14). However, interestingly, two recent studies (9,11) have reported decreased hepatic insulin sensitivity in subjects with diabetes-associated genotypes, possibly attributed to the lower insulin levels and/or a direct or indirect effect of TCF7L2 on endogenous glucose production. The latter findings await confirmation in other studies (29).
In summary, the present study provides evidence that TCF7L2 affects the insulin secretory response to oral glucose with an impaired incretin effect (30% reduction) due to impaired GIP- and GLP-1–induced insulin secretion rather than incretin deficiency in subjects with the at-risk TCF7L2 rs7903146 genotype. In contrast, the β-cell dose response to intravenous glucose over a physiologic range of glucose concentrations is normal. We also found no evidence of impairment in whole-body insulin sensitivity. Further studies are needed to elucidate the mechanisms for the lack of response to incretins as a function of TCF2L7 genotype. Our findings raise the possibility that increasing incretin levels through pharmacotherapy might overcome incretin resistance and thus decrease the risk of type 2 diabetes due to genetic variation in TCF7L2.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health Grants DK-031842, DK-056341 (Clinical Nutrition Research Unit), DK-20579, and DK-20595 (Washington University and University of Chicago Diabetes Research and Training Centers) and the Clinical and Translational Science Award UL1RR024992 to Washington University. The Kovler Family Foundation also provided generous support.
No potential conflicts of interest relevant to this article were reported.
We thank the participants for their cooperation, the staff of the Intensive Research Unit of the Institute of Clinical and Translational Sciences for their skilled assistance in the performance of this study, and Veronica Paz for her technical assistance with genotyping.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K: Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 2006;38:320–323 [DOI] [PubMed] [Google Scholar]
- 2.Cauchi S, El Achhab Y, Choquet H, Dina C, Krempler F, Weitgasser R, Nejjari C, Patsch W, Chikri M, Meyre D, Froguel P: TCF7L2 is reproducibly associated with type 2 diabetes in various ethnic groups: a global meta-analysis. J Mol Med 2007;85:777–782 [DOI] [PubMed] [Google Scholar]
- 3.Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PI, Shuldiner AR, Knowler WC, Nathan DM, Altshuler DDiabetes Prevention Program Research Group. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med 2006; 355:241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin T, Liu L: The Wnt signaling pathway effector TCF7L2 and type 2 diabetes mellitus. Mol Endocrinol 2008; 22: 2383– 2392 [DOI] [PubMed] [Google Scholar]
- 5.Yi F, Brubaker PL, Jin T: TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem 2005;280:1457–1464 [DOI] [PubMed] [Google Scholar]
- 6.Saxena R, Gianniny L, Burtt NP, Lyssenko V, Giuducci C, Sjögren M, Florez JC, Almgren P, Isomaa B, Orho-Melander M, Lindblad U, Daly MJ, Tuomi T, Hirschhorn JN, Ardlie KG, Groop LC, Altshuler D: Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes 2006;55:2890–2895 [DOI] [PubMed] [Google Scholar]
- 7.Damcott CM, Pollin TI, Reinhart LJ, Ott SH, Shen H, Silver KD, Mitchell BD, Shuldiner AR: Polymorphisms in the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in the Amish: replication and evidence for a role in both insulin secretion and insulin resistance. Diabetes 2006;55:2654–2659 [DOI] [PubMed] [Google Scholar]
- 8.Lyssenko V, Lupi R, Marchetti P, Del Guerra S, Orho-Melander M, Almgren P, Sjögren M, Ling C, Eriksson KF, Lethagen AL, Mancarella R, Berglund G, Tuomi T, Nilsson P, Del Prato S, Groop L: Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 2007;117:2155–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilgaard K, Jensen CB, Schou JH, Lyssenko V, Wegner L, Brøns C, Vilsbøll T, Hansen T, Madsbad S, Holst JJ, Vølund A, Poulsen P, Groop L, Pedersen O, Vaag AA: The T allele of rs7903146 TCF7L2 is associated with impaired insulinotropic action of incretin hormones, reduced 24 h profiles of plasma insulin and glucagon, and increased hepatic glucose production in young healthy men. Diabetologia 2009;52:1298–1307 [DOI] [PubMed] [Google Scholar]
- 10.Munoz J, Lok KH, Gower BA, Fernandez JR, Hunter GR, Lara-Castro C, De Luca M, Garvey WT: Polymorphism in the transcription factor 7-like 2 (TCF7L2) gene is associated with reduced insulin secretion in nondiabetic women. Diabetes 2006;55:3630–3634 [DOI] [PubMed] [Google Scholar]
- 11.Wegner L, Hussain MS, Pilgaard K, Hansen T, Pedersen O, Vaag A, Poulsen P: Impact of TCF7L2 rs7903146 on insulin secretion and action in young and elderly Danish twins. J Clin Endocrinol Metab 2008;93:4013–4019 [DOI] [PubMed] [Google Scholar]
- 12.Palmer ND, Langefeld CD, Bryer-Ash M, Rotter JI, Taylor KD, Bowden DW: Association of the Kir6.2 E23K variant with reduced acute insulin response in African-Americans. J Clin Endocrinol Metab 2008;93:4979–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer ND, Lehtinen AB, Langefeld CD, Campbell JK, Haffner SM, Norris JM, Bergman RN, Goodarzi MO, Rotter JI, Bowden DW: Association of TCF7L2 gene polymorphisms with reduced acute insulin response in Hispanic Americans. J Clin Endocrinol Metab 2008;93:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schäfer SA, Tschritter O, Machicao F, Thamer C, Stefan N, Gallwitz B, Holst JJ, Dekker JM, 't Hart LM, Nijpels G, van Haeften TW, Häring HU, Fritsche A: Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia 2007;50:2443–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe RM, Allayee H, Xiang AH, Trigo E, Hartiala J, Lawrence JM, Buchanan TA: Transcription factor 7-like 2 (TCF7L2) is associated with gestational diabetes mellitus and interacts with adiposity to alter insulin secretion in Mexican Americans. Diabetes 2007;56:1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne MM, Sturis J, Polonsky KS: Insulin secretion and clearance during low-dose graded glucose infusion. Am J Physiol 1995;268:E21–E27 [DOI] [PubMed] [Google Scholar]
- 17.Villareal DT, Koster JC, Robertson H, Akrouh A, Miyake K, Bell GI, Patterson BW, Nichols CG, Polonsky KS: Kir6.2 variant E23K increases ATP-sensitive K+ channel activity and is associated with impaired insulin release and enhanced insulin sensitivity in adults with normal glucose tolerance. Diabetes 2009;58:1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones CN, Pei D, Staris P, Polonsky KS, Chen YD, Reaven GM: Alterations in the glucose-stimulated insulin secretory dose-response curve and in insulin clearance in nondiabetic insulin-resistant individuals. J Clin Endocrinol Metab 1997;82:1834–1838 [DOI] [PubMed] [Google Scholar]
- 19.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX: The use of areas under curves in diabetes research. Diabetes Care 1995;18:245–250 [DOI] [PubMed] [Google Scholar]
- 20.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C: Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001;50:150–158 [DOI] [PubMed] [Google Scholar]
- 21.Breda E, Toffolo G, Polonsky KS, Cobelli C: Insulin release in impaired glucose tolerance: oral minimal model predicts normal sensitivity to glucose but defective response times. Diabetes 2002;51(Suppl. 1):S227– S233 [DOI] [PubMed] [Google Scholar]
- 22.Van Cauter E, Mestrez F, Sturis J, Polonsky KS: Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 23.Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W: Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986;63:492–498 [DOI] [PubMed] [Google Scholar]
- 24.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C: Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 2004;287:E637–E643 [DOI] [PubMed] [Google Scholar]
- 25.Dalla Man C, Caumo A, Cobelli C: The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 2002;49:419–429 [DOI] [PubMed] [Google Scholar]
- 26.Dalla Man C, Yarasheski KE, Caumo A, Robertson H, Toffolo G, Polonsky KS, Cobelli C: Insulin sensitivity by oral glucose minimal models: validation against clamp. Am J Physiol Endocrinol Metab 2005;289:E954–E959 [DOI] [PubMed] [Google Scholar]
- 27.Meier JJ, Nauck MA, Kranz D, Holst JJ, Deacon CF, Gaeckler D, Schmidt WE, Gallwitz B: Secretion, degradation, and elimination of glucagon-like peptide 1 and gastric inhibitory polypeptide in patients with chronic renal insufficiency and healthy control subjects. Diabetes 2004;53:654–662 [DOI] [PubMed] [Google Scholar]
- 28.Shu L, Matveyenko AV, Kerr-Conte J, Cho JH, McIntosh CH, Maedler K: Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function. Hum Mol Genet 2009;18:2388–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson ER: Translating TCF7L2: from gene to function. Diabetologia 2009;52:1227–1230 [DOI] [PubMed] [Google Scholar]