Abstract
OBJECTIVE
We have previously shown that lack of thioredoxin-interacting protein (TXNIP) protects against diabetes and glucotoxicity-induced β-cell apoptosis. Because the role of TXNIP in lipotoxicity is unknown, the goal of the present study was to determine whether TXNIP expression is regulated by fatty acids and whether TXNIP deficiency also protects β-cells against lipoapoptosis.
RESARCH DESIGN AND METHODS
To determine the effects of fatty acids on β-cell TXNIP expression, INS-1 cells and isolated islets were incubated with/without palmitate and rats underwent cyclic infusions of glucose and/or Intralipid prior to islet isolation and analysis by quantitative real-time RT-PCR and immunoblotting. Using primary wild-type and TXNIP-deficient islets, we then assessed the effects of palmitate on apoptosis (transferase-mediated dUTP nick-end labeling [TUNEL]), mitochondrial death pathway (cytochrome c release), and endoplasmic reticulum (ER) stress (binding protein [BiP], C/EBP homologous protein [CHOP]). Effects of TXNIP deficiency were also tested in the context of staurosporine (mitochondrial damage) or thapsigargin (ER stress).
RESULTS
Glucose elicited a dramatic increase in islet TXNIP expression both in vitro and in vivo, whereas fatty acids had no such effect and, when combined with glucose, even abolished the glucose effect. We also found that TXNIP deficiency does not effectively protect against palmitate or thapsigargin-induced β-cell apoptosis, but specifically prevents staurosporine- or glucose-induced toxicity.
CONCLUSIONS
Our results demonstrate that unlike glucose, fatty acids do not induce β-cell expression of proapoptotic TXNIP. They further reveal that TXNIP deficiency specifically inhibits the mitochondrial death pathway underlying β-cell glucotoxicity, whereas it has very few protective effects against ER stress–mediated lipoapoptosis.
Pancreatic β-cell loss by apoptosis is a major factor in the pathogenesis of type 1 and type 2 diabetes (1–5). Two highly interconnected intrinsic signaling pathways, the mitochondrial death pathway and endoplasmic reticulum (ER) stress, can lead to β-cell apoptosis (6). In addition, although multiple processes can activate either one or both pathways and thereby contribute to the phenomenon of β-cell loss, glucotoxicity and lipotoxicity are key stimuli especially in type 2 diabetes (7,8). However, the detailed molecular mechanisms involved have just begun to be unraveled.
Recently, we discovered that thioredoxin-interacting protein (TXNIP) acts as a critical link between glucotoxicity and pancreatic β-cell apoptosis (9) and that TXNIP deficiency protects against streptozotocin- as well as against obesity-induced diabetes (10). TXNIP (also called vitamin D3-upregulated gene 1 [VDUP1], or thioredoxin-binding protein 2 [TBP-2]) is a ubiquitously expressed 50-kDa protein (11,12). As suggested by its name, TXNIP binds and inhibits thioredoxin, a thiol-oxidoreductase and major cellular reducing system member, and thereby promotes oxidative stress and regulates the cellular redox state (13–17).
Originally, we identified TXNIP as the most dramatically upregulated gene in response to glucose in a human islet oligonucleotide microarray study (18), found that its expression was increased in islets of diabetic mice (9), and further demonstrated that it induces pancreatic β-cell death (19). More recently, we found that TXNIP is essential for glucotoxic β-cell death (9) and discovered that the functional β-cell mass was significantly increased in TXNIP-deficient HcB-19 mice harboring a nonsense mutation in their TXNIP gene as well as in β-cell–specific TXNIP knockout mice (bTKO) (10). Of note, this was the case despite the fact that HcB-19 mice are hyperlipidemic (10,20,21). Moreover, generation of a double-mutant congenic BTBRlepob/obtxniphcb/hcb mouse lacking leptin as well as TXNIP revealed that TXNIP deficiency was able to reduce β-cell apoptosis >50-fold, to increase pancreatic β-cell mass, and to prevent diabetes in this very severe model of type 2 diabetes associated with marked obesity, insulin resistance, and hyperlipidemia (10). Together these findings raised the possibility that TXNIP deficiency may not only play a role in glucotoxicity, but also be protective against lipotoxicity. The aim of the present study was therefore to assess whether fatty acids regulate β-cell TXNIP expression in vitro and in vivo and to ascertain whether lack of TXNIP protects β-cells against lipoapoptosis.
RESEARCH DESIGN AND METHODS
Animal studies.
All animal studies were approved by the respective Institutional Animal Care and Use Committees and the National Institutes of Health principles of laboratory animal care were followed. The C3H congenic TXNIP-deficient HcB-19 (HcB) mice harboring a naturally occurring nonsense mutation in the TXNIP gene and the control C3H/DiSnA (C3H) strain have been described previously (12,21,22). bTKO and lox/lox control littermates were generated by the Cre-LoxP system and are described in detail elsewhere (10).
Male Wistar rats (Charles River, Saint-Constant, Quebec, Canada) were housed under standard conditions. In vivo infusion studies were performed as described previously (23). In brief, indwelling catheters were placed in the left carotid artery and the right jugular vein under general anesthesia and animals were allowed to recover for 5 days. They were then randomized into four groups receiving either 0.9% saline, 50% glucose, 20% Intralipid (with 20 units/ml of heparin), or glucose plus Intralipid through Harvard infusion pumps (Harvard Apparatus, Holliston, MA). These were administered in alternating 4-h cycles of glucose or saline followed by Intralipid or saline for 4 h and the infusion profile was repeated for a total of 72 h until sacrifice. All animals received the same volume of fluid and had free access to food and water during the infusions.
Islet isolation.
Mouse pancreatic islets were isolated by collagenase digestion as described previously (24,25). In brief, immediately after sacrifice pancreata were inflated with 5 ml collagenase solution (0.40 mg/ml type XI collagenase [Sigma, St. Louis, MO] in Hanks balanced salt solution [HBSS; Invitrogen, Carlsbad, CA] with 0.02% radioimmunoassay-grade BSA [Sigma]) and placed in 25 ml of the same solution, gassed with 95% O2/5% CO2 for 5 min, and vigorously shaken at 37°C for 14 min. After a quick spin, the tissue pellet was washed twice with 10 ml cold HBSS, passed through a 925-micron Spectra mesh filter (Fisher, St. Louis, MO) to remove large debris, and resuspended in 5 ml of 25% Ficoll (type 400-DL; Sigma) prepared with HBSS in a 50-ml conical tube. Then, 2.5 ml of 23%, 20.5%, and 11% Ficoll were layered carefully on top of each other, and the gradient was centrifuged for 15 min at 800g. Layers above the 25% Ficoll containing the isolated islets were collected and washed with HBSS, and the islets were pelleted by a 5-min centrifugation at 800g. To further exclude contamination by exocrine tissue, islets were handpicked under stereomicroscopic observation and incubated at low or high glucose with or without palmitate.
Rat pancreatic islets were isolated by collagenase digestion and gradient centrifugation as described previously (23).
Tissue culture.
Mouse islets (C3H, HcB-19, lox/lox, and bTKO) were incubated in RPMI 1640 (Invitrogen) supplemented with 1% BSA and 1% penicillin-streptomycin and 2.8 mmol/l or 16.7 mmol/l glucose for 24 h at 37°C in the presence or absence of 1 mmol/l palmitate. Rat islets were cultured for 24 h in the presence of 2.8 or 16.7 mmol/l glucose with or without 0.5 mmol/l palmitate as described previously (26).
INS-1 cells were grown in RPMI 1640 (Invitrogen) containing 11.1 mmol/l glucose and supplemented with 10% FBS, 1% penicillin-streptomycin, 1 mmol/l sodium pyruvate, 2 mmol/l l-glutamine, 10 mmol/l HEPES, and 0.05 mmol/l 2-mercaptoethanol.
Culture media containing palmitate were prepared as described previously (27), with minor modifications. The stock solution was prepared by dissolving sodium palmitate (Sigma) in ethanol/water (1:1, vol/vol) at 65°C for 15 min at a final concentration of 150 mmol/l. Aliquots of stock solution were complexed with fatty-acid–free BSA (10% in water; Sigma) by incubation for 1 h at 37°C and then diluted in culture media. The final molar ratio of fatty acid/BSA was 5:1 for all experiments. The final ethanol concentration was ≤0.33% (vol/vol). The control condition included a solution of vehicle (ethanol/water) mixed with fatty acid–free BSA at the same concentration as the palmitate solution. Staurosporine and thapsigargin were from Invitrogen and were dissolved in DMSO.
TUNEL.
For transferase-mediated dUTP nick-end labeling (TUNEL), ∼100 isolated mouse islets were mixed with 15 μl of Affi-Gel Blue Gel (Biorad, Hercules, CA), fixed in 4% formaldehyde, and washed in PBS, and the pellet was resuspended in 0.5 ml of warm 2% Difco-Agar in an Eppendorf tube and centrifuged for 10 s at 10,000 rpm. After solidification, the agar containing the islet pellet was removed from the tube, trimmed, refixed, and processed in an automated Shandon Citadel 100 machine before paraffin embedding and preparation of 5-μm sections.
The DeadEnd Fluorometric TUNEL System Kit (Promega, Madison, WI) was used according to the manufacturer's instructions, but including a permeabilization step (5 min in a 1% Triton X-100 PBS solution). β-Cells were visualized by insulin staining using guinea pig anti-insulin antibody (ZYMED, San Francisco, CA) and cyanin 3–conjugated anti–guinea pig IgG (1:500; Jackson ImmunoResearch, Westgrove, PA). The Vectashield with DAPI mounting solution (VECTOR, Burlingame, CA) was used for visualization of nuclei.
Quantitative real-time RT-PCR.
RNA was extracted using the RNeasy Mini kit (Qiagen), converted to cDNA with the SuperScript III First-Strand Synthesis Super Mix (Invitrogen), and analyzed on a Prism 7000 Sequence Detection System (Applied Biosystems). TXNIP was measured using primers recognizing rat TXNIP, forward: 5′-CGAGTCAAAGCCGTCAGGAT-3′, reverse: 5′-TTCATAGCGCAAGTAGTCCAAGGT-3′. Binding protein (BiP) was amplified using the forward primer 5′-ACGTCCAACCCGGAGAACA-3′ and the reverse primer 5′-TTCCAAGTGCGTCCGATGA-3′ and C/EBP homologous protein (CHOP), with 5′-TGGCACAGCTTGCTGAAGAG-3′ and 5′-TCAGGCGCTCGATTTCCT-3′, respectively. All samples were corrected for the 18S ribosomal subunit (Applied Biosystems) run as an internal standard.
Immunoblotting.
Protein extraction and immunoblotting were performed as described previously (9) using the following antibodies: TXNIP (JY2; MBL International, Woburn, MA) (1:400), monoclonal cleaved caspase-3 (Cell Signaling, Boston, MA) (1:200), β-actin (Abcam, Cambridge, MA) (1:200), anti-mouse IgG (1:5,000) (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-rabbit IgG (Biorad).
Cytochrome c release.
INS-1 cells were incubated at 5 mmol/l or 25 mmol/l glucose with or without 1 mmol/l palmitate for 24 h prior to cell fractionation.
Cytosolic and mitochondrial cell fractions were obtained and analyzed for cytochrome c by immunoblotting as described previously (9) using a rabbit cytochrome c antibody (Cell Signaling) and anti-rabbit IgG (Santa Cruz Biotechnology).
Statistical analysis.
To calculate the significance of a difference between two means, we used two-sided Student t tests and a P value of < 0.05 was considered statistically significant. To compare data sets of more than two groups, one-way ANOVA was used followed by Holm-Sidak tests for all pairwise multiple comparisons.
RESULTS
Fatty acids do not induce TXNIP expression in vitro or in vivo.
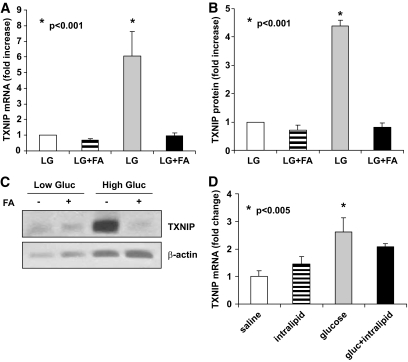
We previously showed that glucose acts as a potent stimulus of β-cell TXNIP expression (9,18,19). However, the effects of fatty acids on TXNIP expression have not been studied. To first examine whether fatty acids modulate TXNIP expression in vitro, isolated rat or mouse islets were incubated in the presence or absence of palmitate at low or high glucose concentrations and TXNIP expression was assessed by quantitative real-time RT-PCR and immunoblotting (Fig. 1A–C). Fatty acids did not alter TXNIP expression in these experiments and, interestingly, the concomitant presence of palmitate completely abolished the stimulatory effect of glucose on TXNIP expression. This was also confirmed in vivo in islets isolated from rats after cyclic 20% Intralipid infusions with and without additional glucose infusions as described in Research Design and Methods. Whereas cyclic glucose infusions led to a significant increase in TXNIP expression compared with saline, Intralipid infusions alone had no effect and, when combined with glucose, suppressed the glucose-stimulated TXNIP induction (Fig. 1D). This suggests that unlike glucose, fatty acids do not increase TXNIP expression in islets and, further, block the effect of glucose on TXNIP expression.
FIG. 1.
In vitro and in vivo effects of fatty acids (FA) on islet TXNIP expression. A: Isolated rat islets were incubated at 2.8 mmol/l glucose (LG) or 2.8 mmol/l glucose (Gluc) and 0.5 mmol/l palmitate (LG+FA), 16.7 mmol/l glucose (HG), or 16.7 mmol/l and 0.5 mmol/l palmitate (HG+FA) for 24 h and TXNIP expression was analyzed by real-time RT-PCR, n = 4. B and C: Isolated wild-type mouse islets were incubated at LG or HG with our without palmitate (1 mmol/l) for 24 h and analyzed for changes in TXNIP protein levels by immunoblotting. Bars represent mean fold change ± SEM in TXNIP protein corrected for β-actin; three independent experiments were performed; one representative immunoblot is shown. D: Male Wistar rats received cyclic infusions of saline 0.9%, glucose 50%, Intralipid 20%, or glucose and Intralipid for a total of 72 h as described in research design and methods prior to sacrifice. Their islets were isolated and analyzed for TXNIP mRNA expression using quantitative real-time RT-PCR. Bars represent mean fold change ± SEM compared with saline. Four independent experiments were performed.
TXNIP deficiency does not protect against β-cell lipoapoptosis.
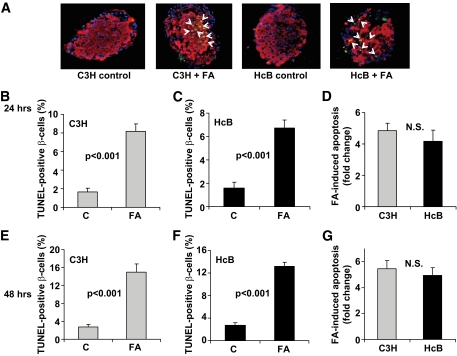
TXNIP deficiency effectively protects pancreatic β-cells against glucotoxic cell death (9). To test whether it may also protect against lipotoxic β-cell apoptosis, we incubated isolated islets of TXNIP-deficient HcB-19 and control C3H mice in the presence or absence of 1 mmol/l palmitate. Palmitate led to a significant fivefold increase in TUNEL-positive β-cells in islets from both C3H- and TXNIP-deficient HcB-19 islets (Fig. 2). We also performed a palmitate dose-response curve and assessed β-cell apoptosis by cleaved caspase-3 measurements as well as by TUNEL. With both methods, a very similar dose-dependent increase in apoptosis was seen (supplementary Fig. 1, available in an online appendix at http://diabetes.diabetesjournals.org/content/full/db09-0949/DC1), confirming that our TUNEL experiments were providing adequate quantification of β-cell apoptosis. In addition, we analyzed the effects of palmitate at 24 h (Fig. 2A–D) as well as at 48 h (Fig. 2E–G). In both C3H and HcB-19 islets, we observed that the percentage of apoptotic β-cells almost doubled at 48 h, demonstrating that we were still in the dynamic range with our 24 h our findings and suggesting that lack of TXNIP is not able to protect against lipoapoptosis.
FIG. 2.
β-Cell apoptosis in TXNIP-deficient HcB-19 and control C3H mice in response to palmitate. Isolated islets were incubated at 11.1 mmol/l glucose in the absence or presence of 1 mmol/l palmitate (FA) and the effects on β-cell apoptosis were assessed by TUNEL. A: Representative islet images. White arrows point at TUNEL-positive apoptotic nuclei; red = insulin; blue = nuclei. Quantification of palmitate-induced β-cell apoptosis after 24 h (B–D) and after 48 h (E–G); bars represent means ± SEM. More than 12 islets and more than 700 β-cell nuclei were analyzed per group. (A high-quality digital representation of this figure is available in the online issue.)
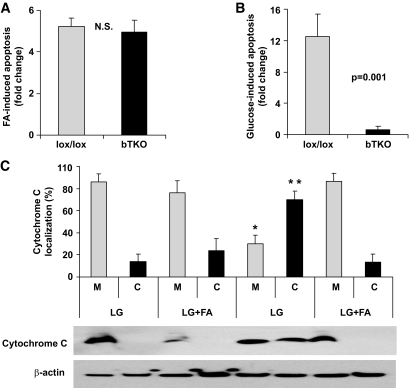
To further substantiate this observation, we performed analogous experiments using isolated islets from bTKO and control lox/lox mice. As observed using HcB-19 islets, β-cell–specific deletion of TXNIP in bTKO islets was not able to prevent the more than fivefold increase in β-cell apoptosis induced by palmitate (Fig. 3A), although it completely prevented glucotoxicity-induced β-cell apoptosis (Fig. 3B), consistent with our earlier findings (9). These results suggest that the protective effect of TXNIP deficiency might be pathway specific and that glucose and fatty acids might affect different features of β-cell apoptosis. The two major intrinsic pathways implicated in pancreatic β-cell death are mitochondrial damage and ER stress (6). Because we previously showed that TXNIP is involved in mitochondria-mediated apoptosis (9), we first compared the effects of glucotoxicity and lipotoxicity on this pathway. Indeed we found that although incubation at high glucose induced the mitochondrial death pathway as shown by the pronounced release of cytochrome c from the mitochondria into the cytosol, palmitate had no such effect and, when added to high glucose, even normalized the cytochrome c distribution (Fig. 3C), suggesting that mitochondria-mediated apoptosis does not play a major role in lipoapoptosis. In contrast, we found that palmitate increased the expression of the ER stress markers BiP and CHOP in INS-1 cells and rat islets (supplementary Fig. 2). Although these findings are consistent with previous studies and suggest that β-cell lipoapoptosis is associated primarily with ER stress (28–36), there is also a significant body of work implicating mitochondria in lipotoxicity (37–41), a discrepancy that is most likely due to the intricate cross talk between mitochondria and the ER (42,43).
FIG. 3.
Different effects of lipotoxicity and glucotoxicity on TXNIP knockout (bTKO) β-cells and mitochondria-mediated apoptosis. Isolated islets of bTKO and control (lox/lox) mice were incubated at (A) 11.1 mmol/l glucose ± palmitate (1 mmol/l) or (B) at 5 versus 25 mmol/l glucose for 24 h and analyzed by TUNEL. At least 12 islets and more than 700 β-cell nuclei were analyzed per group. Bars represent mean fold change ± SEM in β-cell apoptosis. C: Cytochrome c release from the mitochondria (M) into the cytosol (C) was measured by subcellular fractionation and immunoblotting in INS-1 cells incubated for 24 h at low glucose (LG; 5 mmol/l) or high glucose (HG; 25 mmol/l) with or without palmitate (FA). Bars represent means ± SEM of three independent experiments and a representative immunoblot is shown. *P < 0.01 (M) HG versus LG; **P < 0.01 (C) HG versus LG.
Lack of TXNIP protects from staurosporine-induced but not thapsigargin-induced β-cell apoptosis.
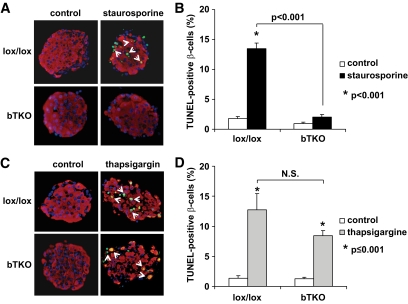
Combined with the finding that TXNIP deficiency protected against only glucotoxic but not lipotoxic cell death, our results suggest that lack of TXNIP may specifically inhibit mitochondria- but not ER stress–mediated β-cell apoptosis. To directly test this hypothesis, we treated isolated islet of bTKO and lox/lox mice with staurosporine, a well-known stimulus of the mitochondrial death pathway. Although staurosporine led to a significant >10-fold increase in β-cell apoptosis in lox/lox islets compared with islets incubated with DMSO vehicle only, TXNIP-deficient bTKO islets were completely protected against staurosporine-induced apoptosis (Fig. 4A and B). (Of note, staurosporine did not increase TXNIP expression [1.0 vs. 1.0-fold ±0.02], demonstrating that the protection conferred by TXNIP deficiency is not limited to stimuli that increase TXNIP expression, but rather to those that induce mitochondrial apoptosis.)
FIG. 4.
Different response of bTKO β-cells to mitochondria (staurosporine) or ER stress (thapsigargin)-mediated apoptosis. Islets isolated from lox/lox and bTKO mice were incubated for 24 h in the presence or absence of staurosporine (0.5 μmol/l) (A and B) or thapsigargin (25 μmol/l) (C and D), and β-cell apoptosis was assessed by TUNEL. More than 12 islets and more than 700 β-cell nuclei were analyzed per group. Representative images are shown. Insulin, red; nuclei, blue; TUNEL-positive nuclei, green (white arrows). Bars represent means ± SEM. (A high-quality digital representation of this figure is available in the online issue.)
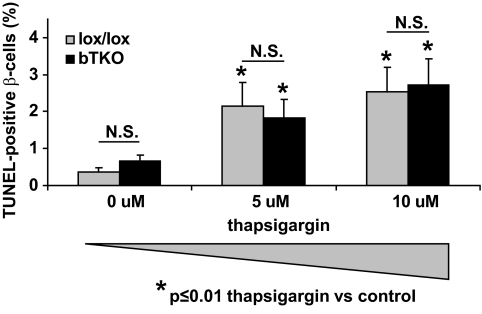
In marked contrast, incubation with the ER stress-inducer thapsigargin led to an ∼10-fold increase in β-cell apoptosis in both lox/lox and bTKO islets and although the percentage of apoptotic β-cells was slightly lower in the bTKO islets, TXNIP deficiency had no significant protective effect (P = 0.119) (Fig. 4C and D), indicating that the protective effects of TXNIP deficiency are largely restricted to mitochondria-mediated apoptosis. To compare the effects of staurosporine and thapsigargin, we had to achieve similar levels of β-cell apoptosis and therefore used this higher dose of 25 μmol/l of thapsigargin. However, dose-response experiments at significantly lower thapsigargin concentrations confirmed that lack of TXNIP was not effective in protecting against thapsigargin-induced β-cell death even at apoptosis rates as low as 2% (Fig. 5).
FIG. 5.
Comparison of β-cell apoptosis in bTKO and control lox/lox islets exposed to different doses of thapsigargin. Islets isolated from lox/lox and bTKO mice were incubated for 24 h in the presence or absence of thapsigargin at the designated concentrations and β-cell apoptosis was assessed by TUNEL. More than 12 islets and more than 700 β-cell nuclei were analyzed per group. Bars represent means ± SEM.
DISCUSSION
The results of this study uniquely identify TXNIP as a specific mediator of the mitochondrial death pathway in β-cells under glucotoxic conditions, while revealing that TXNIP does not play a significant role in ER stress–mediated lipoapoptosis.
Recently, we discovered that TXNIP represents a critical link between glucotoxicity and β-cell apoptosis (9) and that lack of TXNIP protects against streptozotocin- and obesity-induced diabetes, raising the possibility that TXNIP deficiency might also be protective against lipotoxicity. However, using islets of TXNIP-deficient HcB-19 and bTKO mice, the results of the present study indicate that lack of TXNIP is unable to prevent or inhibit β-cell apoptosis induced by fatty acids, although it effectively protects against glucose-induced β-cell death (Figs. 2 and 3).
Even though both glucotoxicity and lipotoxicity play important roles in the pathogenesis of diabetes and diabetic β-cell loss (8) and culminate in β-cell apoptosis, different signaling pathways are involved, and the relative contribution from mitochondrial and ER stress remains under discussion (28–33,37–41,44). This controversy is most likely due to the intricate cross talk between these two organelles and the apoptosis pathways that ultimately lead to β-cell death (42,43) (Fig. 6). In fact, mitochondria represent the major source of ATP and reactive oxygen species, which in turn can stimulate ER stress and activate apoptosis signal-regulating kinase 1 (ASK1) (45). On the other hand, the ER supplies the mitochondria with calcium, a process that is now believed to require physical interaction between the organelles mediated by mitofusin 2 (46). In addition, CHOP has been reported to promote reactive oxygen species formation, whereas CHOP deletion reduced oxidative stress and enhanced β-cell survival (47). Moreover, ER stress also activates ASK1 through formation of an IRE1 (serine-threonine protein kinase)–TRAF2 (tumor necrosis factor receptor-associated factor 2)–ASK1 complex, and ASK1 leads to mitochondria-dependent caspase activation and apoptosis (48). Interestingly, thioredoxin (Trx) directly binds to and inactivates ASK1, and TXNIP deficiency increases the availability of Trx for this interaction (49), suggesting that it may thereby decrease ASK1 activity and apoptosis. Finally, Trx in conjunction with glutaredoxin and NADPH has also been shown to control exocytosis, insulin secretion, and β-cell signaling (50).
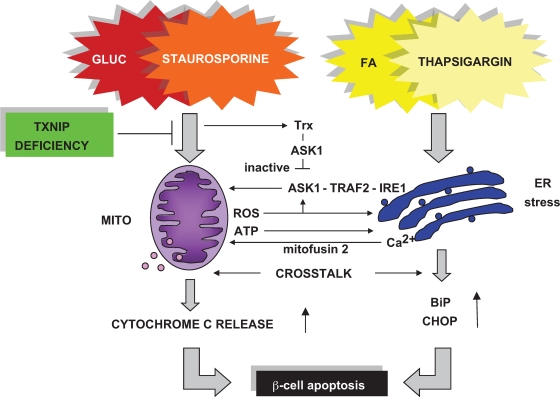
FIG. 6.
Schematic representation of TXNIP deficiency-mediated protection against β-cell apoptosis. Excess glucose (GLUC) or fatty acids (FA) ultimately lead to β-cell apoptosis by mitochondrial (staurosporine-induced) or ER stress (thapsigargin-induced) pathways. Despite extensive cross talk, TXNIP deficiency primarily blocks glucose-induced mitochondria-mediated β-cell apoptosis. Mito, mitochondria; ROS, reactive oxygen species.
Given this extensive signaling network, a clear separation between the pathways involved is, in general, impossible. However, our results demonstrate that the protective effects of TXNIP deficiency are predominantly limited to the prevention of mitochondria-mediated β-cell apoptosis, whereas ER stress–induced apoptosis remained largely unaffected (Fig. 4–5). Consistent with this observation, TXNIP deficiency was ineffective in preventing β-cell lipotoxicity, although it had significant beneficial effects in the context of β-cell glucotoxicity, which is in alignment with our previous findings (9). Nevertheless, and despite the fact that at lower thapsigargin concentrations TXNIP deficiency had absolutely no protective effects (Fig. 5), there was a trend to slightly lower levels of apoptosis in bTKO islets at the higher thapsigargin dose (Fig. 4D), suggesting that lack of TXNIP might be able to partially reduce cell death associated with severe ER stress, potentially by inhibiting mitochondrial pathways activated by ER stress and/or those involved in the ER-mitochondrial cross talk (Fig. 6).
Although we have previously shown that incubation of β-cells and primary islets at high glucose induces TXNIP expression and that diabetic mice have elevated islet TXNIP levels (9,18,19), this is the first demonstration that repeated infusions of glucose can lead to a significant increase in β-cell TXNIP expression (Fig. 1D). These in vivo infusion experiments were not designed to induce or assess β-cell apoptosis (26), and previous work has suggested that glucose infusion does not cause β-cell apoptosis (51). However, it is tempting to speculate that postprandial glucose excursions in diabetic or pre-diabetic patients may result in a similar increase in β-cell TXNIP expression, and given the strong proapoptotic properties of TXNIP this may, over time, contribute to the gradual β-cell loss observed (52) as well as to the overall detrimental effects of postprandial hyperglycemia appreciated clinically (53).
Surprisingly, fatty acids not only failed to induce TXNIP expression, but essentially blocked glucose-induced TXNIP expression both in vitro and in vivo (Fig. 1). This is consistent with a recent observation in INS-1E cells, where, fatty acid–induced insulin secretion also led to inhibition of TXNIP expression (54). In addition, palmitate has been shown to lead to exclusion of carbohydrate responsive element-binding protein (ChREBP) out of the nucleus and thereby to cause a fatty acid “sparing” effect on glucose-induced transcription (55). Because we have shown that glucose-induced β-cell TXNIP expression is mediated by ChREBP (56), these processes may also contribute to the observed reduction in glucose-induced TXNIP expression in response to palmitate. The inhibition of glucose-induced TXNIP expression and cytochrome c release by the concomitant presence of fatty acids also suggests that when both fuels are elevated under glucolipotoxic conditions, the TXNIP-independent, fatty acid–mediated ER stress apoptotic pathway may take precedence over TXNIP-mediated mitochondrial cell death.
In summary, we have found that lack of TXNIP protects primarily against β-cell apoptosis mediated by the mitochondrial death pathway under glucotoxic conditions, whereas lipoapoptosis mediated by ER stress could not be prevented by TXNIP deficiency. Nevertheless, it is likely that TXNIP deficiency also affects components of the ER-mitochondrial cross talk. Moreover, our results indicate that, unlike glucose, fatty acids do not induce TXNIP expression, revealing the specificity of both the upstream control of TXNIP expression as well as the downstream TXNIP effects on apoptotic signaling. Together, these findings provide new insight into the specific regulation and function of TXNIP that further helps in establishing the role of TXNIP as a target for diabetes therapy. In addition, this work enhances our understanding of the molecular pathways controlling the life and death of the pancreatic β-cell.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-078752 to A.S. and R01-DK-58096 to V.P.), the American Diabetes Association (7-07-CD-22), the Juvenile Diabetes Research Foundation (1-2007-790), and National Heart, Lung, and Blood Institute (R21HL-089205) to A.S. The study is the result of work supported with resources and use of facilities at the William S. Middleton Memorial Veterans Hospital (Madison, WI). G.F. is supported by a postdoctoral fellowship from the Canadian Diabetes Association. V.P. holds the Canada Research Chair in Diabetes and Pancreatic β-Cell Function.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Mandrup-Poulsen T: Beta-cell apoptosis: stimuli and signaling. Diabetes 2001;50(Suppl. 1):S58–S63 [DOI] [PubMed] [Google Scholar]
- 2.Mandrup-Poulsen T: Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol 2003;66:1433–1440 [DOI] [PubMed] [Google Scholar]
- 3.Bonner-Weir S: Life and death of the pancreatic beta cells. Trends Endocrinol Metab 2000;11:375–378 [DOI] [PubMed] [Google Scholar]
- 4.Pick A, Clark J, Kubstrup C, et al. : Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes 1998;47:358–364 [DOI] [PubMed] [Google Scholar]
- 5.Mathis D, Vence L, Benoist C: Beta-cell death during progression to diabetes. Nature 2001;414:792–798 [DOI] [PubMed] [Google Scholar]
- 6.Cnop M, Welsh N, Jonas JC, et al. : Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005;54(Suppl. 2):S97–S107 [DOI] [PubMed] [Google Scholar]
- 7.Kaiser N, Leibowitz G, Nesher R: Glucotoxicity and beta-cell failure in type 2 diabetes mellitus. J Pediatr Endocrinol Metab 2003;16:5–22 [DOI] [PubMed] [Google Scholar]
- 8.Poitout V, Robertson RP: Minireview: secondary beta-cell failure in type 2 diabetes—a convergence of glucotoxicity and lipotoxicity. Endocrinology 2002;143:339–342 [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Saxena G, Mungrue IN, et al. : Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes 2008;57:938–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Hui ST, Couto FM, et al. : Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J 2008;22:3581–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen KS, DeLuca HF: Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta 1994;1219:26–32 [DOI] [PubMed] [Google Scholar]
- 12.Bodnar JS, Chatterjee A, Castellani LW, et al. : Positional cloning of the combined hyperlipidemia gene Hyplip1. Nat Genet 2002;30:110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Junn E, Han SH, Im JY, et al. : Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol 2000;164:6287–6295 [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama A, Masutani H, Nakamura H, et al. : Redox regulation by thioredoxin and thioredoxin-binding proteins. IUBMB Life 2001;52:29–33 [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama A, Matsui M, Iwata S, et al. : Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem 1999;274:21645–21650 [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka H, Maehira F, Oshiro M, et al. : A possible interaction of thioredoxin with VDUP1 in HeLa cells detected in a yeast two-hybrid system. Biochem Biophys Res Commun 2000;271:796–800 [DOI] [PubMed] [Google Scholar]
- 17.Patwari P, Higgins LJ, Chutkow WA, et al. : The interaction of thioredoxin with Txnip: evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem 2006;281:21884–21891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shalev A, Pise-Masison CA, Radonovich M, et al. : Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology 2002;143:3695–3698 [DOI] [PubMed] [Google Scholar]
- 19.Minn AH, Hafele C, Shalev A: Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology 2005;146:2397–2405 [DOI] [PubMed] [Google Scholar]
- 20.Castellani LW, Weinreb A, Bodnar J, et al. : Mapping a gene for combined hyperlipidaemia in a mutant mouse strain. Nat Genet 1998;18:374–377 [DOI] [PubMed] [Google Scholar]
- 21.Hui TY, Sheth SS, Diffley JM, et al. : Mice lacking thioredoxin-interacting protein provide evidence linking cellular redox state to appropriate response to nutritional signals. J Biol Chem 2004;279:24387–24393 [DOI] [PubMed] [Google Scholar]
- 22.Sheth SS, Castellani LW, Chari S, et al. : Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J Lipid Res 2005;46:123–134 [DOI] [PubMed] [Google Scholar]
- 23.Hagman DK, Latour MG, Chakrabarti SK, et al. : Cyclical and alternating infusions of glucose and intralipid in rats inhibit insulin gene expression and Pdx-1 binding in islets. Diabetes 2008;57:424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minn AH, Lan H, Rabaglia ME, et al. : Increased insulin translation from an insulin splice-variant overexpressed in diabetes, obesity, and insulin resistance. Mol Endocrinol 2005;19:794–803 [DOI] [PubMed] [Google Scholar]
- 25.Lan H, Rabaglia ME, Stoehr JP, et al. : Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes 2003;52:688–700 [DOI] [PubMed] [Google Scholar]
- 26.Fontés G, Semache M, Hagman DK, et al. : Involvement of Per-Arnt-Sim Kinase and extracellular-regulated kinases-1/2 in palmitate inhibition of insulin gene expression in pancreatic beta-cells. Diabetes 2009;58:2048–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briaud I, Harmon JS, Kelpe CL, et al. : Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes 2001;50:315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunha DA, Hekerman P, Ladrière L, et al. : Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci 2008;121:2308–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeffrey KD, Alejandro EU, Luciani DS, et al. : Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc Natl Acad Sci U S A 2008;105:8452–8457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gwiazda KS, Yang TL, Lin Y, et al. : Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am J Physiol Endocrinol Metab 2009;296:E690–E701 [DOI] [PubMed] [Google Scholar]
- 31.Martinez SC, Tanabe K, Cras-Méneur C, et al. : Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes 2008;57:846–859 [DOI] [PubMed] [Google Scholar]
- 32.Kharroubi I, Ladrière L, Cardozo AK, et al. : Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology 2004;145:5087–5096 [DOI] [PubMed] [Google Scholar]
- 33.Laybutt DR, Preston AM, Akerfeldt MC, et al. : Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 2007;50:752–763 [DOI] [PubMed] [Google Scholar]
- 34.Karaskov E, Scott C, Zhang L, et al. : Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 2006;147:3398–3407 [DOI] [PubMed] [Google Scholar]
- 35.Akerfeldt MC, Howes J, Chan JY, et al. : Cytokine-induced beta-cell death is independent of endoplasmic reticulum stress signaling. Diabetes 2008;57:3034–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi SE, Lee YJ, Jang HJ, et al. : A chemical chaperone 4-PBA ameliorates palmitate-induced inhibition of glucose-stimulated insulin secretion (GSIS). Arch Biochem Biophys 2008;475:109–114 [DOI] [PubMed] [Google Scholar]
- 37.Koshkin V, Dai FF, Robson-Doucette CA, et al. : Limited mitochondrial permeabilization is an early manifestation of palmitate-induced lipotoxicity in pancreatic beta-cells. J Biol Chem 2008;283:7936–7948 [DOI] [PubMed] [Google Scholar]
- 38.Maestre I, Jordán J, Calvo S, et al. : Mitochondrial dysfunction is involved in apoptosis induced by serum withdrawal and fatty acids in the beta-cell line INS-1. Endocrinology 2003;144:335–345 [DOI] [PubMed] [Google Scholar]
- 39.Maedler K, Spinas GA, Dyntar D, et al. : Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes 2001;50:69–76 [DOI] [PubMed] [Google Scholar]
- 40.Maedler K, Oberholzer J, Bucher P, et al. : Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 2003;52:726–733 [DOI] [PubMed] [Google Scholar]
- 41.Shimabukuro M, Zhou YT, Levi M, et al. : Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A 1998;95:2498–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breckenridge DG, Germain M, Mathai JP, et al. : Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 2003;22:8608–8618 [DOI] [PubMed] [Google Scholar]
- 43.Luciani DS, Gwiazda KS, Yang TL, et al. : Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes 2009;58:422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai E, Bikopoulos G, Wheeler MB, et al. : Differential activation of ER stress and apoptosis in response to chronically elevated free fatty acids in pancreatic beta-cells. Am J Physiol Endocrinol Metab 2008;294:E540–E550 [DOI] [PubMed] [Google Scholar]
- 45.Soberanes S, Urich D, Baker CM, et al. : Mitochondrial complex III-generated oxidants activate ASK1 and JNK to induce alveolar epithelial cell death following exposure to particulate matter air pollution. J Biol Chem 2009;284:2176–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Brito OM, Scorrano L: Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008;456:605–610 [DOI] [PubMed] [Google Scholar]
- 47.Song B, Scheuner D, Ron D, et al. : Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 2008;118:3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishitoh H, Matsuzawa A, Tobiume K, et al. : ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 2002;16:1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saitoh M, Nishitoh H, Fujii M, et al. : Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 1998;17:2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivarsson R, Quintens R, Dejonghe S, et al. : Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 2005;54:2132–2142 [DOI] [PubMed] [Google Scholar]
- 51.Tang C, Han P, Oprescu AI, et al. : Evidence for a role of superoxide generation in glucose-induced beta-cell dysfunction in vivo. Diabetes 2007;56:2722–2731 [DOI] [PubMed] [Google Scholar]
- 52.Rhodes CJ: Type 2 diabetes-a matter of beta-cell life and death? Science 2005;307:380–384 [DOI] [PubMed] [Google Scholar]
- 53.Tibaldi J: Importance of postprandial glucose levels as a target for glycemic control in type 2 diabetes. South Med J 2009;102:60–66 [DOI] [PubMed] [Google Scholar]
- 54.Shaked M, Ketzinel-Gilad M, Ariav Y, et al. : Insulin counteracts glucotoxic effects by suppressing thioredoxin-interacting protein production in INS-1E beta cells and in Psammomys obesus pancreatic islets. Diabetologia 2009;52:636–644 [DOI] [PubMed] [Google Scholar]
- 55.Kawaguchi T, Osatomi K, Yamashita H, et al. : Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem 2002;277:3829–3835 [DOI] [PubMed] [Google Scholar]
- 56.Cha-Molstad H, Saxena G, Chen J, et al. : Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. J Biol Chem 2009;284:16898–16905 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.