Abstract
OBJECTIVE
Diabetic nephropathy is one of the most common causes of end-stage renal failure. Inhibition of ACE2 function accelerates diabetic kidney injury, whereas renal ACE2 is downregulated in diabetic nephropathy. We examined the ability of human recombinant ACE2 (hrACE2) to slow the progression of diabetic kidney injury.
RESEARCH DESIGN AND METHODS
Male 12-week-old diabetic Akita mice (Ins2WT/C96Y) and control C57BL/6J mice (Ins2WT/WT) were injected daily with placebo or with rhACE2 (2 mg/kg, i.p.) for 4 weeks. Albumin excretion, gene expression, histomorphometry, NADPH oxidase activity, and peptide levels were examined. The effect of hrACE2 on high glucose and angiotensin II (ANG II)–induced changes was also examined in cultured mesangial cells.
RESULTS
Treatment with hrACE2 increased plasma ACE2 activity, normalized blood pressure, and reduced the urinary albumin excretion in Akita Ins2WT/C96Y mice in association with a decreased glomerular mesangial matrix expansion and normalization of increased α-smooth muscle actin and collagen III expression. Human recombinant ACE2 increased ANG 1–7 levels, lowered ANG II levels, and reduced NADPH oxidase activity. mRNA levels for p47phox and NOX2 and protein levels for protein kinase Cα (PKCα) and PKCβ1 were also normalized by treatment with hrACE2. In vitro, hrACE2 attenuated both high glucose and ANG II–induced oxidative stress and NADPH oxidase activity.
CONCLUSIONS
Treatment with hrACE2 attenuates diabetic kidney injury in the Akita mouse in association with a reduction in blood pressure and a decrease in NADPH oxidase activity. In vitro studies show that the protective effect of hrACE2 is due to reduction in ANG II and an increase in ANG 1–7 signaling.
Chronic kidney disease is recognized as an increasing global public health problem due in part to the increasing prevalence of diabetes (1–3). Activation of the renin-angiotensin system (RAS) and the generation of angiotensin II (ANG II) play an important pathogenic role in diabetic nephropathy, and blockade of the RAS attenuates the development of diabetic kidney injury (4–8). The discovery of a homologue of the classical ACE, ACE2, has introduced a new enzyme in ANG peptide metabolism (9–12). Like ACE, ACE2 is membrane bound, but it is a monocarboxypeptidase that generates ANG (1–7) from the octapeptide ANG II (9,10,12,13). As such, ACE2 serves as an endogenous negative regulator of the renin-angiotensin system.
In animal models of diabetes, early increases in ACE2 mRNA levels, protein expression, and ACE2 activity occurs (14,15), whereas ACE2 mRNA and protein levels have been found to decrease in older streptozotocin-induced diabetic rats (16). Loss of ACE2 is associated with age-dependent glomerulosclerosis and albuminuria (17) and exacerbation of diabetic kidney injury in Akita mice (18) and is preventable by angiotensin type 1 (AT1) receptor blockade. In patients with type 2 diabetes, glomerular and tubular ACE2 expressions are reduced in the setting of increased ACE expression (19,20). Taken together, these studies suggest that ACE2 may play an early protective role against the development of diabetic nephropathy (18,21,22). We hypothesized that treatment with human recombinant ACE2 (hrACE2) will target the diabetic glomerulus and slow progression of diabetic nephropathy in the Akita mouse (Ins2WT/C96Y), a model of type 1 diabetes.
RESEARCH DESIGN AND METHODS
Experimental animals and protocol.
C57BL/6J and diabetic heterozygous Akita (Ins2WT/C96Y) mice were purchased from The Jackson Laboratory and bred in our animal facility. Throughout the period of study, animals were provided with free access to water and standard 18% protein rodent chow (Harlan Teklad, Madison, WI). Ins2WT/C96Y (Akita) and Ins2WT/WT mice were treated from 3 months of age with daily injections of placebo or human recombinant ACE2 at a daily dose of 2 mg/kg for 4 weeks. Twenty-four–hour urine volumes were collected at the end of 4 months and animals were killed. All experiments were conducted in accordance with the Canadian Council of Animal Care and Institutional Guidelines.
Generation and characterization of human recombinant ACE2.
The extracellular domain of human ACE2 (amino acid residues 1–740, molecular wt = 101 kDa) (9) was expressed recombinantly in CHO cells under serum-free conditions in a chemically defined medium. The expression product was purified to homogeneity by applying a capture step on a DEAE Sepharose anion exchanger resin (Pharmacia Biotech AB, Uppsala, Sweden). The eluted fractions containing the expression product were submitted to a polishing step on a Superdex 200 gel filtration column (Pharmacia Biotech AB). The expression product was compared with the commercially available ACE2 standard 933-ZN (R&D Systems, Minneapolis, MN). Chemical and immunological properties of both products were almost identical, although rhACE2 showed a 93% enzymatic activity with Mca-APK-(Dnp)-OH substrate in comparison with rhACE2 standard 933-ZN (R&D Systems). The enzymatic turnover of hrACE2 with ANG II substrate was 5.2 ± 0.1 μmol · mg−1 · min−1, and the elimination half-life of hrACE2 was 10.4 h in rhesus monkeys. The purity of the expression product was 99.99% measured by high-performance liquid chromatography.
Plasma ACE2 activity and detection of anti-ACE2 antibodies.
Plasma collected from mice injected with hrACE2 (2 mg/kg, i.p.) for 2 weeks were stored at −80°C. The enzymatic activity of rhACE2 in plasma samples was measured by its ability to cleave the fluorescent peptide substrate Mca-Ala-Pro-Lys(Dnp)-OH. Cleavage was measured in 1:5 diluted samples (final assay dilution) using excitation and emission wavelengths of 320 and 430 nm, respectively, in presence of 100 μmol/l substrate in 50 mmol/l MES, 300 mmol/l NaCl, 10 μmol/l ZnCl2, and 0.01% Brij-30 at pH 6.5. Evaluation was performed by comparing the maximal slope of the fluorescence/time curve to respective maximal slopes of a serial rhACE2 dilution in normal mouse plasma. The response to the specific peptide ACE2 inhibitor DX600 (23) (Phoenix Pharmaceuticals, Burlingame, CA) on the ACE2 activity in murine plasma was also examined.
Serum samples of mice were analyzed using an ACE2 antigen–specific enzyme-linked immunosorbent assay (ELISA) recognizing total anti–ACE2-specific IgG. Recombinant human soluble ACE2 was presented as antigen, coated at 10 μg/ml onto Maxisorp adsorption plates (Nunc, Vedbaek, Denmark) diluted in coating buffer. Remaining active groups were blocked by incubation with 3% skim milk (Difco) in PBS. Induced antibodies were detected by their constant domains using a rabbit anti-mouse IgG or a rabbit anti-mouse IgM peroxidase-labeled antibody (Zymed). Staining was performed by o-phenylenediamine dihydrochloride (OPD; Sigma-Aldrich) in staining buffer (PAA Laboratories) using H2O2 as substrate according to the manufacturer's instructions. Absorbance at 492 nm was measured using 620 nm as reference wavelength. Quantification was performed by comparison with a commercially available monoclonal mouse anti-human ACE2 antibody (R&D Systems).
Blood glucose, urinary albumin excretion, and tail-cuff blood pressure measurements.
Blood glucose levels were obtained weekly between 8:30 and 10:30 am using an Ascensia Breeze glucometer (Bayer, Toronto, ON, Canada), and hyperglycemia was stable and sustained in Ins2WT/C96Y mice, as previously reported (18). Twenty-four–hour urine collections were obtained from mice prior to sacrifice by housing them in individual mouse metabolic cages (Nalgene, model 650-0311; Nalge Nunc International, Rochester, NY) with free access to water and rodent mash. Urinary albumin concentration was measured using an indirect competitive ELISA according to the manufacturer's instructions (Albuwell M; Exocell, Philadelphia, PA).
For the measurement of tail-cuff systolic blood pressure (TC-SBP), conscious mice were placed in the restrainers and their body temperature was maintained at ∼34°C by the warming chamber. The IITC tail-cuff sensor containing both the inflation cuff and the photoelectric sensor was placed on the tail and attached to the restrainer. The cuff was inflated to a pressure of 200 mmHg and then deflated slowly. Upon reappearance of pulse signals, TC-SBP data from the IITC amplifier were recorded, analyzed, and reported by the IITC software (IITC Life Science Blood Pressure System, Woodland Hills, CA). The mice were trained on three occasions before actual recordings were made, and the corresponding TC-SBPs were averaged from three readings and used for the averaged comparisons.
Histopathology and electron microscopy.
Kidneys were harvested for pathological examination and one section was fixed in 10% neutral-buffered formalin (Sigma-Aldrich, St. Louis, MO) for 24 h and then transferred to 90% ethanol for light microscopy and immunohistochemistry, and the remaining sections were used for electron microscopy or snap-frozen for RNA extraction. The formalin-fixed tissue was embedded in paraffin and 3-micron sections were stained with periodic acid Schiff stain. A detailed methodology is included in the online appendix (available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1218/DC1).
Biochemistry, peptide analysis, and Western blot analysis.
Mice were injected intraperitoneally 10–15 min prior to sacrifice with 0.1 ml of heparin sodium (500 IU/ml; LEO Pharma, Thornhill, ON, Canada) to prevent blood clotting. Whole blood was collected from the carotid artery and jugular vein in syringes containing a mixture of rat renin inhibitor and protease inhibitors. Samples were centrifuged at 3,000 rpm at 4°C for 20 min and the plasma was stored at −80°C until analysis. Plasma potassium, glucose and creatinine, and urine creatinine were measured by VITA-TECH (Markham, ON, Canada), as previously described (18). For the measurement of peptides, isolated kidneys were quickly perfused with ice-cold saline and the renal cortices dissected and snap frozen in liquid nitrogen. Renal cortical and plasma ANG II and ANG 1–7 concentrations were measured by radioimmunoassay in the Hypertension and Vascular Disease Centre Core Laboratory at Wake Forest University School of Medicine, Winston-Salem, North Carolina, as previously described (18). Western blot analysis for protein kinase Cα (PKCα) and protein kinase B βI isoforms was carried out as previously described (24). The extracted proteins were separated using 10% SDS-PAGE gels and then transferred to nitrocellulose membrane (Millipore). The membrane was blocked in 5% milk for 2 h and incubated overnight at 4°C with specific antibody against PKCα, PKCβ1, and β-actin (Santa Cruz Biotechnology and Cell Signaling Technology). The β-actin was used as an endogenous loading control.
Real-time Taqman PCR.
mRNA expression levels of various genes were determined by TaqMan Real-time PCR using 18S rRNA as the internal standard as previously described (see supplementary Table 1 for primers and probes) (17,25). Briefly, kidney samples from mice were snap frozen in liquid nitrogen, the cortex was later dissected in an RNA-stabilizing solution (RNAlater; Ambion, Austin, TX), and RNA was extracted using TRIZOL Reagent (Invitrogen, Carlsbad, CA). Total RNA (1 μg) was reverse transcribed, and RNA expression levels were quantified by Taqman RT-PCR using a sequence detection system (Prism 7700; Applied Biosystems, Foster City, CA).
NADPH oxidase activity and dihydroethidium fluorescence.
Harlan Sprague-Dawley rat mesangial cells were cultured in Dulbecco's modified Eagle's medium supplemented with 20% FCS, penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37°C in 95% air and 5% CO2. Experiments were carried out in cells between passages 12 and 20. Sprague-Dawley rat mesangial cells were plated on 60-mm dishes with growth medium. The cells were cultured for 72 h to 90% confluence. A detailed methodology is included in the online appendix.
Statistical analysis.
Results are expressed as means ± SEM, unless otherwise specified. Student t test was used for comparison between two groups. Comparisons among multiple groups were performed by one-way ANOVA followed by multiple comparison testing (Student-Newman-Keuls test) using SPSS software (version 10.1; Chicago, IL).
RESULTS
Human recombinant ACE2 increases serum ACE2 activity and reduces urinary albumin excretion.
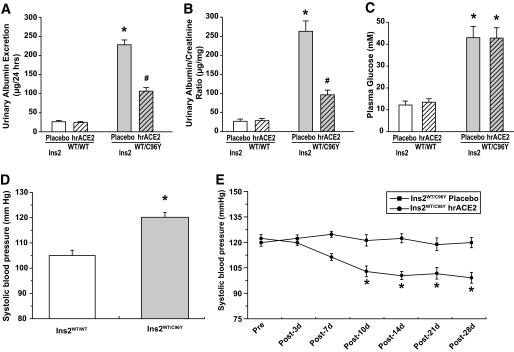
Male Ins2WT/WT (control C57BL/6J mice) and Ins2WT/C96Y (mutant diabetic Akita mice) were studied at 3 months of age (18,26). Whereas plasma ACE2 activity in Akita mice injected with placebo was undetectable, daily injection of 2 mg/kg of hrACE2 for 2 weeks resulted in measurable serum ACE2 activity of 3,138 ± 721 fluorescence unit/min (n = 6) in Akita mice that was equivalent to 7.14 ± 2.1 μg/ml of hrACE2 (n = 6). The specific ACE2 inhibitor, DX600 (1 μmol/l), suppressed 95 ± 4% of the murine plasma ACE2 activity (n = 3). We hypothesized that the large size of hrACE2 and the increased serum ACE2 activity would target the diabetic glomeruli. Treatment with hrACE2 for 4 weeks reduced the urinary albumin excretion rate by 60% in the diabetic Akita mice (Ins2WT/C96Y) compared with the placebo-treated diabetic Akita mice (Fig. 1A and B). There were no significant differences in the plasma glucose concentrations of the Ins2WT/C96Y + placebo and Ins2WT/C96Y + hrACE2 mice (Fig. 1C). Despite severe hyperglycemia in the Ins2WT/C96Y mice, body weights were similar in all four groups of mice (Table 1). Assessment of TC-SBP in conscious mice revealed mild hypertension in the Akita mice (Fig. 1D) that declined over a 4-week period in response to daily administration of hrACE2 (Fig. 1E). Human recombinant ACE2 did not affect the serum creatinine concentrations or potassium levels (Table 1).
FIG. 1.
Human recombinant ACE2 reduces the increased urinary albumin excretion rates in diabetic Akita mice independent of hyperglycemia and with a mild blood pressure–lowering effect. A and B: Urinary albumin excretion rate (A) and urinary albumin/creatinine ratio (B) based on 24-h urine samples showing marked reduction in albuminuria after 4 weeks of daily treatment with hrACE2. n = 8 and 10 for urine albumin measurements in Ins2WT/WT and Ins2WT/C96Y groups, respectively. *P < 0.05 compared with all other groups and #P < 0.05 compared with placebo + Ins2WT/C96Y group using ANOVA and multiple comparison testing. C–E: Plasma glucose and tail-cuff systolic blood pressure showing no effect of hrACE2 on the marked hyperglycemia (C) and mild elevation in systolic blood pressure in diabetic Akita mice (D) that was normalized over a 4-week period in response to daily hrACE2 administration (2 mg · kg−1 · day−1) (E). n = 10 for plasma glucose and n = 12 for systolic blood pressure measurements. *P < 0.05 compared with corresponding Ins2WT/WT group (C and D) or with Ins2WT/C96Y + placebo group (E) using Student t test.
TABLE 1.
Morphometry and plasma biochemistry in 4-month-old mice
| Ins2WT/WT+ placebo | Ins2WT/WT+ hrACE2 | Ins2WT/C96Y+ placebo | Ins2WT/C96Y+ hrACE2 | |
|---|---|---|---|---|
| n | 8 | 10 | 8 | 10 |
| BW (g) | 25.0 ± 1.2 | 25.3 ± 1.4 | 23.1 ± 0.8 | 23.4 ± 1.3 |
| KW (g) | 0.145 ± 0.06 | 0.151 ± 0.08 | 0.262 ± 0.09* | 0.254 ± 0.07* |
| KW/BW (mg/g) | 0.72 ± 0.15 | 0.63 ± 0.18 | 1.12 ± 0.23* | 1.10 ± 0.29* |
| KW/TL (mg/mm) | 7.25 ± 0.83 | 7.14 ± 0.91 | 11.23 ± 1.65* | 10.64 ± 1.47* |
| Plasma K+ (mM) | 4.12 ± 0.32 | 4.5 ± 0.36 | 4.58 ± 0.41 | 4.2 ± 0.46 |
| Creatinine (μM) | 42.7 ± 5.7 | 36.7 ± 8.4 | 45.3 ± 5.1 | 33.7 ± 8.9 |
Data are means ± SEM.
*P < 0.05 compared with corresponding nondiabetic control group. BW, body weight; KW, kidney weight; TL, tibial length.
Recombinant human ACE2 reduces mesangial matrix expansion.
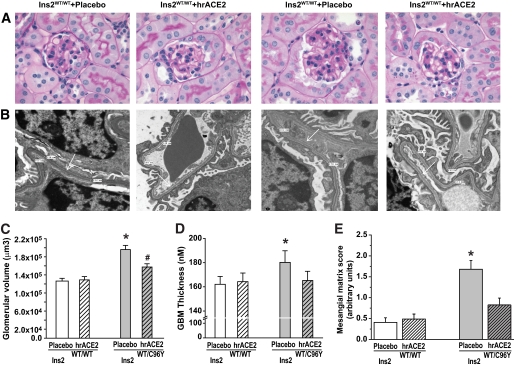
Given the marked protective effect of hrACE2 on the urinary albumin excretion in the diabetic mice, we sought to relate this functional change to kidney histomorphology. As expected, kidney hypertrophy (Table 1) was associated with an increase in glomerular volume in the Ins2WT/C96Y + placebo mice compared with the control Ins2WT/WT mice, and glomerular volume was reduced by hrACE2 treatment (Fig. 2A and C). In accordance with the light microscopic changes, increased glomerular basement membrane (GBM) thickness in the Akita Ins2WT/C96Y mice was also significantly reduced in response to hrACE2 treatment (Fig. 2B and D). Diabetic nephropathy is characterized by an accumulation of extracellular matrix proteins in the glomerular mesangium. A semiquantitative and blinded assessment of the mesangial matrix expansion showed a significant increase in the diabetic Akita mice that was reduced by treatment with hrACE2 (Fig. 2E).
FIG. 2.
Glomerular mesangial expansion and thickening of basement membrane were reduced by treatment with human recombinant ACE2. A–C: Representative light micrographs of periodic acid Schiff–stained kidney sections from each group of mice (magnification ×630) (A) with quantification of the glomerular volume (C) showing glomerular expansion in the diabetic Akita mice and a marked reduction in response to hrACE2. B and D: Transmission electron microscopy of the glomeruli (B) with quantification of the glomerular basement thickness (D) showing increased glomerular basement membrane thickness in the Akita Ins2WT/C96Y mice, which was normalized by treatment with hrACE2. White arrows indicate the glomerular basement membrane. E: Mesangial matrix expansion score showing increased mesangial expansion in diabetic Akita kidneys, which was prevented by hrACE2. n = 5 for all groups. *P < 0.05 compared with all other groups and #P < 0.05 compared with placebo + Ins2WT/WT group using ANOVA with multiple comparison testing. (A high-quality digital representation of this figure is available in the online issue.)
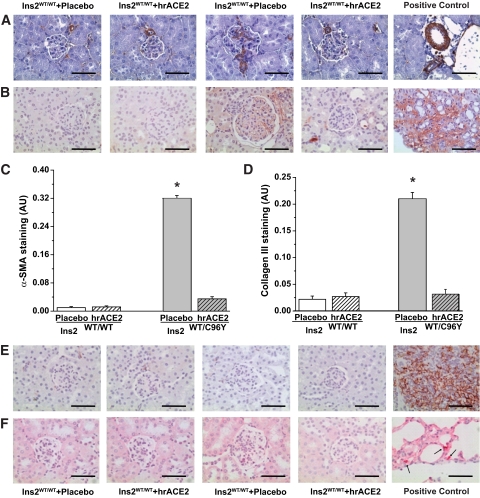
Immunohistochemical staining for α-smooth muscle actin (α-SMA) (Fig. 3A and C) and collagen III (Fig. 3B and D) was significantly increased in the glomeruli of diabetic mice, and expression was normalized by hrACE2 treatment. Inflammation and the accumulation of kidney macrophages can play an important role in diabetic kidney injury (27) and we also observed that anti-ACE2 IgG antibodies developed in 50% of the mice injected with hrACE2, with a mean IgG titer of 11 ± 7.2 ng/ml. We therefore performed immunohistochemical studies of macrophage and neutrophil infiltration in the kidneys (Fig. 3E and F). There was no evidence of glomerular or tubulointerstitial infiltration by macrophages or neutrophils in the untreated and treated diabetic Akita mice. In addition, the expression profiles of the proinflammatory cytokines, tumor necrosis factor-α, interleukin-1β, and interleukin-6, and the chemokine, monocyte chemoattractant protein-1, were similar in all four groups of mice (supplementary Table 2).
FIG. 3.
Increased glomerular expression of α-SMA and collagen III in the diabetic Akita kidneys without evidence of inflammatory changes in response to human recombinant ACE2. A–-D: Increased glomerular immunostaining for α-SMA (A) and collagen III (B) in diabetic Akita mice that was quantified based on computer image analysis scores of glomerular immunostaining of α-SMA (C) and collagen III (D). Positive controls are shown as staining in renal blood vessels for α-SMA and from a kidney after 14 days of ureteral obstruction for collagen III. n = 5 for all groups. *P < 0.01 compared with all other groups using ANOVA and multiple comparison testing. E and F: Immunohistochemical-specific staining of neutrophil and macrophage revealed no evidence of inflammation in the diabetic Akita mice without a differential impact with treatment with hrACE2. Positive controls were taken from mouse spleen and lung tissue. Scale bar, 100 μm. (A high-quality digital representation of this figure is available in the online issue.)
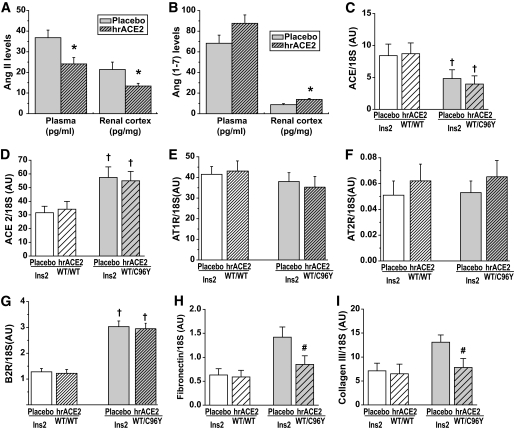
Recombinant human ACE2 reduces ANG II levels in the plasma and renal cortex.
Plasma ACE2 activity was increased in the treated mice, so we measured plasma and renal cortical levels of ANG II, a substrate for ACE2 and ANG 1–7, a product of ACE2, in response to the exogenous hrACE2. In the Akita mice, plasma and renal cortical ANG II levels were significantly reduced by 4 weeks of hrACE2 treatment (Fig. 4A). Consistent with the biochemical action of ACE2, renal ANG 1–7 levels were increased after 4 weeks of treatment with hrACE2. There was a numeric increase in plasma ANG 1–7 levels in the treated mice but the difference did not reach statistical significance (P = 0.092) (Fig. 4B). Renal cortical expression of ace (Fig. 4C) was reduced in the diabetic mice, whereas expression of ace2 (Fig. 4D) was increased in the diabetic Akita mice. The administration of hrACE2 did not influence ace and ace2 expression levels in the kidney, suggesting that the treatment-induced changes in peptide levels were due to exogenous ACE2 activity rather than changes in endogenous kidney expression of the key angiotensin processing enzymes. Similarly, the expression of other components of the RAS known to play a key role in diabetic nephropathy such as the AT1 receptor (Fig. 4E), AT2 receptor (Fig. 4F), bradykinin2 receptor (Fig. 4G), and the Mas receptor (supplementary Table 2) was not influenced by hrACE2 treatment. Consistent with the measures of mesangial matrix expansion, the mRNA expression of the ANG II–sensitive genes fibronectin (Fig. 4H) and pro–collagen III α-1 (Fig. 4I) was increased in Akita diabetic mice and normalized by treatment with hrACE2.
FIG. 4.
Human recombinant ACE2 alters angiotensin peptide metabolism without a differential impact on expression of the genes of the renin-angiotensin system while normalizing matrix gene expression in diabetic Akita mice. A and B: Reduction in plasma and renal cortical ANG II levels (A) and increases in plasma and renal cortical ANG 1–7 levels (B) in diabetic Akita mice after treatment with hrACE2. n = 10 for placebo group and n = 12 for hrACE2-treated group. *P < 0.05 compared with corresponding placebo group using Student t test. C–G: Decreased renal cortical ace (C) and increased ace2 (D) expression, unaltered ANG II type 1 receptor, AT1R (E), and type 2 receptor, AT2R (F), expression, and increased bradykinin type 2 receptor, B2R (G), expression in Akita mice were not affected by treatment with hrACE2. n = 8 for placebo groups; n = 10 for hrACE2 groups. †P < 0.05 compared with corresponding Ins2WT/WT group using Student t test. H and I: Increased renal cortical expression of extracellular matrix genes, fibronectin (H) and pro–collagen III α-1 (I), in diabetic Akita mice was suppressed in response to hrACE2. n = 8 for placebo groups; n = 10 for hrACE2 groups. #P < 0.05 compared with placebo + Ins2WT/C96Y group using ANOVA and multiple comparison testing.
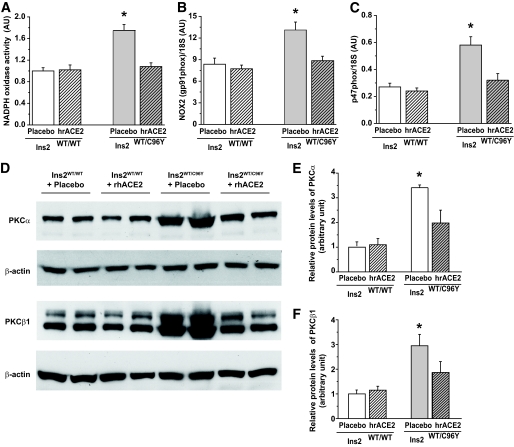
Increased NADPH oxidase activity and PKC expression were suppressed by hrACE2.
Increased renal NADPH oxidase activity and activation of the PKC system play key roles in the pathophysiology of diabetic nephropathy (5,28–30). Given the protective effect of hrACE2 on diabetic kidney injury, we examined the effect of hrACE2 treatment on renal cortical NADPH oxidase activity and the protein expression of PKCα and PKCβ1 isoforms. In the diabetic Akita mice, renal cortical NADPH activity based on the lucigenin chemiluminescence assay was significantly increased compared with nondiabetic mice (Fig. 5A) in association with increased renal cortical mRNA expression of the NADPH oxidase subunits, NOX2 (gp91phox) (Fig. 5B) and p47phox (Fig. 5C). The cortical expression of the other NADPH subunits including NOX1, NOX4, p22phox, p40phox, and p67phox was not significantly altered in our diabetic model (supplementary Table 3). Treatment with hrACE2 normalized NADPH oxidase activity and mRNA expression of NOX2 and p47phox subunits in the diabetic mice (Fig. 5A–C). In the diabetic Akita mice, protein levels of PKCα (Fig. 5D and E) and PKCβ1 (Fig. 5D and F) increased threefold compared with nondiabetic C57BL/6J mice and this effect was attenuated by hrACE2 treatment.
FIG. 5.
Reduction of NADPH oxidase activity and PKC protein levels in response to human recombinant ACE2 in diabetic Akita mice. A–C: Elevated renal cortical NADPH activity (A) and increased expression of NOX2 (gp91phox) (B) and p47phox mRNA (C) in Akita mice were completely suppressed and normalized by treatment with hrACE2. n = 6 and 8 in Ins2WT/WT and Ins2WT/C96Y groups, respectively. *P < 0.05 compared with all other groups using ANOVA with multiple comparison testing. D–F: Western blot analysis of PKCα and PKCβ1 protein levels (D) showing a marked elevation in PKCα (E) and PKCβ1 (F) protein levels that was significantly reduced by treatment with hrACE2. β-actin was used as the loading control; n = 5 for all groups. *P < 0.05 compared with all other groups using ANOVA with multiple comparison testing.
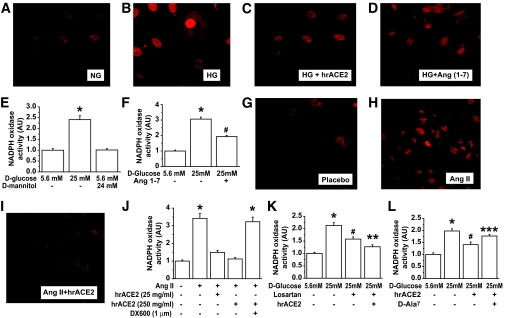
Human recombinant ACE2 reduces high glucose and ANG II–induced NADPH oxidase activity in mesangial cells: evidence for the potential role of ANG 1–7.
To address mechanisms responsible for the protective effect of hrACE2 in the diabetic mice, we used cultured primary rat mesangial cells to study the effects of hrACE2 on high glucose and ANG II–induced dihydroethidium fluorescence (DHF) fluorescence and NADPH oxidase activity. High glucose–induced DHF fluorescence was attenuated by both hrACE2 and ANG 1–7 (Fig. 6A–D). NADPH oxidase was also activated by high glucose (Fig. 6E), and this effect was attenuated by pretreatment with rhACE2 (Fig. 6F). As an osmotic control, d-mannitol did not activate NADPH oxidase (Fig. 6E). Consistent with the ability of hrACE2 to metabolize ANG II, hrACE2 also suppressed ANG II–induced DHF fluorescence (Fig. 6G–I) and reduced NADPH oxidase activity in a dose-dependent manner (Fig. 6J) in mesangial cells. Pretreatment with the specific ACE2 inhibitor, DX600 (1 μmol/l), prevented the ability of hrACE2 to suppress ANG II–mediated activation of NADPH oxidase (Fig. 6J). High glucose concentrations can activate the intrarenal RAS and increase the generation of ANG II in mesangial cells (31–33), so we studied the effects of hrACE2 on high glucose–induced NADPH oxidase activity in mesangial cells pretreated with either the ANG II type I receptor antagonist, losartan, or the Mas receptor peptide antagonist, d-Ala7-ANG 1–7 (34). Treatment with the ANG II type 1 receptor antagonist attenuated the high glucose–induced increase in NADPH oxidase activity with hrACE2, leading to incremental suppression (Fig. 6K). The attenuation of high glucose–induced NADPH oxidase activity by hrACE2 was partially prevented by the Mas receptor antagonist, d-Ala7-ANG 1–7, suggesting that part of the effect was mediated by ANG 1–7 (Fig. 6L). Taken together, these results support the hypothesis that the protective effect of hrACE2 is mediated, at least in part, by a reduction in ANG II and an increase in ANG 1–7 and that together these changes reduce oxidative stress in the diabetic kidney.
FIG. 6.
Recombinant human ACE2 prevents high glucose (HG) and ANG II–induced oxidative stress and NADPH oxidase activation in cultured rat mesangial cells. A–D: DHF staining in cultured mesangial cells treated for 24 h with normal glucose (NG; 5.6 mmol/l) (A) or high glucose (25 mmol/l) (B) and pretreated with 250 ng/ml of ACE2 for 1 h (C) or 100 nmol/l of ANG 1–7 for 15 min (D) and then exposed to 25 mmol/l of high d-glucose for 24 h. E: NADPH oxidase activity in response to high d-glucose (25 mmol/l) or d-mannitol for 24 h. F: NADPH oxidase activity in response to 24 h of high d-glucose (25 mmol/l) and the effects of pretreatment with 100 nmol/l ANG 1–7. G–J: Dihydroethidium fluorescence in cultured mesangial cells treated with placebo (G) or stimulated by 100 nmol/l of ANG II for 18 h (H) and with pretreatment with hrACE2 (25 ng/ml) (I). Suppression of ANG II–induced NADPH oxidase activity in cultured mesangial cells by pretreatment with 25 and 250 ng/ml of hrACE2 was preventable by 1 μmol/l of the specific ACE2 inhibitor, DX600 (J). K: The effects of pretreatment with AT1 receptor blocker, losartan (10 μmol/l), and hrACE2 (250 ng/ml) on high-glucose–induced NADPH oxidase activity. L: The effects of hrACE2 (250 ng/ml) with and without the ANG 1–7 blocker, d-Ala-ANG 1–7 (10 μmol/l), on high glucose–induced NADPH oxidase activity. n = 5 for all groups. *P < 0.05 compared with all other groups, #P < 0.05 compared with the 5.6 mmol/l d-glucose group, **P < 0.05 compared with the 25 mmol/l d-glucose– and losartan-treated group, and ***P < 0.05 compared with the 25 mmol/l d-glucose– and hrACE2-treated group using ANOVA with multiple comparison testing. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
Diabetic nephropathy continues to be the most common cause of end-stage renal disease in North America. Activation of the RAS and ANG II play an important role in the development of experimental and clinical diabetic nephropathy, and blockade of the RAS in both experimental and clinical diabetes attenuates the development of diabetic kidney injury (6–8,18). However, ACE inhibitors and angiotensin receptor blockers provide only partial long-term benefits in patients with type 1 (35) and type 2 (6,7,36) diabetes. The recent discovery of an ACE homologue, ACE2, has revised our understanding of the renin-angiotensin system (11,12,37). In a long-standing diabetic rat model, renal ACE2 expression is reduced (16), whereas there is an early increase in ACE2 expression and activity in the kidneys of the diabetic db/db (15) and Akita (18) mice. Deletion of the AceII gene and pharmacological inhibition of ACE2 is associated with accelerated glomerular injury in Akita diabetic mice (18) and in streptozocin-induced diabetes (38,39), providing definitive evidence that ACE2 is renoprotective and that reduced ACE2 activity contributes to the progression of kidney disease (19,20). Kidney disease in patients with type 2 diabetes is associated with a reduction in ACE2 mRNA and protein expression (20). Accordingly, we evaluated the ability of hrACE2 to reduce the functional and structural changes of diabetic nephropathy in male Akita (Ins2WT/C96Y) mice, a model of type 1 diabetes that is associated with the development of changes in the kidney that are similar to human diabetic nephropathy (18,26,40).
We observed early and sustained increases in the blood glucose concentrations in our Akita mice, as reported previously (18,26,40). Our major finding is that treatment with exogenous hrACE2 slows the progression of diabetic nephropathy. The Akita diabetic mice develop an increase in the urinary albumin excretion rate in association with renal and glomerular hypertrophy, mesangial matrix expansion, and an increase in GBM thickness compared with littermate nondiabetic mice. The increase in albumin excretion, an early functional abnormality in the natural history of nephropathy in patients with diabetes (41,42), was markedly reduced by hrACE2 treatment. ACE2 activity increased in the plasma of treated mice; plasma and renal ANG II levels declined whereas ANG 1–7 levels rose in the treated Akita mice. These observations are consistent with the hypothesis that ACE2 plays an important role in the processing of angiotensin peptides in the plasma and kidney (13,15,16) and that ANG II–dependent injury (via the AT1 receptors) in the diabetic kidney is accelerated by reduced ACE2 activity (18). Whether the changes in renal angiotensin peptide levels reflect the changes in plasma angiotensin levels and/or an active intrarenal process remains to be clarified. Consistent with previous studies (18,40), we observed mild hypertension in the diabetic Akita mice and hrACE2 treatment lowered blood pressure in association with the decrease in plasma ANG II levels, an effect that may contribute to renal protection.
Glomerular hypertrophy and mesangial matrix expansion, early features of human diabetic nephropathy, were reduced by hrACE2 treatment, confirming that modulation of angiotensin peptide metabolism and its downstream effects can attenuate diabetic kidney injury in the diabetic mouse. The RAS is activated in the diabetic milieu and increasing ACE2 activity may provide an alternate and important strategy to limit the role of the RAS in progressive diabetic nephropathy. Increased renal NADPH oxidase activity and activation of the PKC system are two canonical pathways known to play a fundamental role in the pathophysiology of diabetic nephropathy (28–30). Expression analysis of the renal cortical NADPH subunits revealed that both NOX2 (gp91phox) and p47 subunits were increased in diabetic Akita mice in agreement with previous findings in a type 1 diabetic model (29). Along with these changes in NADPH oxidase subunit expression, NADPH oxidase activity increased in the kidney cortex of our diabetic Akita mice. Importantly, hrACE2 treatment reduced renal cortical protein levels of PKCα and PKCβ1 and normalized NADPH oxidase subunit expression and activity in diabetic Akita mice.
We used an in vitro system of cultured primary rat mesangial cells to provide further insights into the mechanisms responsible for renoprotective effect of hrACE2 treatment in our diabetic Akita mice. Both high glucose concentrations and ANG II increased NADPH oxidase activity in vitro, and hrACE2 treatment attenuated high glucose– and ANG II–induced DHF staining and NADPH oxidase activation in the mesangial cells. Blockade of ANG 1–7 signaling with a Mas receptor peptide antagonist limited the protective effect of hrACE2 in vitro. Taken together with our finding that kidney cortical levels of ANG 1–7 were increased in the treated diabetic mice, these in vitro findings support the hypothesis that the protective effect of hrACE2 on diabetic injury was mediated, at least in part, by an increase in ANG 1–7 levels and attenuation of oxidative stress. Indeed, treatment with ANG 1–7 reduces renal NADPH oxidase activity and urinary albumin excretion in diabetic hypertensive rats (43), whereas the loss of ANG 1–7 receptor (Mas receptor) leads to glomerular hyperfiltration and albuminuria (44), changes that are characteristic of early diabetic nephropathy. Finally, our data also suggest that hrACE2-induced reduction in NADPH oxidase activity in vivo is due in part to the decrease in plasma and kidney ANG II levels.
In summary, we have shown that hrACE2 treatment improves kidney function and structure in a murine model of diabetic nephropathy. The ability of hrACE2 to suppress high glucose– and ANG II–induced activation of NADPH oxidase and to limit diabetic nephropathy is consistent with the notion that it functions as a negative regulator of the RAS. These beneficial effects of hrACE2 were not due to changes in plasma glucose levels, although there was a normalizing effect on blood pressure that may contribute to the renoprotection. Enhancing ACE2 activity may represent a novel therapeutic strategy to minimize the rate of progression of diabetic kidney disease. Additional work will be required to determine whether hrACE2 provides an incremental benefit over AT1 receptor blockade and/or ACE inhibition.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the financial support from the Canadian Institute for Health Research (CIHR) (G.Y.O., Grant 86602; Z.K., Grant 84279), the Canadian Diabetes Association (J.W.S., G.Y.O., and A.M.H., Grant OG-3-08-2559-JS), the EuGeneHeart (EU 6th Framework programs), a GEN-AU grant, and the Institute of Molecular Biotechnology (IMBA), Vienna, Austria (J.M.P.). G.Y.O. is a Clinician-Investigator Scholar of the Alberta Heritage Foundation for Medical Research, and Z.K. is a New Investigator of the Heart and Stroke Foundation of Canada. J.W.S. is supported by a CIHR AMGEN Canada Incorporated Chair in Kidney Research.
Apeiron Biologics is a biotechnology company founded by J.M.P. H.L. owns stock in and is the chief executive officer for Apeiron Biologics. M.S. owns stock in and is the chief operating officer of Apeiron Biologics. E.J. owns stock in and is employed by Apeiron Biologics. J.M.P. owns stock in and is a member of the supervisory board for Apeiron Biologics. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in an oral presentation at the 2009 Scientific Sessions of the American Heart Association, Orlando, Florida, 14–18 November 2009.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 2007;72:247–259 [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ: Chronic kidney disease: common, harmful, and treatable—World Kidney Day 2007. J Am Soc Nephrol 2007;18:374–378 [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–2047 [DOI] [PubMed] [Google Scholar]
- 4.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy: the Collaborative Study Group. N Engl J Med 1993;329:1456–1462 [DOI] [PubMed] [Google Scholar]
- 5.Cooper ME: Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet 1998;352:213–219 [DOI] [PubMed] [Google Scholar]
- 6.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz ICollaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860 [DOI] [PubMed] [Google Scholar]
- 7.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar SRENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861– 869 [DOI] [PubMed] [Google Scholar]
- 8.Huang W, Gallois Y, Bouby N, Bruneval P, Heudes D, Belair MF, Krege JH, Meneton P, Marre M, Smithies O, Alhenc-Gelas F: Genetically increased angiotensin I-converting enzyme level and renal complications in the diabetic mouse. Proc Natl Acad Sci U S A 2001;98:13330–13334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ: A human homolog of angiotensin-converting enzyme: cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 2000;275:33238–33243 [DOI] [PubMed] [Google Scholar]
- 10.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S: A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 2000;87:E1–E9 [DOI] [PubMed] [Google Scholar]
- 11.Oudit GY, Crackower MA, Backx PH, Penninger JM: The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med 2003;13:93–101 [DOI] [PubMed] [Google Scholar]
- 12.Hamming I, Cooper ME, Haagmans BL, Hooper NM, Korstanje R, Osterhaus AD, Timens W, Turner AJ, Navis G, van Goor H: The emerging role of ACE2 in physiology and disease. J Pathol 2007;212:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chappell MC, Modrall JG, Diz DI, Ferrario CM: Novel aspects of the renal renin-angiotensin system: angiotensin-(1–7), ACE2 and blood pressure regulation. Contrib Nephrol 2004;143:77–89 [DOI] [PubMed] [Google Scholar]
- 14.Ye M, Wysocki J, Naaz P, Salabat MR, LaPointe MS, Batlle D: Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination? Hypertension 2004;43:1120–1125 [DOI] [PubMed] [Google Scholar]
- 15.Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D: ACE and ACE2 activity in diabetic mice. Diabetes 2006;55:2132–2139 [DOI] [PubMed] [Google Scholar]
- 16.Tikellis C, Johnston CI, Forbes JM, Burns WC, Burrell LM, Risvanis J, Cooper ME: Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension 2003;41:392–397 [DOI] [PubMed] [Google Scholar]
- 17.Oudit GY, Herzenberg AM, Kassiri Z, Wong D, Reich H, Khokha R, Crackower MA, Backx PH, Penninger JM, Scholey JW: Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol 2006;168:1808–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW: Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol 2007;171:438–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizuiri S, Hemmi H, Arita M, Ohashi Y, Tanaka Y, Miyagi M, Sakai K, Ishikawa Y, Shibuya K, Hase H, Aikawa A: Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis 2008;51:613–623 [DOI] [PubMed] [Google Scholar]
- 20.Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM: Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int 2008;74:1610–1616 [DOI] [PubMed] [Google Scholar]
- 21.Soler MJ, Barrios C, Oliva R, Batlle D: Pharmacologic modulation of ACE2 expression. Curr Hypertens Rep 2008;10:410–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oudit GY, Imai Y, Kuba K, Scholey JW, Penninger JM: The role of ACE2 in pulmonary diseases—relevance for the nephrologist. Nephrol Dial Transplant 2009;24:1362–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang L, Sexton DJ, Skogerson K, Devlin M, Smith R, Sanyal I, Parry T, Kent R, Enright J, Wu QL, Conley G, DeOliveira D, Morganelli L, Ducar M, Wescott CR, Ladner RC: Novel peptide inhibitors of angiotensin-converting enzyme 2. J Biol Chem 2003;278:15532–15540 [DOI] [PubMed] [Google Scholar]
- 24.Redling S, Pfaff IL, Leitges M, Vallon V: Immunolocalization of protein kinase C isoenzymes alpha, beta I, beta II, delta, and epsilon in mouse kidney. Am J Physiol Renal Physiol 2004;287:F289–F298 [DOI] [PubMed] [Google Scholar]
- 25.Kassiri Z, Oudit GY, Kandalam V, Awad A, Wang X, Ziou X, Maeda N, Herzenberg AM, Scholey JW: Loss of TIMP3 enhances interstitial nephritis and fibrosis. J Am Soc Nephrol 2009;20:1223–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakoki M, Takahashi N, Jennette JC, Smithies O: Diabetic nephropathy is markedly enhanced in mice lacking the bradykinin B2 receptor. Proc Natl Acad Sci U S A 2004;101:13302–13305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tesch GH: MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol 2008;294:F697–F701 [DOI] [PubMed] [Google Scholar]
- 28.Kelly DJ, Zhang Y, Hepper C, Gow RM, Jaworski K, Kemp BE, Wilkinson-Berka JL, Gilbert RE: Protein kinase C beta inhibition attenuates the progression of experimental diabetic nephropathy in the presence of continued hypertension. Diabetes 2003;52:512–518 [DOI] [PubMed] [Google Scholar]
- 29.Ohshiro Y, Ma RC, Yasuda Y, Hiraoka-Yamamoto J, Clermont AC, Isshiki K, Yagi K, Arikawa E, Kern TS, King GL: Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase C beta-null mice. Diabetes 2006;55:3112–3120 [DOI] [PubMed] [Google Scholar]
- 30.Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM: Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes 2008;57:460–469 [DOI] [PubMed] [Google Scholar]
- 31.Carey RM, Siragy HM: The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab 2003;14:274–281 [DOI] [PubMed] [Google Scholar]
- 32.Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA: High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol 2004;286:F1039–F1045 [DOI] [PubMed] [Google Scholar]
- 33.Cristovam PC, Arnoni CP, de Andrade MC, Casarini DE, Pereira LG, Schor N, Boim MA: ACE-dependent and chymase-dependent angiotensin II generation in normal and glucose-stimulated human mesangial cells. Exp Biol Med (Maywood) 2008;233:1035–1043 [DOI] [PubMed] [Google Scholar]
- 34.Su Z, Zimpelmann J, Burns KD: Angiotensin-(1–7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int 2006;69:2212–2218 [DOI] [PubMed] [Google Scholar]
- 35.Suissa S, Hutchinson T, Brophy JM, Kezouh A: ACE-inhibitor use and the long-term risk of renal failure in diabetes. Kidney Int 2006;69:913–919 [DOI] [PubMed] [Google Scholar]
- 36.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G: Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000;342:145–153 [DOI] [PubMed] [Google Scholar]
- 37.Turner AJ, Hiscox JA, Hooper NM: ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci 2004;25:291–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D: ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int 2007;72:614–623 [DOI] [PubMed] [Google Scholar]
- 39.Tikellis C, Bialkowski K, Pete J, Sheehy K, Su Q, Johnston C, Cooper ME, Thomas MC: ACE2 deficiency modifies renoprotection afforded by ACE inhibition in experimental diabetes. Diabetes 2008;57:1018–1025 [DOI] [PubMed] [Google Scholar]
- 40.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM: Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 2006;290:F214–F222 [DOI] [PubMed] [Google Scholar]
- 41.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S: HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426 [DOI] [PubMed] [Google Scholar]
- 42.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS: Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348: 2285– 2293 [DOI] [PubMed] [Google Scholar]
- 43.Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI: Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol 2008;28:25–33 [DOI] [PubMed] [Google Scholar]
- 44.Pinheiro SV, Ferreira AJ, Kitten GT, da Silveira KD, da Silva DA, Santos SH, Gava E, Castro CH, Magalhães JA, da Mota RK, Botelho-Santos GA, Bader M, Alenina N, Santos RA, Simoes e Silva AC: Genetic deletion of the angiotensin-(1–7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int 2009;75:1184–1193 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.