Abstract
OBJECTIVE
The response of ventromedial hypothalamic (VMH) glucose-inhibited neurons to decreased glucose is impaired under conditions where the counterregulatory response (CRR) to hypoglycemia is impaired (e.g., recurrent hypoglycemia). This suggests a role for glucose-inhibited neurons in the CRR. We recently showed that decreased glucose increases nitric oxide (NO) production in cultured VMH glucose-inhibited neurons. These in vitro data led us to hypothesize that NO release from VMH glucose-inhibited neurons is critical for the CRR.
RESEARCH DESIGN AND METHODS
The CRR was evaluated in rats and mice in response to acute insulin-induced hypoglycemia and hypoglycemic clamps after modulation of brain NO signaling. The glucose sensitivity of ventromedial nucleus glucose-inhibited neurons was also assessed.
RESULTS
Hypoglycemia increased hypothalamic constitutive NO synthase (NOS) activity and neuronal NOS (nNOS) but not endothelial NOS (eNOS) phosphorylation in rats. Intracerebroventricular and VMH injection of the nonselective NOS inhibitor NG-monomethyl-l-arginine (l-NMMA) slowed the recovery to euglycemia after hypoglycemia. VMH l-NMMA injection also increased the glucose infusion rate (GIR) and decreased epinephrine secretion during hyperinsulinemic/hypoglycemic clamp in rats. The GIR required to maintain the hypoglycemic plateau was higher in nNOS knockout than wild-type or eNOS knockout mice. Finally, VMH glucose-inhibited neurons were virtually absent in nNOS knockout mice.
CONCLUSIONS
We conclude that VMH NO production is necessary for glucose sensing in glucose-inhibited neurons and full generation of the CRR to hypoglycemia. These data suggest that potentiating NO signaling may improve the defective CRR resulting from recurrent hypoglycemia in patients using intensive insulin therapy.
Intensive insulin therapy significantly reduces the onset and progression of hyperglycemia-related complications in patients with type 1 and advanced type 2 diabetes. However, intensive insulin therapy also causes a clinically adverse effect: hypoglycemia (1). Powerful neuroendocrine and autonomic counterregulatory mechanisms protect the brain from hypoglycemia (2,3). These protective mechanisms, known as the counterregulatory response (CRR) to hypoglycemia, involve the release of hormones (e.g., glucagon, epinephrine) that restore euglycemia by stimulating hepatic glucose production and inhibiting peripheral glucose uptake (3). Although the physiology of the CRR is well understood, the underlying cellular mechanisms by which the brain senses hypoglycemia and initiates the CRR remain elusive.
During hypoglycemia, central and peripheral glucose sensors detect declining glucose levels (4). In the brain, the ventromedial hypothalamus, which includes the arcuate nucleus and the ventromedial nucleus (VMN), is important in the initiation of the CRR (5–7). This region contains specialized glucose-sensing neurons (GSNs). Ventromedial hypothalamic (VMH) GSN electrical activity is regulated by physiologically relevant changes in extracellular glucose levels (8–11). Glucose-excited neurons decrease, whereas glucose-inhibited neurons increase, their input resistance, membrane potential, and action potential frequency when extracellular glucose is reduced (10). Many studies suggest that VMH glucose-inhibited neurons play a critical role in the control of the CRR (4). For example, the response of VMH glucose-inhibited neurons to decreased glucose is impaired under conditions where the CRR is impaired (e.g., recurrent hypoglycemia) (12,13).
Nitric oxide (NO) is a gaseous messenger produced by NO synthase (NOS). Two classes of NOS have been identified in the brain: the inducible NOS (iNOS) and the constitutive NOS, which includes the neuronal NOS (nNOS) and endothelial NOS (eNOS) isoforms (14). Hypothalamic NO is involved in the regulation of food intake and glucose homeostasis (15–18). In support of this, we have recently shown that VMH glucose-inhibited neurons produce NO via nNOS in response to decreased extracellular glucose levels (19,20). Therefore, in this study, we test the hypothesis that NO production by VMH glucose-inhibited neurons is necessary for the CRR to hypoglycemia. We tested this hypothesis using a combination of in vivo and in vitro techniques in wild-type rats and mice as well as in transgenic nNOS and eNOS knockout mice.
RESEARCH DESIGN AND METHODS
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Medicine and Dentistry of New Jersey. Adult male Sprague-Dawley rats were purchased from Charles River. Adult 5- to 8-week-old C57BL/6J wild-type, nNOS knockout (B6.129S4-Nos1tm1Plh/J), and eNOS knockout (B6.129P2-Nos3tm1Unc/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were housed individually and maintained on a 12-h light/12-h dark schedule at 22–23°C with ad libitum access to food and water.
In vivo experiments
Surgical procedures.
Rats were anesthetized with sodium pentobarbital (50 mg/kg i.p.; Ovation) and mice, with ketamine/xylazine (80/8 mg/kg i.p.; BionichePharma/Lloyd Laboratories). Vascular catheters were surgically implanted in the left carotid and/or the right jugular vein in rats, and a vascular catheter was implanted in the right jugular vein in mice. The catheters were filled with heparin (10 units/ml) and flushed every other day. Additionally, rats received a stereotaxic implantation of microinjection cannula guide positioned 1-mm dorsal to the ventromedial hypothalamus or in the right lateral ventricle according to stereotaxic coordinates (VMH cannulation; from bregma: −2.5 mm anterior-posterior, −2.8 mm medial-lateral, and −8.5 mm dorsal-ventral, at an angle of 20°; intracerebroventricular [ICV] cannulation; from bregma: −1.0 mm anterior-posterior, −1.4 mm medial-lateral, and −4.0 mm dorsal-ventral). Animals were allowed 5–7 days to recover from surgery and were handled every day. Animals that did not recover to their presurgery body weights were excluded from the study. For probe placement, at the end of each experiment, cannula placement was verified by methyl-blue (Sigma) injection.
Experimental procedures.
Animals undergoing hyperinsulinemic/hypoglycemic clamps were either fasted overnight (rats) or for 5 h (9:00 a.m. to 2:00 p.m.; mice). Two hours before the start of the study, catheters were externalized outside the cage to minimize investigator interaction and were connected to infusion pumps. Starting 30 min before insulin injection (see below), one group of rats was infused intracerebroventricularly (0.4 μl/min, 2 h), whereas another group was injected in the ventromedial hypothalamus (0.1 μl/min, 10 min) with one of the following compounds in artificial cerebrospinal fluid (aCSF; containing in mM: 135 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, pH = 7.4): NG-monomethyl-l-arginine (l-NMMA; 50 mmol/l in aCSF), 1H-[1,2,4]-oxadiazolo-[4,3-a]quinoxalin-1–1 (ODQ; 0.1 mmol/l in aCSF containing 0.1% DMSO). The control for l-NMMA was injected with aCSF, whereas the control for ODQ was injected with DMSO (0.1% in aCSF).
Acute insulin infusion.
Rats (100–150 g) were injected with an insulin bolus (1 unit/kg; regular human insulin; Eli-Lilly) through the jugular catheter 30 min after ICV or VMH infusion. Blood glucose was monitored every 15 min from −30 to 120 min after insulin infusion via tail prick.
Hyperinsulinemic/hypoglycemic clamp.
Starting 30 min after VMH or ICV infusion, rats (300–350 g) or mice (7–8 weeks old) were injected through the jugular catheter with an insulin bolus (rats: 0.4 units/kg; mice: 1 unit/kg) to decrease glycemia to ∼50 mg/dl within 30–40 min. This time course was used based on the results of Saberi et al. (21), suggesting that brain versus peripheral glucose sensors predominate in CRR initiation when blood glucose decreases rapidly. After this bolus, animals were perfused with insulin at 1.2 units · kg−1 · h−1 for 90 (rats) or 120 (mice) min. Glucose (20%) was co-perfused with insulin to maintain plasma glucose level of ∼50 mg/dl. The concentration of blood glucose was measured every 10 min via tail prick. For clamps carried out in rats, arterial blood samples (500 μl) taken from the carotid catheter were collected at 0, 30, 60, and 90 min for subsequent measurement of plasma glucagon, epinephrine, and norepinephrine. Glucocorticoid levels were not measured because they are not an essential aspect of the recovery from an acute hypoglycemic challenge (for review, see [22]). For glucagon, 250 μl of blood was collected in chilled tubes containing EGTA (1.6 mg/ml; Sigma) and aprotinin (250 KIU/ml; Sigma). For catecholamines, blood was collected in chilled tubes containing reduced glutathione (1.2 mg/ml; Sigma) and EDTA (1.8 mg/ml; Sigma). After removal of plasma, erythrocytes from experimental rats were resuspended in an equivalent volume of sterile NaCl 0.9% and reinfused after each blood sampling to prevent volume depletion. For mice clamp, trunk blood was collected at the end of the clamp in chilled tubes containing reduced glutathione (1.2 mg/ml; Sigma) and EDTA (1.8 mg/ml; Sigma) for plasma epinephrine and norepinephrine measurement.
Plasma glucagon and catecholamine determination.
Plasma glucagon concentrations were determined using commercially available radioimmunoassay kits (Linco Research). Plasma epinephrine and norepinephrine concentrations were analyzed by high-performance liquid chromatography using electrochemical detection (ESA Biosciences, Acton, MA).
Phosphorylated-NOS Western blot.
Rats (100–150 g) were injected with saline or insulin (2 units/kg, s.c.) and killed 60 min after by an overdose of sodium pentobarbital (Euthasol, Virbac, Fort Worth, TX). The ventral hypothalamus was quickly harvested, snap frozen, and stored at −80°C. Brain samples were lysed over ice in lysis buffer (150 mmol/l NaCl, 0.02% sodium azide, 10 mmol/l HEPES, 50 mmol/l NaF, 0.1% SDS, 0.5% deoxycholic acid, 1% Nonidet P-40, 0.2 mmol/l phenylmethylsulfonyl fluoride, 2 μg/ml pepstatin-A, 2 μg/ml leupeptin, and 2 μg/ml aprotinin). Cytosolic lysate supernatants were collected by centrifugation at 14,000g for 10 min at 4°C. Protein (15 μg) was electrophoresed and transferred to nitrocellulose membranes. Immunodetection with primary antibodies was performed for 12 h at 4°C: phosphorylated-NOS (P-nNOS; nNOS-Ser 1717) 1:5,000 (Millipore), phosphorylated eNOS (eNOS-Ser 1177) 1:5,000, and nNOS and eNOS 1:2,500 (Cell Signaling). After washing, secondary antibody (donkey anti-rabbit; Jackson ImmunoResearch) was added at 1:1,000 for 1 h at room temperature. Signals are visualized using ECL kit (Thermo) and quantified using Scion Image. Results are presented as percentage of control after normalization to total nNOS/eNOS.
NOS activity.
NOS activity was quantified using the radiodetection kit (Calbiochem) based on the biochemical conversion of [3H-]l-arginine to [3H-]l-citrulline by NOS. To distinguish Ca2+-dependent constitutive NOS activity (nNOS + eNOS), from Ca2+-independent iNOS activity, hypothalamic homogenates were prepared as above and divided into two sets of samples, one of which omitted calcium in the assay medium for measurement of iNOS activity.
In vitro experiments
Electrophysiology.
Coronal brain slices (250 μm) from wild-type and nNOS knockout mice (5–7 weeks old) were prepared as previously described (8,23). Briefly, viable neurons were visualized under infrared differential-interference contrast microscopy (DM LFS microscope; Leica Microsystems). Current clamp recordings (standard whole-cell configuration) from VMN neurons were performed using a MultiClamp 700A (Axon Instruments) and analyzed using pCLAMP9 software. During recording, brain slices were perfused at 10 ml/min with normal oxygenated artificial cerebrospinal fluid containing (in mM): 126 NaCl, 1.9 KCl, 1.2 KH2PO4, 26 NaHCO3, 2.4 CaCl2, 1.3 MgCl2, 2.5 glucose; 300–310 mOsM, pH 7.4). Borosilicate pipettes (3–5 MΩ; Sutter Instrument) were filled with an intracellular solution containing (in mM): 128 K-gluconate, 10 KCl, 4 KOH, 10 HEPES, 4 MgCl2, 0.5 CaCl2, 5 EGTA, and 2 Na2ATP (pH 7.2; 290–300 mOsM). Membrane potential, action potential frequency, and input resistance in response to constant hyperpolarizing pulse (20 pA) were monitored as extracellular glucose level was changed from 2.5 to 0.1 mmol/l as described in figures.
Cellular imaging.
VMH neurons were prepared using a protocol modified from Murphy et al. (24,25) (see supplementary data for detailed protocol, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09hyphen]0421/DC1). VMH neurons were perfused in a closed chamber at 0.6 ml/min with oxygenated extracellular solution containing (in mM): 132 NaCl, 5 KCl, 0.45 KH2PO4, 0.45 NaH2PO4, 1.2 CaCl2, 0.5 MgCl2, 0.4 MgSO4, 5 HEPES, 2.5 glucose (pH 7.3; osmolarity adjusted to 300–310 mOsM) in the presence of 0.5% membrane potential dye (FLIPR-MPD; Molecular Devices, Sunnyvale, CA). After 10 min of equilibration, VMH neurons were perfused with the same extracellular solution containing 0.1 mmol/l glucose for 15 min followed by 15 min at 2.5 mmol/l glucose. Image acquisition and analysis were performed as previously described (24,25). Neurons were considered as glucose-inhibited neurons when their fluorescence intensity reversibly increased more than 25% in response to 0.1 mmol/l glucose. Data are expressed in percentage of glucose-inhibited neurons detected per dish.
Hypothalamic NO real-time measurement.
Wild-type mice were killed by decapitation without anesthesia. The hypothalamus was quickly harvested and maintained in 200 μl Krebs-Ringer oxygenated solution containing 2.5 mmol/l glucose at 37°C. A NO-specific amperometric probe (ISO-NOPF100; World Precision Instruments [WPI], Sarasota, FL) was implanted directly in the tissue and NO release was monitored. The hypothalamus was exposed to the following sequence of glucose concentrations (15 min each): 2.5, 0.1, and 2.5 mmol/l. The concentration of NO gas in the tissue was measured in real time with the data acquisition system LabTrax (WPI) connected to the free radical analyzer Apollo1000 (WPI). Data acquisition and analysis were performed with DataTrax2 software (WPI). The NO-specific amperometric probe was calibrated as previously described (26).
Data analysis.
All data are presented as mean ± SEM. Statistical analysis was performed using Graphpad Prism 4.0 by two-way ANOVA followed by Bonferroni post hoc test, one-way ANOVA followed by Dunnett post hoc test, or unpaired t test as described in the figure legends. P < 0.05 indicates statistical significance.
RESULTS
Hypoglycemia activates ventral hypothalamic nNOS.
We have previously shown that decreased glucose concentration increases NO production in cultured VMH glucose-inhibited neurons in vitro using a membrane sensitive dye (20). To confirm that decreased glucose increases hypothalamic NO production, we performed amperometric measurement of NO release in hypothalamic chunks ex vivo using an NO-sensitive electrode. As shown in Figure 1, decreased glucose from 2.5 to 0.1 mmol/l significantly increases the amplitude (3.5-fold; P < 0.05) and frequency (2.1-fold; P < 0.05) of NO release. NO release returned to baseline when extracellular solution was subsequently raised to 2.5 mmol/l glucose (Fig. 1).
FIG. 1.
Decreased glucose increases VMH NO release. A: Representative trace of ex vivo amperometric measurements of NO release from mouse hypothalamus in response to an extracellular glucose decrease from 2.5 to 0.1 mmol/l. B: Mean frequency and (C) mean amplitude of NO release calculated during the last 10-min recording for each glucose level (n = 4). *P < 0.05 vs. 2.5 mmol/l glucose (one-way ANOVA).
To provide in vivo evidence that hypoglycemia increases hypothalamic NO production, constitutive (nNOS and eNOS) and inducible (iNOS) activity was determined in ventral hypothalamus from rats 60 min after insulin injection. Insulin-hypoglycemia significantly increased constitutive NOS activity by 1.45 ± 0.11-fold. iNOS activity was not changed (Fig. 2 A). Cortical constitutive NOS activity was not changed in insulin-induced hypoglycemia treated rats versus control (data not shown). To determine whether nNOS or eNOS is primarily responsible for hypoglycemia-induced hypothalamic NO production, Western blots against the phosphorylated nNOS and eNOS forms were performed. nNOS phosphorylation was significantly increased by 7.26 ± 0.36-fold, whereas eNOS phosphorylation was not changed (Fig. 2B), suggesting that nNOS activation was responsible for increased VMH constitutive NOS activity during insulin-induced hypoglycemia. These data strongly suggest that insulin-induced hypoglycemia stimulates nNOS-derived VMH NO production.
FIG. 2.
Hypoglycemia increases ventral hypothalamic nNOS activity. A: Ventral hypothalamic constitutive (e/nNOS) or inducible (iNOS) NOS activity from rats injected subcutaneously with saline (control, n = 6) or insulin (2 units/kg; n = 6) 60 min after injection. B: Representative Western blot (left panel) of ventral hypothalamic total nNOS, phosphorylated nNOS (P-nNOS), total eNOS, and P-eNOS from control or insulin-treated rats injected subcutaneously with saline (n = 5) or insulin (n = 5) 60 min after injection. The right panel shows the quantification of the ratio between P-nNOS or P-eNOS and total nNOS or eNOS, respectively. Data are means ± SEM and represented as percentage of saline where the control group was considered to be 100%. *P < 0.05 vs. control (unpaired t test).
Inhibition of VMH NO signaling impairs the CRR to hypoglycemia.
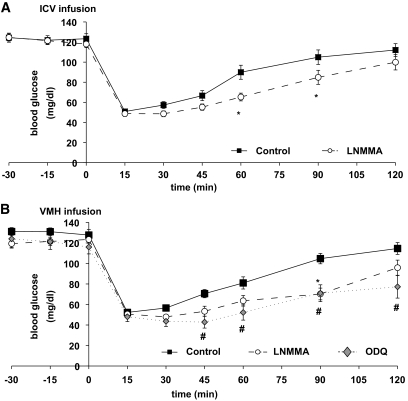
We first evaluated the effect of brain NO on the counterregulatory response to acute insulin-induced hypoglycemia. As shown in Fig. 3, rats infused with the nonselective NOS inhibitor l-NMMA either intracerebroventricularly or into the ventromedial hypothalamus showed significantly lower glycemia at 60 and 90 min after insulin injection compared with control. Many of the effects of NO are mediated by its receptor, soluble guanylyl cyclase (sGC) (14). Inhibition of VMH sGC with ODQ decreased the glycemia at 45, 60, 90, and 120 min after insulin injection (Fig. 3B).
FIG. 3.
VMH NO signaling is necessary for recovery to euglycemia after insulin-induced hypoglycemia. Blood glucose levels in response to insulin-induced hypoglycemia (1 unit/kg, i.v.) in rats receiving (A) ICV perfusion of aCSF (controls; n = 14) or l-NMMA (50 mmol/l; n = 14), or (B) unilateral VMH injection of aCSF (n = 7), l-NMMA (50 mmol/l, n = 7), or ODQ (0.1 mmol/l, n = 5). *, #P < 0.05 vs. control (two-way ANOVA).
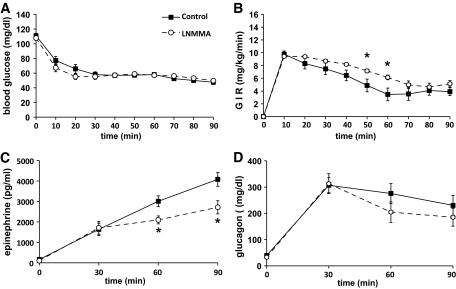
To confirm that VMH NO production is involved in the CRR, we performed hyperinsulinemic/hypoglycemic clamps (5,6,21,27). During the hypoglycemic clamp, blood glucose was decreased to similar levels in control (52 ± 1.1 mg/dl) and treated (54 ± 1.0 mg/dl) animals (Fig. 4). Administration of the nonselective NOS inhibitor l-NMMA in the ventromedial hypothalamus significantly increased the glucose infusion rate (GIR) necessary to maintain the hypoglycemia plateau (Fig. 4). Changes in GIR were associated with significant decreases in epinephrine levels at 60 and 90 min in l-NMMA–treated animals (Fig. 4). Glucagon (Fig. 4) and norepinephrine (data not shown) levels were not significantly reduced. Taken together, these data show that the VMH NO-sGC signaling pathway is necessary for the full generation of the sympathoadrenal response to hypoglycemia.
FIG. 4.
VMH NOS inhibition impairs the CRR to hypoglycemia. Blood glucose level (A); GIR (B); plasma epinephrine (C), and glucagon levels (D) during hyperinsulinemic/hypoglycemic clamp (1.2 units · kg−1 · h−1) of animals injected bilaterally in the ventromedial hypothalamus with aCSF (controls; n = 8) or l-NMMA (50 mmol/l; n = 6). *P < 0.05 vs. controls (two-way ANOVA).
VMH nNOS is involved in the CRR to hypoglycemia.
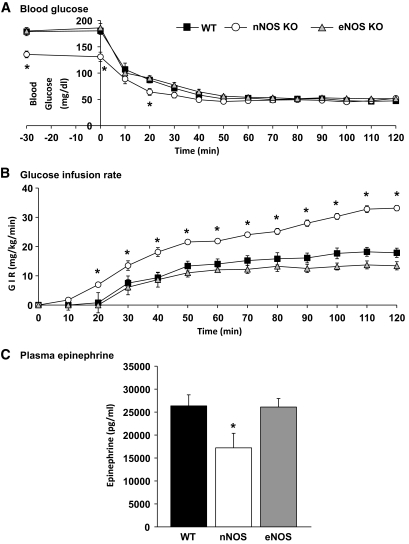
To confirm that VMH nNOS derived-NO is involved in the CRR, we performed hyperinsulinemic/hypoglycemic clamps in wild-type, nNOS, and eNOS knockout mice. The GIR required to maintain the hypoglycemic plateau over the last 30 min was significantly higher in nNOS knockout and lower in eNOS knockout mice compared with wild type (wild type: 17.4 ± 1.6 mg · kg−1 · min−1; nNOS: 31.1 ± 1.7 mg · kg−1 · min−1; eNOS: 12.8 ± 1.1 mg · kg−1 · min−1; P < 0.05; Fig. 5B). At the end of the clamp, epinephrine levels were significantly reduced in the nNOS knockout compared with wild-type or eNOS knockout mice (Fig. 5C). There was no difference in plasma norepinephrine levels between groups (data not shown). Because the initial blood glucose level was lower in nNOS knockout than wild-type mice (nNOS knockout: 136 ± 6.4 mg/dl versus wild type: 179 ± 7.5 mg/dl; P < 0.05), we measured plasma insulin and liver glycogen content in another group of mice after 5-h fast. Neither plasma insulin nor liver glycogen concentration was different between nNOS knockout and wild-type mice (insulin: wild type: 0.58 ± 0.2 versus nNOS: 0.52 ± 0.1 ng/ml; glycogen: wild type: 23.5 ± 2.8 versus nNOS: 24.7 ± 5.3 mg/g of liver; n = 4; P > 0.05). These data show that NO produced specifically by the nNOS isoform is necessary for the full generation of the CRR.
FIG. 5.
nNOS is necessary for full initiation of the CRR. Blood glucose concentration (A), glucose infusion rate (B), and plasma epinephrine taken at the end of the clamp (C) of wild-type (WT) (n = 14), eNOS (n = 6), and nNOS (n = 7) knockout mice during hyperinsulinemic/hypoglycemic clamp (1.2 units · kg−1 · h−1). *P < 0.05 vs. wild type (two-way ANOVA).
nNOS is necessary for glucose sensing by VMH glucose-inhibited neurons.
Data from our laboratory and others suggest that VMN GSNs play a role in sensing hypoglycemia and initiating the CRR (9,13,27–32). Because we showed above that the CRR is impaired in nNOS knockout mice, we wanted to determine whether the glucose sensitivity of GSNs is also impaired. We used whole-cell current clamp recording techniques to measure the membrane potential, action potential frequency (APF), and input resistance of VMN neurons in response to decreased glucose levels from 2.5 to 0.1 mmol/l in wild-type and nNOS knockout mice. In wild-type mice, three neurons (3 of 36, 8%) were identified as glucose-excited neurons by a decrease in their membrane potential, APF, and input resistance in response to 2.5–0.1 mmol/l glucose decrease, whereas 11 neurons (11 of 36, 30%) increased membrane potential, APF, and input resistance in response to decreased glucose and were identified as glucose-inhibited neurons (Fig. 6A). In nNOS knockout mice, four neurons (4 of 25, 16%) were identified as glucose-excited neurons (Fig. 6B). In contrast, no glucose-inhibited neurons (0 of 25) were found in nNOS knockout mice VMN. Results are summarized in Fig. 6C. We confirmed these electrophysiology data using a membrane potential sensitive dye in cultured VMH neurons. Whereas 13.0 ± 1.2% of VMH neurons were glucose-inhibited neurons in wild-type mice (14 dishes; 1,352 neurons; 7 mice), only 2.4 ± 0.6% were glucose-inhibited neurons in nNOS knockout mice (12 dishes; 961 neurons; 3 mice; P < 0.05). These data suggest that VMH glucose-inhibited neuron glucose sensing is impaired in nNOS knockout mice.
FIG. 6.
nNOS is necessary for glucose sensing by VMN glucose-inhibited neurons. Representative whole-cell current-clamp recordings of VMN glucose-excited and glucose-inhibited neurons in brain slices from wild-type (WT) mice (A) or nNOS knockout mice (B). The dotted lines represent the resting membrane potential. Glucose concentration changes are schematically displayed below each recording. Downward deflections in whole-cell current-clamp recordings represent the membrane voltage responses to constant hyperpolarizing currents. (C) Table summarizing the number (and %) of VMN glucose-excited (GE), glucose-inhibited (GI), or nonglucose-sensitive (NG) neurons in wild-type or nNOS knockout mice.
DISCUSSION
This study confirms that decreased glucose increases VMH NO production in vivo. Moreover, this study supports our novel hypothesis that NO production is necessary for the full generation of the CRR and glucose sensing in VMH glucose-inhibited neurons. Pharmacological inhibition of VMH NO signaling decreases blood glucose recovery and impairs the CRR after hypoglycemia. Interestingly, the impaired CRR in mice lacking nNOS is associated with an almost complete loss of VMH glucose-inhibited neurons, consistent with our recently published data showing that NO production is required for glucose-inhibited neurons to sense glucose (24). We have previously shown that VMH glucose-inhibited neurons are less sensitive to decreased glucose under conditions where the CRR is also impaired. These data suggested a role for VMH glucose-inhibited neurons in the CRR (12,13,28,31,33). Our current data strengthen the hypothesis that detection of hypoglycemia by VMH glucose-inhibited neurons is a necessary step in the full generation of the CRR.
We found previously, using in vitro cellular imaging, that among cultured VMH neurons only glucose-inhibited neurons produce NO in response to decreased glucose. nNOS, but not eNOS, mediates NO production in VMH glucose-inhibited neurons (20). In the present study, we confirm this finding by showing that decreased glucose increases VMH NO release using an NO-sensitive electrode. Moreover, insulin-induced hypoglycemia in vivo increases VMH NOS activity and nNOS phosphorylation. Because insulin increases nNOS-derived NO production in cultured VMH neurons (20), insulin injection may contribute to the increased VMH NO production during this clinically relevant form of hypoglycemia. These data strongly support our hypothesis that nNOS activation during insulin-induced hypoglycemia induces VMH NO production in vivo. Cabou et al. (16) recently suggested that cerebral insulin injection during euglycemia increases hypothalamic NO production through eNOS. Insulin-induced hypoglycemia did not increase eNOS activity in our study. Moreover, because Cabou et al. did not evaluate nNOS activity, they did not rule out a role for this NOS isoform in response to cerebral insulin injection. It is possible that prolonged hyperinsulinemia and/or recurrent episodes of insulin-induced hypoglycemia further increase VMH NO production through a combined increase in nNOS and eNOS activity.
What is the role of VMH NO production in energetic homeostasis during energy deficit? One putative function for VMH NO production is to increase cerebral blood flow, leading to increased local nutrient availability. Human and animal studies show that insulin-induced hypoglycemia is associated with increased cerebral blood flow in many brain areas including the hypothalamus (34–36). For example, Page et al. (37) recently showed that decreased blood glucose increased hypothalamic blood flow prior to the release of CRR hormones. One of the main physiological functions of NO is related to the vascular system. The role of eNOS-mediated NO production in peripheral vasorelaxation is well established (38). One of the unique features of NO as a neurotransmitter is the ability to diffuse across cell membranes (14). Thus, although we did not see an increase in eNOS activity in our studies, NO produced in VMH glucose-inhibited neurons may diffuse to adjacent vascular smooth muscle cells lining cerebral vasculature and cause vasodilatation. However, we think that this is unlikely because Horinaka et al. (39) and Paulson (40) showed that increased cerebral blood flow in response to hypoglycemia was NO independent. These data suggest that VMH nNOS-mediated NO production does not play a role in blood flow regulation. This is consistent with other studies that suggest a role for the β-adrenergic receptor and/or the ATP-sensitive K+ channel (KATP) in hypoglycemia-induced increases in cerebral blood flow (41,42).
Another function of VMH NO production is through the CRR. We used two complementary approaches to show that VMH NO production is a physiologically required step in the full generation of the CRR. First, inhibition of VMH NO production slows down the recovery to euglycemia in response to acute insulin-induced hypoglycemia. Although this is the most physiological evaluation of the CRR, it is difficult to reliably compare the levels of counterregulatory hormones between treatments due to variation in the actual degree of hypoglycemia. Thus, we also used the “gold standard” technique for studying the CRR: hyperinsulinemic/hypoglycemic clamps. Here we found that VMH NOS inhibition increases the GIR and decreases epinephrine production during hypoglycemic clamps. Moreover, the GIR is significantly greater and epinephrine production lower in nNOS knockout versus wild-type mice. These data confirm our hypothesis that VMH NO plays an important role in the control of the CRR. However, it is also clear that the CRR was not completely abolished by either l-NMMA injection or in the nNOS knockout mice. These findings are consistent with parallel regulation of the CRR by other central or peripheral glucose sensors. Finally, both eNOS and nNOS knockout mice exhibit insulin resistance (18,43). In eNOS knockout mice, there was a decrease in the GIR to maintain the hypoglycemic plateau that may reflect insulin resistance (18,43). The milder insulin resistance in nNOS knockout mice probably did not affect the GIR due to the high insulin concentration used for the hypoglycemic clamp.
The next step was to explore the molecular and cellular mechanisms by which VMH NO production contributes to the CRR. Our previous studies suggested a role for VMN glucose-inhibited neurons in the generation of the CRR because their response to decreased glucose is impaired when the CRR is impaired (12,13,28,31,33). We have recently shown that NO production via nNOS is necessary for VMN glucose-inhibited neurons to depolarize in response to decreased glucose (24). In the present study, VMH glucose-inhibited neurons were not detected in nNOS knockout mice in response to decreased extracellular glucose from 2.5 to 0.1 mmol/l. This glucose concentration decrease, although supraphysiologic, was necessary because we have previously shown that recurrent episodes of hypoglycemia decrease the response of VMH glucose-inhibited neurons to decreased glucose. In fact, after recurrent hypoglycemia the response of VMH glucose-inhibited neurons to a glucose decrease from 2.5 to 0.5 mmol/l was almost undetectable; however their response to a glucose decrease from 2.5 to 0.1 mmol/l was intact (13). Thus, using a glucose decrease to 0.1 mmol/l suggests that functional VMH glucose-inhibited neurons are almost absent in nNOS knockout mice. The CRR was also impaired in nNOS knockout mice. These data reinforce our hypothesis that activation of VMH glucose-inhibited neurons in response to decreased glucose is critical for the full generation of the CRR. Restoration of VMH NO expression in nNOS knockout mice would lend further strength to this conclusion. However, the effects of NO are highly dependent on the localization of intracellular NO production, which, in turn, is highly dependent on intracellular NOS localization (14). Overexpressing nNOS or injecting NO donors into the ventromedial hypothalamus of nNOS knockout mice would not mimic physiological NO production and could lead to difficulties in data interpretation. Our data suggest also that the NO receptor sGC mediates the effect of VMH NO on the CRR. sGC is expressed in all VMH neurons including glucose-inhibited neurons (20). Cyclic guanosine monophosphate produced by sGC has been shown to modulate neuronal activity (17,44). Taken together, these data suggest that decreased glucose depolarizes VMH glucose-inhibited neurons through NO-sGC signaling and leads to full generation of the CRR.
On the other hand, our data suggest that VMH glucose-inhibited neurons are not the only mediator of the CRR because the CRR is still present, albeit impaired, in the absence of NO signaling. VMH glucose-excited neurons are normal in nNOS knockout mice. Moreover, Miki et al. (30) showed that the CRR was impaired and VMH glucose-excited neurons were absent in KATP-deficient mice. Therefore, it is likely that VMH glucose-inhibited and glucose-excited neurons as well as extrahypothalamic glucose sensors are needed for the full generation of the CRR. Interestingly, glucagon but not epinephrine secretion in response to hypoglycemia was impaired in the KATP-deficient mice (30). In contrast our data indicate that inhibition of VMH NO signaling impairs epinephrine but not glucagon or norepinephrine secretion in response to hypoglycemia. This suggests that different glucose sensors may control unique elements of the CRR.
In conclusion, the VMH NO-sGC signaling pathway is a key component in the generation of the CRR. Moreover, our data provide strong support for our hypothesis that VMH glucose-inhibited neurons play a crucial role in the central detection of hypoglycemia and generation of the CRR. These data also suggest that potentiating NO signaling may enhance epinephrine secretion and glucose recovery in diabetic patients exposed to recurrent hypoglycemia. The role of NO signaling in epinephrine secretion in response to hypoglycemia is extremely relevant for patients with type 1 diabetes who lack a glucagon response. Thus, the NO-sGC signaling pathway may offer new therapeutic targets to improve the treatment of patients with type 1 and advanced type 2 diabetes using intensive insulin therapy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the Juvenile Diabetes Research Foundation (X.F. and V.H.R.) and the National Institutes of Health (2RO1-DK-55619 and 1RO1-DK-64566) (V.H.R.). X.F. was also supported in part by the Philippe Foundation.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS: Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med 1987;316:1376–1383 [DOI] [PubMed] [Google Scholar]
- 3.Cryer PE: Glucose counterregulation in man. Diabetes 1981;30:261–264 [DOI] [PubMed] [Google Scholar]
- 4.Routh VH, Song Z, Liu X: The role of glucosensing neurons in the detection of hypoglycemia. Diabetes Technol Ther 2004;6:413–421 [DOI] [PubMed] [Google Scholar]
- 5.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI: Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 1997;99:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI: Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest 1994;93:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI: Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 1995;44:180–184 [DOI] [PubMed] [Google Scholar]
- 8.Fioramonti X, Contié S, Song Z, Routh VH, Lorsignol A, Pénicaud L: Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes 2007;56:1219–1227 [DOI] [PubMed] [Google Scholar]
- 9.Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE: Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 2004;53:549–559 [DOI] [PubMed] [Google Scholar]
- 10.Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH: Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 2001;50:2673–2681 [DOI] [PubMed] [Google Scholar]
- 11.Wang R, Liu X, Hentges ST, Dunn-Meyhell AA, Levin BE, Wang W, Routh VH: The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding relevant peptides. Diabetes 2004;53:1959–1965 [DOI] [PubMed] [Google Scholar]
- 12.Powell AM, Sherwin RS, Shulman GI: Impaired hormonal responses to hypoglycemia in spontaneously diabetic and recurrently hypoglycemic rats: reversibility and stimulus specificity of the deficits. J Clin Invest 1993;92:2667–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song Z, Routh VH: Recurrent hypoglycemia reduces the glucose sensitivity of glucose-inhibited neurons in the ventromedial hypothalamus nucleus. Am J Physiol Regul Integr Comp Physiol 2006;291:R1283–R1287 [DOI] [PubMed] [Google Scholar]
- 14.Guix FX, Uribesalgo I, Coma M, Muñoz FJ: The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol 2005;76:126–152 [DOI] [PubMed] [Google Scholar]
- 15.Cabou C, Campistron G, Marsollier N, Leloup C, Cruciani-Guglielmacci C, Pénicaud L, Drucker DJ, Magnan C, Burcelin R: Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes 2008;57:2577–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabou C, Cani PD, Campistron G, Knauf C, Mathieu C, Sartori C, Amar J, Scherrer U, Burcelin R: Central insulin regulates heart rate and arterial blood flow: an endothelial nitric oxide synthase-dependent mechanism altered during diabetes. Diabetes 2007;56:2872–2877 [DOI] [PubMed] [Google Scholar]
- 17.Riediger T, Giannini P, Erguven E, Lutz T: Nitric oxide directly inhibits ghrelin-activated neurons of the arcuate nucleus. Brain Res 2006;1125:37–45 [DOI] [PubMed] [Google Scholar]
- 18.Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD: Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes 2000;49:684–687 [DOI] [PubMed] [Google Scholar]
- 19.Canabal DD, Potian JG, Duran RG, McArdle JJ, Routh VH: Hyperglycemia impairs glucose and insulin regulation of nitric oxide production in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 2007;293:R592–R600 [DOI] [PubMed] [Google Scholar]
- 20.Canabal DD, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH: Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 2007;292:R1418–R1428 [DOI] [PubMed] [Google Scholar]
- 21.Saberi M, Bohland M, Donovan CM: The locus for hypoglycemic detection shifts with the rate of fall in glycemia: the role of portal-superior mesenteric vein glucose sensing. Diabetes 2008;57:1380–1386 [DOI] [PubMed] [Google Scholar]
- 22.Cryer PE: Role of growth hormone in glucose counterregulation. Horm Res 1996;46:192–194 [DOI] [PubMed] [Google Scholar]
- 23.Fioramonti X, Lorsignol A, Taupignon A, Pénicaud L: A new ATP-sensitive K+ channel-independent mechanism is involved in glucose-excited neurons of mouse arcuate nucleus. Diabetes 2004;53:2767–2775 [DOI] [PubMed] [Google Scholar]
- 24.Murphy BA, Fakira KA, Song Z, Beuve A, Routh VH: AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am J Physiol Cell Physiol 2009;297:C750–C758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy BA, Fioramonti X, Jochnowitz N, Fakira K, Gagen K, Contie S, Lorsignol A, Penicaud L, Martin WJ, Routh VH: Fasting enhances the response of arcuate neuropeptide Y-glucose-inhibited neurons to decreased extracellular glucose. Am J Physiol Cell Physiol 2009;296:C746–C756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knauf C, Prevot V, Stefano GB, Mortreux G, Beauvillain JC, Croix D: Evidence for a spontaneous nitric oxide release from the rat median eminence: influence on gonadotropin-releasing hormone release. Endocrinology 2001;142:2343–2350 [DOI] [PubMed] [Google Scholar]
- 27.McCrimmon RJ, Fan X, Cheng H, McNay E, Chan O, Shaw M, Ding Y, Zhu W, Sherwin RS: Activation of AMP-activated protein kinase within the ventromedial hypothalamus amplifies counterregulatory hormone responses in rats with defective counterregulation. Diabetes 2006;55:1755–1760 [DOI] [PubMed] [Google Scholar]
- 28.Borg MA, Tamborlane WV, Shulman GI, Sherwin RS: Local lactate perfusion of the ventromedial hypothalamus suppresses hypoglycemic counterregulation. Diabetes 2003;52:663–666 [DOI] [PubMed] [Google Scholar]
- 29.Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE: Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 2006;55:412–420 [DOI] [PubMed] [Google Scholar]
- 30.Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S: ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 2001;4:507–512 [DOI] [PubMed] [Google Scholar]
- 31.Song Z, Routh VH: Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 2005;54:15–22 [DOI] [PubMed] [Google Scholar]
- 32.Levin BE, Becker TC, Eiki J, Zhang BB, Dunn-Meynell AA: Ventromedial hypothalamic glucokinase is an important mediator of the counterregulatory response to insulin-induced hypoglycemia. Diabetes 2008;57:1371–1379 [DOI] [PubMed] [Google Scholar]
- 33.McCrimmon RJ, Song Z, Cheng H, McNay EC, Weikart-Yeckel C, Fan X, Routh VH, Sherwin RS: Corticotrophin-releasing factor receptors within the ventromedial hypothalamus regulate hypoglycemia-induced hormonal counterregulation. J Clin Invest 2006;116:1723–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryan RM, Jr, Eichler MY, Johnson TD, Woodward WT, Williams JL: Cerebral blood flow, plasma catecholamines, and electroencephalogram during hypoglycemia and recovery after glucose infusion. J Neurosurg Anesthesiol 1994;6:24–34 [DOI] [PubMed] [Google Scholar]
- 35.Bryan RM, Jr, Hollinger BR, Keefer KA, Page RB: Regional cerebral and neural lobe blood flow during insulin-induced hypoglycemia in unanesthetized rats. J Cereb Blood Flow Metab 1987;7:96–102 [DOI] [PubMed] [Google Scholar]
- 36.Kennan RP, Takahashi K, Pan C, Shamoon H, Pan JW: Human cerebral blood flow and metabolism in acute insulin-induced hypoglycemia. J Cereb Blood Flow Metab 2005;25:527–534 [DOI] [PubMed] [Google Scholar]
- 37.Page KA, Arora J, Qiu M, Relwani R, Constable RT, Sherwin RS: Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes 2009;58:448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moncada S, Palmer RM, Higgs EA: Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991;43:109–142 [PubMed] [Google Scholar]
- 39.Horinaka N, Artz N, Jehle J, Takahashi S, Kennedy C, Sokoloff L: Examination of potential mechanisms in the enhancement of cerebral blood flow by hypoglycemia and pharmacological doses of deoxyglucose. J Cereb Blood Flow Metab 1997;17:54–63 [DOI] [PubMed] [Google Scholar]
- 40.Paulson OB: Blood-brain barrier, brain metabolism and cerebral blood flow. Eur Neuropsychopharmacol 2002;12:495–501 [DOI] [PubMed] [Google Scholar]
- 41.Hollinger BR, Bryan RM: Beta-receptor-mediated increase in cerebral blood flow during hypoglycemia. Am J Physiol 1987;253:H949–H955 [DOI] [PubMed] [Google Scholar]
- 42.Horinaka N, Kuang TY, Pak H, Wang R, Jehle J, Kennedy C, Sokoloff L: Blockade of cerebral blood flow response to insulin-induced hypoglycemia by caffeine and glibenclamide in conscious rats. J Cereb Blood Flow Metab 1997;17:1309–1318 [DOI] [PubMed] [Google Scholar]
- 43.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U: Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 2001;104:342–345 [DOI] [PubMed] [Google Scholar]
- 44.Ahern GP, Klyachko VA, Jackson MB: cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci 2002;25:510–517 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.