Abstract
OBJECTIVE
To evaluate the direct impact of n-3 polyunsaturated fatty acids (n-3 PUFAs) on the functions and viability of pancreatic β-cells.
RESEARCH DESIGN AND METHODS
We developed an mfat-1 transgenic mouse model in which endogenous production of n-3 PUFAs was achieved through overexpressing a C. elegans n-3 fatty acid desaturase gene, mfat-1. The islets and INS-1 cells expressing mfat-1 were analyzed for insulin secretion and viability in response to cytokine treatment.
RESULTS
The transgenic islets contained much higher levels of n-3 PUFAs and lower levels of n-6 PUFAs than the wild type. Insulin secretion stimulated by glucose, amino acids, and glucagon-like peptide-1 (GLP-1) was significantly elevated in the transgenic islets. When challenged with tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and γ-interferon (IFN-γ), the transgenic islets completely resisted cytokine-induced cell death. Adenoviral transduction of mfat-1 gene in wild-type islets and in INS-1 cells led to acute changes in the cellular levels of n-3- and n-6 PUFAs and recapitulated the results in the transgenic islets. The expression of mfat-1 led to decreased production of prostaglandin E2 (PGE2), which in turn contributed to the elevation of insulin secretion. We further found that cytokine-induced activation of NF-κB and extracellular signal–related kinase 1/2 (ERK1/2) was significantly attenuated and that the expression of pancreatic duodenal hemeobox-1 (PDX-1), glucokinase, and insulin-1 was increased as a result of n-3 PUFA production.
CONCLUSIONS
Stable cellular production of n-3 PUFAs via mfat-1 can enhance insulin secretion and confers strong resistance to cytokine-induced β-cell destruction. The utility of mfat-1 gene in deterring type 1 diabetes should be further explored in vivo.
Polyunsaturated fatty acids (PUFAs) are synthesized from the modification of saturated fatty acid precursors by different desaturases and elongation enzymes. Mammals have neither the desaturase necessary to synthesize the precursors of other PUFAs, linoleic acid (LA, n-6), and α-linolenic acid (ALA, n-3), nor the n-3 fatty acid desaturase to convert n-6 PUFAs to n-3 PUFAs. Therefore, LA and ALA and their elongation products are essential fatty acids to mammals and must be taken from diets (1,2). Physiologically, n-3 PUFAs play critical roles in the development and functions of retina, spermatozoa, and the central nervous system (1,3).
A series of epidemiological studies have established the health benefits of dietary intake of n-3 PUFAs in preventing cardiovascular diseases and type 2 diabetes (4,5). Two recent large-scale clinical studies also demonstrated that long-term dietary intake of fish oil starting at 1 year of age lowers the risk of type 1 diabetes and islet autoimmunity (6,7). Consistent with such epidemiological evidence, recent studies in rodents indicated that dietary gain or direct administration of n-3 PUFAs could restore palmitate acid– or linoleic acid–impaired insulin secretion (8,9). An issue central to this study is whether the effects of n-3 PUFAs on type 1 diabetes are related to the direct impact on the functions and viability of β-cells. To evaluate such effects as well as the underlying mechanisms, we developed a transgenic mouse model that expresses a C. elegans gene, fat-1, encoding a n-3 fatty acid desaturase (10). Since this enzyme can convert n-6 PUFAs into the corresponding n-3 forms, transgenic expression of fat-1 will render the host endogenous capability of producing n-3 PUFAs while concomitantly reducing the levels of n-6 PUFAs. Using such a unique animal model, we are able to evaluate the impact of stable cellular elevation of n-3 PUFAs on insulin secretion and viability of β-cells without the need of lengthy feeding of fish oil. The positive outcomes from our studies may reveal the potential utility of this type of gene to deter and mitigate type 1 diabetes.
RESEARCH DESIGN AND METHODS
Generation of mfat-1 transgenic mice.
The coding region of fat-1 cDNA was optimized for mammalian cell expression, and the resultant cDNA, renamed mfat-1, was synthesized by Geneart. The mfat-1 expression cassette, consisting of the mfat-1 cDNA driven by a cytomegalovirus (CMV) enhancer and chicken β-actin promoter tethered with a muscle creatine kinase (MCK) enhancer (supplemental Fig. 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0284/DC1), was introduced into C57BL/6 mice by pronuclear microinjection. All animal protocols were approved by the University of Pittsburgh Institutional Animal Care and Usage Committee.
Ad-mfat-1 viral vector and infection with adenoviruses.
The mfat-1 cDNA was inserted into an adenoviral shuttle vector under the control of CMV promoter. The generation of Ad-mfat-1 viral vector and the large-scale preparation of Ad-mfat-1 (and Ad-lacZ) vector were carried out by the Viral Core Facility at the University of Pittsburgh. Rat insulinoma INS-1 cells cultured in RPMI-1640 medium were infected in the presence of 7.5 mmol/l glucose (no serum) with either a control (Ad-lacZ) or Ad-mfat1 virus at a multiplicity of infection (MOI) of 100 for 4 h. Isolated islets were cultured overnight after isolation and were then infected for 4 h with Ad-mfat1 or Ad-lacZ at 250–500 MOI (∼103 cells/islet).
Cytokine treatment and imaging analysis.
Adenovirus-infected INS-1 cells or islets (48 h after) were exposed to the following cytokines: 5 ng/ml human interleukin-1β (IL-1β), 100 ng/ml γ-interferon (IFN-γ), and 10 ng/ml tumor necrosis factor-α (TNF-α) (Roche Diagnostics) for 48 h. Each experiment was performed in triplicate. The cells or islets were double stained with propidium iodide (PI) and Hoescst nuclear dye before imaging analysis using a confocal microscope for islets or an inverted fluorescent microscope for INS-1 cells.
Islet isolation, glucose-stimulated insulin secretion in islets or INS-1 cells, and quantitative analysis of gene expression levels.
Briefly, the pancreas was injected through the pancreatic duct with Hanks' buffered saline solution (HBSS) containing collagenase (type V), excised, incubated in HBSS for 15 min at 37°C, and then filtered through a 500-μm wire mesh. The digested pancreas was rinsed with HBSS, and islets were separated by Ficoll-gradient centrifugation. After several washes in HBSS, islets were handpicked under an inverted light microscope. Insulin release from isolated islets or INS-1 cells was measured as previous described (11) with some modifications. INS-1 cells or groups of 10 islets of similar size were preincubated in Krebs-Ringer bicarbonate buffer (KRBB) (supplemented with 10 mmol/l HEPES and 0.5% BSA) and 2.8 mmol/l glucose for 1 h at 37°C, washed once in KRBB plus 1% BSA, and then incubated in 1 ml fresh KRBB containing either low glucose (2.8 mmol/l) or high glucose (11.1 for INS-1 or 22.2 mmol/l for islets) for 30 min. In a parallel set of experiments, the INS-1 cells or the islets were incubated with KRBB containing high glucose plus 10 nmol/l glucagon-like peptide-1 (GLP-1). At the end of incubation, supernatant was collected and the cells or islets were harvested in KRBB for ELISA determination of secreted or cellular insulin content (Linco Research).
Leucine and glutamine-stimulated insulin secretion in isolated islets.
Groups of 10 islets of similar size were preincubated in KRBB and 1.1 mmol/l glucose for 1 h at 37°C, washed once in KRBB, and then incubated in 0.5 ml fresh KRBB containing 1.1 mmol/l glucose plus 10 mmol/l leucine and 10 mmol/l glutamine for 60 min. At the end of incubation, supernatant was collected and the islets were harvested in KRBB for measuring insulin content.
Pertussis toxin and prostaglandin E2 treatment on isolated islets.
Groups of six islets of similar size were preincubated in KRBB containing 2.8 mmol/l glucose with or without 50 ng/ml pertussis toxin (PTX) and 10 μmol/l prostaglandin E2 (PGE2) for 12 h before the study of 22.2 mmol/l glucose-stimulated insulin secretion. The medium was collected and analyzed for insulin concentration.
Western blotting.
INS-1 cells were infected with Ad-mfat-1 and Ad-lacZ for 4 h before being switched to the medium without viral particles. After 48 h the cells were treated with RPMI-1640 medium containing with IL-1β (5 ng/ml) or TNF-α (10 ng/ml) for various lengths of time. Analysis of extra cellular–signal related kinse 1/2 (ERK1/2) phosphorylation and IκB-α phosphorylation were performed using antibodies against phosphor-ERK1/2 and phospo–IκB-α, followed by control blots with antibodies against ERK1/2 and Iκκ-α, respectively. All antibodies were from Cell Signaling.
Quantitative analysis of gene expression levels in INS-1 cells.
Total RNA was prepared using TRIZOL reagent (Invitrogen, Carlsbad, CA), treated with DNase I (Ambion, Austin, TX), and reverse transcribed with a Superscript kit (Invitrogen). The real-time PCRs were carried out on an ABI7900 Taqman machine with the following cycles: 50°C for 2 min, one cycle; 95°C for 10 min, one cycle; and 95°C for 15 s and 60°C for 1 min, 40 cycles. Rat 18s rRNA was used as the internal control for input cDNA quantity. Linearity of each assay was determined based on a co-efficient value of at least 0.99 for the standard curve. The cDNA concentrations of all samples were within the linear range of the standard curve. The design of double-labeled probes and the flanking primers was carried out using Primer-Express software (Applied Biosystems, Foster City, CA). The probes for rat pancreatic duodenal homeobox-1 (PDX-1), insulin 1, glucokinase (GK), and 18s rRNA were labeled with FAM (5′) and BHQ-1 (3′). The probe and primer sequences are as follows. Rat PDX-1: probe, 5′-6FAM-ATGAAATCCACCAAAGCTCACGCGTG-3′; forward primer, 5′-CCGCGTTCATCTCCCTTTC-3′; reverse primer, 5′-CTCCTGCCCACTGGCTTTT-3′. Rat insulin 1: probe, 5′-6FAM-CAGCACCTTTGTGGTCCTCACCTGG-3′; forward primer, 5′-CTGCCCAGGCTTTTGTCAA-3′; reverse primer, 5′-TCCCCACACACCAGGTACAGA-3′. Rat GK: probe, 5′-6FAM-CCGTGTACAAGCTGCACCCGAGC-3′; forward primer, 5′-CATCACTGTGGGCGTGGAT-3′, reverse primer, 5′-GGCGTGAAACCGCTCCTT-3′. Rat 18s rRNA: probe, 5′-6FAM-TGGACCGGCGCAAGACGGAC-3′; forward primer, 5′-CGCCGCTAGAGGTGAAATTC-3′; reverse primer, 5′-TTGGCAAATGCTTTCGC TC-3′.
Gas chromatography analysis of fatty acid compositions.
Lipids extraction from tissues was performed according to the general technique of Bligh and Dyer (12). The gas chromatograph was performed on a Perkin Elmer Clarus 500. Identification of components was by comparison of retention times with those of authentic standards (Sigma).
Statistical analysis.
Data are presented as the mean ± SEM. All paired comparisons were subject to a two-tailed Student t test with P < 0.05 considered statistically significant.
RESULTS
Although the newly generated transgenic mouse model is similar to the one reported earlier (called “fat-1 mice”) (13), the codons of fat-1 cDNA are further optimized for efficient translation in mammalian systems (hence renamed “mfat-1,”) (supplemental Fig. 1). The generation of mfat-1 mice was not only verified through genotyping but also confirmed by examining the relative content of n-6 and n-3 PUFAs in the tails as well as in isolated pancreatic islets (Table 1). In both tissue samples of mfat-1 transgenic mice there was a significant increase in n-3 PUFAs (2.6- and 3.3-fold in tails and islets, respectively), accompanied by a prominent decrease in n-6 PUFAs (2.7- and 3.8-fold in tails and islets, respectively), which resulted in sharply reduced n-6–to–n-3 ratios relative to those in the wild-type tissues (Table 1). Interestingly, the total percentage of n-3 plus n-6 PUFAs in mfat-1 transgenic tissues was modestly reduced, which corresponded to a minor percentage increase in some other fatty acid species, primarily reflected in the most dominant peak of oleic acid (see legend to Table 1). Similar observations have been made in previously reported fat-1–expressing tissues or cells (14,15). Although the underlying reason for this observation is still not clear, it is likely due to the increased preferential usage of n-3 PUFAs as substrates by various enzymes in mfat-1–expressing tissues or cells, which might have contributed to the reduced inflammatory states described below.
TABLE 1.
Analysis of n-6 and n-3 PUFA composition
| Total n-6 | Total n-3 | n-6/n-3 ratio | |
|---|---|---|---|
| Mouse tissues (%) | |||
| Tails | |||
| Wild type | 23.71 ± 0.32 | 5.21 ± 0.21 | 4.76 ± 0.23 |
| mfat-1 | 8.90 ± 1.79* | 13.72 ± 0.87* | 0.66 ± 0.17* |
| Islets | |||
| Wild type | 32.39 ± 0.05 | 6.36 ± 0.02 | 5.10 ± 0.01 |
| mfat-1 | 8.37 ± 0.05* | 21.16 ± 0.01* | 0.39 ± 0.01* |
| Virally infected (%) | |||
| INS-1 | |||
| Adenovirus carrying lac-Z cDNA | 13.13 ± 0.83 | 2.29 ± 0.49 | 5.54 ± 1.78 |
| Adenovirus carrying mfat-1 cDNA | 4.12 ± 0.65* | 8.26 ± 0.27* | 0.50 ± 0.09* |
| Wild-type | |||
| Adenovirus carrying lac-Z cDNA | 28.42 ± 0.30 | 7.73 ± 0.11 | 3.68 ± 0.01 |
| Adenovirus carrying mfat-1 cDNA | 18.85 ± 0.12* | 15.48 ± 0.07* | 1.22 ± 0.01* |
Data are means ± SD. The tissues (50 mg of tail samples or ∼200 islets) were collected from the mfat-1 transgenic or wild-type control mice. Fatty acid analysis was also performed on the INS-1 or the wild-type islets infected with adenoviral vectors carrying either mfat-1 or lacZ cDNA. The compositions of n-6 or n-3 PUFAs were expressed using relative percentages, i.e., the distribution areas of n-3 or n-6 PUFA peaks divided by the total peak areas of all detectable saturated and unsaturated free fatty acids (from the same sample) resolved from the gas chromatography column. In the transgenic tissues, the modest drop in relative percentages of n-3 plus n-6 PUFAs corresponded to a minor percentage increase in some other detectable fatty acid species. However, the increase was primarily reflected in the most dominant peak of oleic acids (tails, 26.9% [wt] vs. 33.0% [mfat-1]; islets, 17.6% [wt] vs. 22.8% [mfat-1]). The specificity of mfat-1 enzymatic activity primarily caused the changes of n-6 and n-3 PUFAs. n = 4.
*P < 0.001 when mfat-1 group compared with corresponding control (wt or lacZ) group.
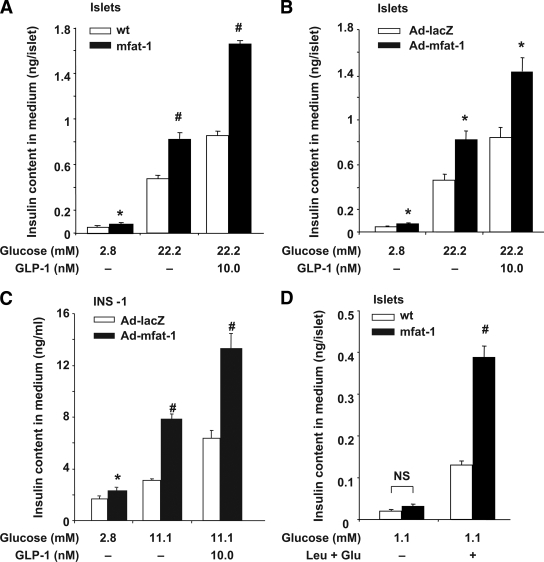
We investigated the impact of cellular production of n-3 PUFAs on insulin secretion. In response to high glucose stimulation, the levels of insulin secretion were significantly higher in the islets isolated from the mfat-1 transgenic than in those from the wild type (Fig. 1A). Addition of GLP-1 further stimulated insulin secretion in both wild-type and transgenic islets, but the level was significantly higher in mfat-1 transgenic islets (Fig. 1A). The enhanced insulin secretion of mfat-1 transgenic islets was unlikely due to enlarged islet size because confocal microscopy analysis of insulin-stained pancreatic sections did not find significant difference in islet sizes between the transgenic and wild-type groups (supplemental Fig. 2). Although the levels of insulin secretion were expressed in absolute concentrations (nanograms of secreted insulin/islet, Fig. 1A), these insulin secretion patterns still held up after we expressed them in relative concentrations (secreted insulin as a percentage of total insulin content, supplemental Fig. 3A). These data depict the enhanced insulin-secreting action of the β-cells that were pre-exposed to a high n-3 PUFA environment (as a result of mfat-1 expression) even long before birth. We wondered if acute elevation of n-3 PUFAs via mFAT-1 activity in the β-cells would also enhance insulin secretion. To this end, we used an adenoviral expression vector to introduce mfat-1 cDNA (or the control bacterial lacZ gene) into the islets originally isolated from wild-type mice and into a rat insulinoma cell line, INS-1. Owing to mfat-1 expression, the cellular levels of n-3 PUFAs were sharply elevated, and the levels of n-6 PUFAs and the ratios of n-6 to n-3 were significantly decreased in these virally transduced cells (Table 1). The virally induced expression of mfat-1 in the islets and INS-1 cells caused significant enhancement in glucose- and GLP-1–stimulated insulin secretion (Fig. 1B and C and supplemental Fig. 3B and C). Interestingly, even at the baseline of 2.8 mmol/l, glucose could evoke significantly higher insulin secretion in mfat-1–expressing islets (though not necessarily in the insulinoma INS-1 cells) than in control islets (Fig. 1A and B). As a result, the relative fold of stimulation did not differ between the transgenic and wild-type islets (supplemental Fig. 3E and F), although this does not seem to be the case for the insulinoma INS-1 cells (supplemental Fig. 3G). Such observation made us wonder if the high glucose concentrations only amplified an enhanced secretion mechanism in the mfat-1–expressing islets and prompted us to inquire if the enhancement of insulin secretion as a result of cell production of n-3 PUFAs is beyond the scope of high glucose and GLP-1. To this end, we tested whether certain types of amino acid–induced insulin secretion could also be elevated in mfat-1 transgenic islets. We adopted a widely established protocol to evaluate this issue by applying leucine and glutamine to the culture of mfat-1 transgenic islets in the presence of low concentrations of glucose (1.1 mmol/l) (16). Although the amino acid mixtures stimulated insulin secretion on top of the very low secretion levels elicited by 1.1 mmol/l glucose, there was a significant increase of insulin secretion in the mfat-1–expressing islets above that in the wild-type islets (Fig. 1D and supplemental Fig. 3D). In addition, the fold of stimulation of insulin secretion by amino acids was also substantially higher in mfat-1–expressing islets (supplemental Fig. 3H). It should be noted that elevation of n-3 PUFAs in islets (or INS-1 cells) via the mfat-1 gene (delivered either transgenically or virally) did not alter total insulin content (supplemental Fig. 3I–L). Thus, the broad positive impact on glucose–, amino acids–, and GLP-1–induced insulin release observed in such engineered islets was likely due to the modulation of secretory mechanisms.
FIG. 1.
Insulin secretion in response to glucose, amino acids, and GLP-1 stimulation in transgenic or adenoviral-driven expression of mfat-1 islets or INS-1 cells. A: Islets isolated from the transgenic (mfat-1) or wild-type (wt) mice were incubated with different concentrations of glucose in the presence or absence of GLP-1 (10 nmol/l) for 30 min. The medium was harvested for the measurement of insulin concentration. B and C: Isolated islets from wild-type mice (B) or INS-1 cells (C) were preinfected with adenovirus carrying mfat-1 or lacZ genes for 24 h before the study of insulin secretion, as outlined in A. D: Islets isolated from wild-type and mfat-1 transgenic mice were incubated with 1.1 mmol/l glucose in the presence or absence of 10 mmol/l leucine (Leu) and 10 mmol/l glutamine (Glu) for 60 min before the medium was collected for measurement of secreted insulin. In A–D, the levels of secreted insulin (ng/islet) in each panel were the means + SEM of three independent experiments with each condition carried out in five or six replicates and 10–20 islets in each replicate. #P < 0.01, *P < 0.05 when compared with the control group.
To assess the impact of cellular production of n-3 PUFAs on the viability of pancreatic islets, we incubated the isolated islets with a mixture of TNF-α, IL-1β, and IFN-γ at the concentrations typically applied in previously published studies (17). Double nuclear staining was applied to the live islets using propidium iodide and Hoechst dye for staining dead cells and all the cells, respectively. As expected, cytokine treatment for 48 h led to a sharply elevated level of cell death in the wild-type islets (58 vs. 9% in the nontreated, Fig. 2A and B); however, the islets derived from the mfat-1 transgenic mice strongly resisted cytokine-induced cell death (13 vs. 8% in the nontreated islets, Fig. 2A and B).
FIG. 2.
Cytokine-induced apoptosis of cultured islets or INS-1 cells. A and B: Isolated islets from transgenic or wild type were challenged with the cytokine mix for 48 h before fluorescent confocal imaging. C and D: Islets derived from wild-type mice (C) or INS-1 cells (D) were infected with adenovirus carrying either mfat-1 or lacZ gene for 24 h before being challenged with the cytokine mix for 48 h. The live islets or cells were incubated with propidium iodide and Hoechst dye before imaging analysis. The images were collected under either a confocal (for islets) or regular (for INS-1) fluoresecent microscope. The rates of cell death were calculated based on at least four randomly selected sections or areas of the images. #P < 0.01 when compared with the corresponding control group. (A high-quality digital representation of this figure is available in the online issue.)
Introduction of mfat-1 cDNA into wild-type islets or cultured β-cells using an adenoviral vector also provided strong protection against cytokine-induced cell death. After infected with the adenovirus carrying mfat-1 cDNA in vitro, the INS-1 cells or pancreatic islets originally derived from the wild-type mice displayed significantly reduced dead cells after cytokine challenge (Fig. 2C and D), which was in sharp contrast to the high death rates observed in the control islets or INS-1 cells infected with the lacZ-carrying adenovirus (Fig. 2C and D).
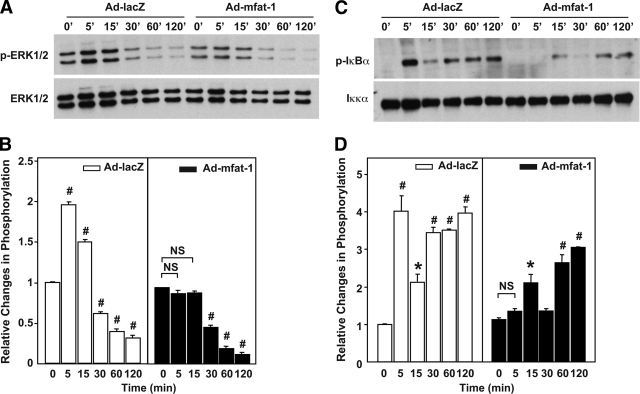
The strong resistance to cytokine-induced cell death led us to investigate the ability of cytokines to signal in mfat-1–expressing β-cells. To this end, we infected INS-1 cells with adenovirus carrying either mfat-1 or lacZ genes and then evaluated the activation of ERK1/2 and NF-κB, two canonical pathways for IL-1β and TNF-α, respectively (18–20). Although, as expected, IL-1β significantly stimulated the activity of ERK1/2 in the lacZ-expressing INS-1 cells, it failed to do so in the mfat-1–expressing cells (Fig. 3A and B). Shortly after treatment (within 5 min), TNF-α evoked a robust and temporally dependent phosphorylation of IκB-α, a critical early step for the activation of NF-κB signaling pathway, in the lacZ-expressing INS-1 cells (Fig. 3C and D). However, the phosphorylation of IκB-α induced by TNF-α was sharply attenuated and even delayed in the mfat-1–expressing cells (Fig. 3C and D). Such differential cytokine signaling pattern is very likely to have contributed to strong resistance of mfat-1–expressing β-cells to cytokine-induced cell death.
FIG. 3.
Activation of ERK1/2 and NF-κB in INS-1 cells infected with Ad-mfat-1 or Ad-lacZ. INS-1 cells were infected with Ad-mfat-1 or Ad-LacZ for 4 h. Approximately 48 h later, the cells were exposed to IL-1β (5 ng/ml) (A and B) or TNF-α (10 ng/ml) (C and D) for the indicated time. The cells were then extracted for protein for Western blot assays of ERK1/2 and IκB-α phosphorylation. The level of phosphorylation was quantified with Image J software. #P < 0.01, *P < 0.05 when compared with the corresponding control group.
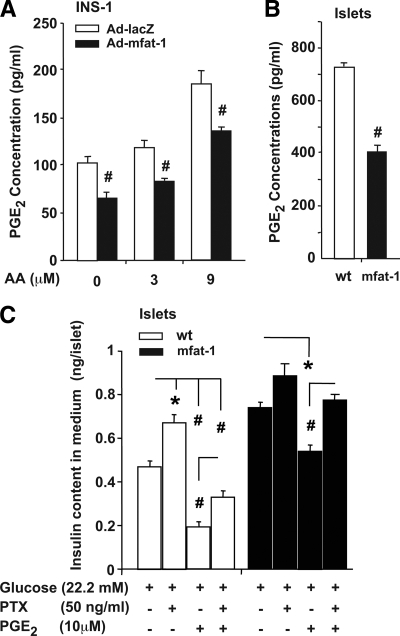
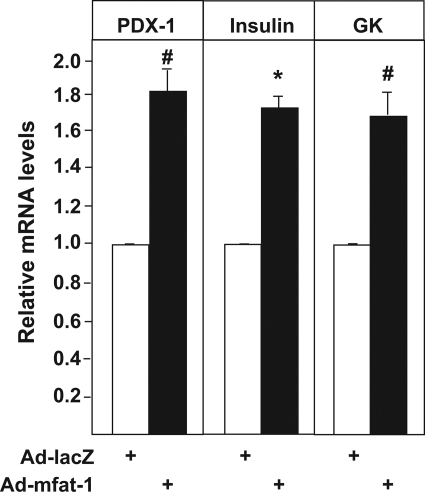
To gain further mechanistic understanding of the aforementioned phenotypes related to insulin secretion, we focused on the roles of inflammation modulation. Elevation of n-3 PUFAs is known to suppress the production of PGE2 (21,22), a negative regulator of glucose-stimulated insulin secretion (23,24). We found that PGE2 concentration was significantly reduced in the medium incubated with the mfat-1–expressing INS-1 cells relative to that incubated with the lacZ-expressing control cells (Fig. 4A). Similarly, the medium culturing the mfat-1 transgenic islets contained less PGE2 than the one culturing the wild-type islets (Fig. 4B). As expected, addition of arachidonic acid (AA, an n-6 PUFA), a substrate of cycloxygenase-2 (COX-2) for PGE2 synthesis, to the incubation medium increased PGE2 secretion from INS-1 cells; however, such an increase was significantly attenuated in mfat-1–expressing INS-1 cells (Fig. 4A). Preincubation with PGE2 (10 μmol/l) for 12 h attenuated glucose-stimulated insulin secretion in the mfat-1 transgenic and wild-type islets (Fig. 4C). Importantly, pretreatment with PGE2 completely neutralized the mfat-1–caused enhancement in insulin secretion (Fig. 4C, no statistical significance when comparing mfat-1+PGE2 vs. wild-type without PGE2), suggesting that the reduction of PGE2 synthesis plays a critical role in n-3 PUFA–regulated insulin secretion. Inhibitory regulatory G-protein (Gi) has been thought as a major signaling component in mediating the actions of PGE2 (25,26). Preincubation of pancreatic islets with pertussis toxin along with PGE2, a known inhibitor of Gi (27,28), partially rescued insulin secretion in the wild-type islets and completely neutralized the inhibitory effect of PGE2 on insulin secretion (Fig. 4C), suggesting that the aforementioned action of PGE2 was mediated at least in part through Gi. Finally, in analyzing gene expression, we further found that elevation of n-3 PUFAs through mFAT-1 activity in the β-cells could lead to changes in the expression of certain genes that are critical to the functions and viability of β-cells. For example, the mRNA levels of PDX-1, GK, and insulin (insulin1) were significantly increased in the INS-1 cells infected with adenoviruses carrying mfat-1 cDNA relative to the lacZ-expressing control cells (Fig. 5).
FIG. 4.
PGE2 production and the effects of PGE2 and pertussis toxin on insulin secretion in mfat-1–expressing INS-1 cells and islets. A: Measurement PGE2 concentrations in the medium incubated with INS-1 cells (preinfected with Ad-mfat-1 or Ad-lacZ) in the presence or absence of AA (3 or 9 μmol/l) for 30 min. B: The concentrations of PGE2 in the medium incubated for 30-min with isolated islets from mfat-1 transgenic or wild-type mice. C: Islets isolated from the transgenic or wild-type mice were preincubated with 10 μmol/l PGE2 in the presence or absence of 50 ng/ml PTX for 12 h before the study of glucose-stimulated insulin secretion. The results are means + SEM from three independent repeats with each condition carried out in eight replicates and six islets in each replicate. #P < 0.01, *P < 0.05 when mfat-1 group compared with corresponding control group.
FIG. 5.
Investigation of gene expression in mfat-1–expressing INS-1 cells. Real-time PCR assays of mRNA expression of PDX-1, GK, and insulin 1 in the INS-1 cells infected with Ad-mfat-1 or Ad-lacZ. n = 4. #P < 0.01, *P < 0.05 when compared with control (Ad-lacZ) cells.
DISCUSSION
In this study, we have shown that cellular increase of n-3 PUFAs and reduction of n-6 PUFAs through transgenic expression of mfat-1 enhances glucose–, amino acid–, and GLP-1–stimulated insulin secretion in isolated pancreatic islets and renders the islets strongly resistant to cytokine-induced cell death. Acute and stable elevation of n-3 PUFAs through adenoviral transduction of mfat-1 gene in isolated islets and in INS-1 cells was able to recapitulate the observations in the mfat-1 transgenic islets.
Although the underlying mechanisms remain to be systematically investigated, initial studies described here offer some mechanistic insights into how mfat-1 expression (and the subsequent changes in PUFA lipid species) impact insulin secretion as well as the survival of pancreatic β-cells. For example, PGE2 has been well demonstrated as a negative regulator of insulin secretion (23,24). The reduction of PGE2 synthesis in the mfat-1–expressing cells or islets was the result of decreased availability of AA, an n-6 PUFA, to COX-2 enzyme, as mFAT-1 activity converted AA to EPA (eicosapentaenoic acid). Consistent with this concept, AA-induced synthesis of PGE2 was also attenuated in mfat-1–expressing cells (Fig. 3A). Though not necessarily the only mechanism, the regulation of PGE2 largely contributed to the elevation of insulin secretion in mfat-1–expressing islets, since addition of 10 μmol/l PGE2 to the islet culture almost completely eliminated the increased portion of insulin secretion (relative to that in the wild-type islets without PGE2 treatment) (Fig. 3C). In strengthening this argument, we have revealed that the negative impact of PGE2 on insulin secretion is mediated at least in part by Gi, as pertussis toxin was able to neutralize the action of PGE2 while co-incubated with mfat-1 transgenic and wild-type islets. Thus, the reduction of PGE2 secretion from the mfat-1–expressing islets and β-cells is critical for the enhanced insulin secretion, suggesting that the control of inflammation through reduction of n-6 PUFAs can have a beneficial effect on β-cell functions.
The strong resistance to cytokine-caused destruction in mfat-1–expressing islets and INS-1 cells was also reflected at the level of cytokine signaling. Specifically, we have shown that cytokine-induced activation of ERK1/2 and NF-κB pathways was significantly suppressed, both of which were critical for cytokines' actions. We should emphasize that the attenuation of TNF-α–activated NF-κB signaling is not necessarily confined to the pancreatic β-cells only. Our recent studies have found similar observations in fat-1 transgenic hepatocytes (J.L. and A.Z., unpublished observations). Thus, the control of cytokine signaling appears to be a common theme that has broad physiological implications. Although it is predicted to enhance insulin signaling and sensitivity in the liver tissue, the reduction of TNF-α–activated NF-κB signaling leads to protection from cytokine-inflicted islet destruction. Finally, from the perspective of gene expression, mfat-1–induced increase in PDX-1, glucokinase, and insulin 1 expression may have also played important roles in improved insulin secretion and β-cell survival.
Modern diets contain excessive n-6 PUFAs but very low levels of n-3 PUFAs, which results in an unhealthy n-6–to–n-3 ratio of ∼20:1 instead of the ideal 1:1 (29). Since the eicosanoid products derived from n-6 PUFAs are more potent mediators of inflammation than the similar products derived from n-3 PUFAs, an imbalanced n-6–to–n-3 ratio in favor of n-6 PUFAs will be highly proinflammatory, which is believed to have contributed to the pathogenesis of such modern diseases as autoimmune disorders and diabetes (1,30). The production of n-3 PUFAs in the β-cells and islets via mFAT-1 activity is achieved by using n-6 PUFAs as the substrates, thus bringing down the ratios of n-6 to n-3 as well as the inflammatory state. Thus, although the increase of n-3 PUFAs is critical, the decrease of n-6 PUFAs may be just as important in determining the phenotypes of mfat-1–expressing islets. In keeping with this concept, we have already shown that the reduction of PGE2 secretion from the mfat-1–expressing islets and β-cells is critical for the enhanced insulin secretion, suggesting that the control of inflammation through reduction of n-6 PUFAs is a main theme in the regulation of β-cell functions by n-3 PUFAs.
Recent clinical studies have established that daily compensation of n-3 PUFAs starting at 1 year of age can have clear benefits against the development of type 1 diabetes and islet autoimmunity (6). Consistent with these clinical observations, studies in rodents have shown that administration of n-3 PUFAs could restore palmitate- or linoleic acid–impaired insulin secretion (8,9). Although we cannot fully equate our transgenic approach to the dietary means of gaining n-3 PUFAs, both methods provide the same types of n-3 PUFAs to the whole body. The results presented here suggest that elevation of cellular n-3 PUFAs has a direct beneficial impact on the survival of pancreatic β-cells from inflammation attack and that they may explain mechanistically why increased intake of n-3 PUFAs in early childhood can help reduce the incidence of type 1 diabetes. Our data may also add n-3 PUFAs to the growing list of factors that can help improve the functions and viability of β-cells. Thus, it warrants further investigation whether mfat-1 expression in disease models such as nonobese diabetic (NOD) mice can mitigate the development of type 1 diabetes.
From a therapeutic perspective, the phenotypes of the islets infected with mfat-1–containing adenovirus bear strong clinical relevance. Engineered islets with enhanced insulin secretion and viability are always sought to overcome inflammatory destruction and the scarcity of available islets (31,32). In this context, the mfat-1–expressing islets should be urgently tested in transplantation settings and may represent an additional tool for the treatment of type 1 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported in part by a U.S. National Institutes of Health Grant (1RO1DK064383-01) and Research Award (7-06-RA-173) from the American Diabetes Association to A.Z., a postdoctoral fellowship award (0725418U) from the American Heart Association to J.L., a scholarship from China Scholarship Council to M.S., and a Chinese National Key Basic Research Program (973 Program, no. 2009CB918904) to Y.D.
No potential conflicts of interest relevant to this article were reported.
We thank Dr. Adolfo Garcia-O'Cana for his assistance in this work. We also thank Dr. Rhobert Evans for his technical assistance in measuring tissue contents of fatty acids.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Simopoulos AP: Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Diet 2003;92:1–22 [DOI] [PubMed] [Google Scholar]
- 2.DeFilippis AP, Sperling LS: Understanding omega-3's. Am Heart J 2006;151:564–570 [DOI] [PubMed] [Google Scholar]
- 3.Lin DS, Connor WE, Wolf DP, Neuringer M, Hachey DL: Unique lipids of primate spermatozoa: desmosterol and docosahexaenoic acid. J Lipid Res 1993;34:491–499 [PubMed] [Google Scholar]
- 4.Petersen M, Pedersen H, Major-Pedersen A, Jensen T, Marckmann P: Effect of fish oil versus corn oil supplementation on LDL and HDL subclasses in type 2 diabetic patients. Diabetes Care 2002;25:1704–1708 [DOI] [PubMed] [Google Scholar]
- 5.Schraer CD, Mayer AM, Vogt AM, Naylor J, Brown TL, Hastie J, Moore J: The Alaska Native diabetes program. Int J Circumpolar Health 2001;60:487–494 [PubMed] [Google Scholar]
- 6.Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, Orton HD, Barón AE, Clare-Salzler M, Chase HP, Szabo NJ, Erlich H, Eisenbarth GS, Rewers M: Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA 2007;298:1420–1428 [DOI] [PubMed] [Google Scholar]
- 7.Stene LC, Joner GNorwegian Childhood Diabetes Study Group. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am J Clin Nutr 2003;78:1128–1134 [DOI] [PubMed] [Google Scholar]
- 8.Kato T, Shimano H, Yamamoto T, Ishikawa M, Kumadaki S, Matsuzaka T, Nakagawa Y, Yahagi N, Nakakuki M, Hasty AH, Takeuchi Y, Kobayashi K, Takahashi A, Yatoh S, Suzuki H, Sone H, Yamada N: Palmitate impairs and eicosapentaenoate restores insulin secretion through regulation of SREBP-1c in pancreatic islets. Diabetes 2008;57:2382–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winzell MS, Pacini G, Ahrén B: Insulin secretion after dietary supplementation with conjugated linoleic acids and n-3 polyunsaturated fatty acids in normal and insulin-resistant mice. Am J Physiol Endocrinol Metab 2006;290:E347–354 [DOI] [PubMed] [Google Scholar]
- 10.Spychalla JP, Kinney AJ, Browse J: Identification of an animal omega-3 fatty acid desaturase by heterologous expression in Arabidopsis. Proc Natl Acad Sci U S A 1997;94:1142–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roccisana J, Reddy V, Vasavada RC, Gonzalez-Pertusa JA, Magnuson MA, Garcia-Ocaña A: Targeted inactivation of hepatocyte growth factor receptor c-met in beta-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of beta-cell mass. Diabetes 2005;54:2090–2102 [DOI] [PubMed] [Google Scholar]
- 12.BLIGH EG, DYER WJ: A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–917 [DOI] [PubMed] [Google Scholar]
- 13.Kang JX, Wang J, Wu L, Kang ZB: Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature 2004;427:504. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Nie D, Witt WT, Chen Q, Shen M, Xie H, Lai L, Dai Y, Zhang J: Expression of the fat-1 gene diminishes prostate cancer growth in vivo through enhancing apoptosis and inhibiting GSK-3 beta phosphorylation. Mol Cancer Ther 2008;7:3203–3211 [DOI] [PubMed] [Google Scholar]
- 15.Nowak J, Weylandt KH, Habbel P, Wang J, Dignass A, Glickman JN, Kang JX: Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids. Carcinogenesis 2007;28:1991–1995 [DOI] [PubMed] [Google Scholar]
- 16.Fahien LA, MacDonald MJ, Kmiotek EH, Mertz RJ, Fahien CM: Regulation of insulin release by factors that also modify glutamate dehydrogenase. J Biol Chem 1988;263:13610–13614 [PubMed] [Google Scholar]
- 17.Su D, Zhang N, He J, Qu S, Slusher S, Bottino R, Bertera S, Bromberg J, Dong HH: Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes 2007;56:2274–2283 [DOI] [PubMed] [Google Scholar]
- 18.Wullaert A, van Loo G, Heyninck K, Beyaert R: Hepatic tumor necrosis factor signaling and nuclear factor-kappaB: effects on liver homeostasis and beyond. Endocr Rev 2007;28:365–386 [DOI] [PubMed] [Google Scholar]
- 19.Larsen CM, Wadt KA, Juhl LF, Andersen HU, Karlsen AE, Su MS, Seedorf K, Shapiro L, Dinarello CA, Mandrup-Poulsen T: Interleukin-1beta-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Biol Chem 1998;273:15294–15300 [DOI] [PubMed] [Google Scholar]
- 20.Eizirik DL, Mandrup-Poulsen T: A choice of death–the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 2001;44:2115–2133 [DOI] [PubMed] [Google Scholar]
- 21.Funahashi H, Satake M, Hasan S, Sawai H, Newman RA, Reber HA, Hines OJ, Eibl G: Opposing effects of n-6 and n-3 polyunsaturated fatty acids on pancreatic cancer growth. Pancreas 2008;36:353–362 [DOI] [PubMed] [Google Scholar]
- 22.Calviello G, Di Nicuolo F, Gragnoli S, Piccioni E, Serini S, Maggiano N, Tringali G, Navarra P, Ranelletti FO, Palozza P: n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1alpha induction pathway. Carcinogenesis 2004;25:2303–2310 [DOI] [PubMed] [Google Scholar]
- 23.Robertson RP: Molecular Regulation of Pancreatic Islet Prostaglandin Synthesis and its Relevance to Diabetes Mellitus. West Sussex, England, John Wiley & Sons, 2004 [Google Scholar]
- 24.Meng ZX, Sun JX, Ling JJ, Lv JH, Zhu DY, Chen Q, Sun YJ, Han X: Prostaglandin E2 regulates Foxo activity via the Akt pathway: implications for pancreatic islet beta cell dysfunction. Diabetologia 2006;49:2959–2968 [DOI] [PubMed] [Google Scholar]
- 25.Chillar A, Wu J, So SP, Ruan KH: Involvement of non-conserved residues important for PGE2 binding to the constrained EP3 eLP2 using NMR and site-directed mutagenesis. FEBS Lett 2008;582:2863–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weller CL, Collington SJ, Hartnell A, Conroy DM, Kaise T, Barker JE, Wilson MS, Taylor GW, Jose PJ, Williams TJ: Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proc Natl Acad Sci U S A 2007;104:11712–11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilman AG: G proteins: transducers of receptor-generated signals. Annu Rev Biochem 1987;56:615–649 [DOI] [PubMed] [Google Scholar]
- 28.Kowluru A, Li G, Metz SA: Glucose activates the carboxyl methylation of gamma subunits of trimeric GTP-binding proteins in pancreatic beta cells. Modulation in vivo by calcium, GTP, and pertussis toxin. J Clin Invest 1997;100:1596–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang JX: The importance of omega-6/omega-3 fatty acid ratio in cell function. The gene transfer of omega-3 fatty acid desaturase. World Rev Nutr Diet 2003;92:23–36 [DOI] [PubMed] [Google Scholar]
- 30.Leaf A, Kang JX, Xiao YF, Billman GE: Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation 2003;107:2646–2652 [DOI] [PubMed] [Google Scholar]
- 31.van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK: Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation 2007;14:288–297 [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Moore DJ, Ketchum RJ, Nunemaker CS, Kovatchev B, McCall AL, Brayman KL: Resolving the conundrum of islet transplantation by linking metabolic dysregulation, inflammation, and immune regulation. Endocr Rev 2008;29:603–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.