Abstract
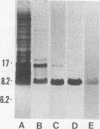
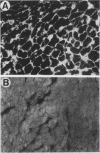
A 9-kDa glycosaminoglycan-binding protein (GAG-BP) was isolated from Streptococcus pyogenes and purified to homogeneity by affinity chromatography on heparin-agarose. The protein selectively bound to the basal laminae of human cardiac muscle and had an apparent dissociation constant of 2.5 x 10(-7) M. Chemical analyses indicated that the GAG-BP was rich in alanine, lysine, and arginine (pI 9.5) and devoid of tyrosine, methionine, histidine, and half-cystine. There were no detectable carbohydrate or phosphate substituents. The amino acid sequence of the N terminus of GAG-BP showed homology with those of histone-like DNA-binding proteins of several other bacteria. Circular dichroism spectroscopy indicated that the protein was made up of 50% beta-sheet and 50% beta-turn and random coil in aqueous solution; however, when the protein complexed with heparin, it adopted a more ordered structure containing 25% alpha-helix, 50% beta-sheet, and 25% beta-turn and random coil. The GAG-BP cross-reacted serologically with a component of similar size in extracts of other group A streptococci and was present in the culture medium during late logarithmic growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergey E. J., Stinson M. W. Heparin-inhibitable basement membrane-binding protein of Streptococcus pyogenes. Infect Immun. 1988 Jul;56(7):1715–1721. doi: 10.1128/iai.56.7.1715-1721.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Choi S. H., Stinson M. W. Binding of a Streptococcus mutans cationic protein to kidney in vitro. Infect Immun. 1991 Feb;59(2):537–543. doi: 10.1128/iai.59.2.537-543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. H., Stinson M. W. Purification of a Streptococcus mutans protein that binds to heart tissue and glycosaminoglycans. Infect Immun. 1989 Dec;57(12):3834–3840. doi: 10.1128/iai.57.12.3834-3840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin W., Deol H., Azadegan A., Lange K. Endostreptosin: isolation of the probable immunogen of acute post-streptococcal glomerulonephritis (PSGN). Clin Exp Immunol. 1989 May;76(2):198–203. [PMC free article] [PubMed] [Google Scholar]
- De Scheerder I., Wulfrank D., Van Renterghem L., Sabbe L., Robbrecht D., Clement D., Derom F., Plum J., Verdonk G. Association of anti-heart antibodies and circulating immune complexes in the post-pericardiotomy syndrome. Clin Exp Immunol. 1984 Aug;57(2):423–428. [PMC free article] [PubMed] [Google Scholar]
- Fillit H., Damle S. P., Gregory J. D., Volin C., Poon-King T., Zabriskie J. Sera from patients with poststreptococcal glomerulonephritis contain antibodies to glomerular heparan sulfate proteoglycan. J Exp Med. 1985 Feb 1;161(2):277–289. doi: 10.1084/jem.161.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glurich I., Winters B., Albini B., Stinson M. Identification of Streptococcus pyogenes proteins that bind to rabbit kidney in vitro and in vivo. Microb Pathog. 1991 Mar;10(3):209–220. doi: 10.1016/0882-4010(91)90055-f. [DOI] [PubMed] [Google Scholar]
- Hol W. G., van Duijnen P. T., Berendsen H. J. The alpha-helix dipole and the properties of proteins. Nature. 1978 Jun 8;273(5662):443–446. doi: 10.1038/273443a0. [DOI] [PubMed] [Google Scholar]
- Imber R., Kimura M., Groch N., Heinemann U. DNA-binding properties and primary structure of HB protein from Bacillus globigii. Eur J Biochem. 1987 Jun 15;165(3):547–552. doi: 10.1111/j.1432-1033.1987.tb11474.x. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Zabriskie J. B. Purification and partial characterization of the nephritis strain-associated protein from Streptococcus pyogenes, group A. J Exp Med. 1986 Mar 1;163(3):697–712. doi: 10.1084/jem.163.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalides N. A., Pegg M. T., Ohno N., Poon-King T., Zabriskie J., Fillit H. Antibodies to basement membrane collagen and to laminin are present in sera from patients with poststreptococcal glomerulonephritis. J Exp Med. 1986 Mar 1;163(3):588–602. doi: 10.1084/jem.163.3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanaka H., Laine B., Sautiere P., Guillaume J. Characterization and primary structures of DNA-binding HU-type proteins from Rhizobiaceae. Eur J Biochem. 1985 Mar 1;147(2):343–349. doi: 10.1111/j.1432-1033.1985.tb08755.x. [DOI] [PubMed] [Google Scholar]
- Kimura M., Wilson K. S. On the DNA binding protein II from Bacillus stearothermophilus. II. The amino acid sequence and its relation to those of homologous proteins from other prokaryotes. J Biol Chem. 1983 Mar 25;258(6):4007–4011. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laine B., Belaiche D., Sautiere P., Biserte G. Characterization and structural study of the DNA-binding protein HRm From Rhizobium meliloti. Biochem Biophys Res Commun. 1982 May 14;106(1):101–107. doi: 10.1016/0006-291x(82)92063-0. [DOI] [PubMed] [Google Scholar]
- Lange K., Seligson G., Cronin W. Evidence for the in situ origin of poststreptococcal glomerulonephritis: glomerular localization of endostreptosin and the clinical significance of the subsequent antibody response. Clin Nephrol. 1983 Jan;19(1):3–10. [PubMed] [Google Scholar]
- Leon O., Panos C. An electron microscope study of kidney basement membrane changes in the mouse by lipoteichoic acid from Streptococcus pyogenes. Can J Microbiol. 1987 Aug;33(8):709–717. doi: 10.1139/m87-124. [DOI] [PubMed] [Google Scholar]
- Makino H., Gibbons J. T., Reddy M. K., Kanwar Y. S. Nephritogenicity of antibodies to proteoglycans of the glomerular basement membrane--I. J Clin Invest. 1986 Jan;77(1):142–156. doi: 10.1172/JCI112269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Ozols J. Amino acid analysis. Methods Enzymol. 1990;182:587–601. doi: 10.1016/0076-6879(90)82046-5. [DOI] [PubMed] [Google Scholar]
- Seligson G., Lange K., Majeed H. A., Deol H., Cronin W., Bovie R. Significance of endostreptosin antibody titers in poststreptococcal glomerulonephritis. Clin Nephrol. 1985 Aug;24(2):69–75. [PubMed] [Google Scholar]
- Stinson M. W., Bergey E. J. Isolation of heart- and kidney-binding protein from group A streptococci. Infect Immun. 1982 Jan;35(1):335–342. doi: 10.1128/iai.35.1.335-342.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder F. J., Barua P. K., Albini B., Stinson M. W. Heart-reactive antibodies in rabbit anti-Streptococcus mutans sera fail to cross-react with Streptococcus mutans. J Immunol. 1988 Feb 1;140(3):954–961. [PubMed] [Google Scholar]
- Tanaka I., Appelt K., Dijk J., White S. W., Wilson K. S. 3-A resolution structure of a protein with histone-like properties in prokaryotes. Nature. 1984 Aug 2;310(5976):376–381. doi: 10.1038/310376a0. [DOI] [PubMed] [Google Scholar]
- Tomlinson K., Leon O., Panos C. Morphological changes and pathology of mouse glomeruli infected with a streptococcal L-form or exposed to lipoteichoic acid. Infect Immun. 1983 Dec;42(3):1144–1151. doi: 10.1128/iai.42.3.1144-1151.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt A., Batsford S., Rodríguez-Iturbe B., García R. Cationic antigens in poststreptococcal glomerulonephritis. Clin Nephrol. 1983 Dec;20(6):271–279. [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]