Abstract
OBJECTIVE
In humans, multiple genes in the interleukin (IL)-2/IL-2 receptor (IL-2R) pathway are associated with type 1 diabetes. However, no link between IL-2 responsiveness and CD4+CD25+FOXP3+ regulatory T-cells (Tregs) has been demonstrated in type 1 diabetic subjects despite the role of these IL-2–dependent cells in controlling autoimmunity. Here, we address whether altered IL-2 responsiveness impacts persistence of FOXP3 expression in Tregs of type 1 diabetic subjects.
RESEARCH DESIGN AND METHODS
Persistence of Tregs was assessed by culturing sorted CD4+CD25hi natural Tregs with IL-2 and measuring FOXP3 expression over time by flow cytometry for control and type 1 diabetic populations. The effects of IL-2 on FOXP3 induction were assessed 48 h after activation of CD4+CD25− T-cells with anti-CD3 antibody. Cytokine receptor expression and signaling upon exposure to IL-2, IL-7, and IL-15 were determined by flow cytometry and Western blot analysis.
RESULTS
Maintenance of FOXP3 expression in CD4+CD25+ Tregs of type 1 diabetic subjects was diminished in the presence of IL-2, but not IL-7. Impaired responsiveness was not linked to altered expression of the IL-2R complex. Instead, IL-2R signaling was reduced in Tregs and total CD4+ T-cells of type 1 diabetic subjects. In some individuals, decreased signal transducer and activator of transcription 5 phosphorylation correlated with significantly higher expression of protein tyrosine phosphatase N2, a negative regulator of IL-2R signaling.
CONCLUSIONS
Aberrant IL-2R signaling in CD4+ T-cells of type 1 diabetic subjects contributes to decreased persistence of FOXP3 expression that may impact establishment of tolerance. These findings suggest novel targets for treatment of type 1 diabetes within the IL-2R pathway and suggest that an altered IL-2R signaling signature may be a biomarker for type 1 diabetes.
There are two major classes of FOXP3+ regulatory T-cells (Tregs): natural Tregs (nTregs), which develop in the thymus and control peripheral immune responses to self-antigens, and induced Tregs (iTregs), which can be generated from peripheral blood CD4+CD25− T-cells (1,2). Although the ontogeny of human peripheral blood FOXP3+ T-cells is still debated, it is clear that interleukin (IL)-2 strongly influences the biology of both nTregs and iTregs (3–5). IL-2 regulates FOXP3 expression in a signal transducer and activator of transcription 5 (STAT5)–dependent manner (6), and both IL-2 and STAT5 are required for the peripheral generation of iTregs (5,7) and maintenance of nTregs (8,9). The dependence of Tregs on IL-2 has been clearly demonstrated in knockout mice where deficiency of IL-2, IL-2 receptor-α (IL-2Rα), or IL-2Rβ leads to early death due to severe autoimmunity caused by a lack of FOXP3+ T-cells (10–12). In rare human cases, deficiency in IL-2Rα results in autoimmunity, lymphadenopathy, and persistent viral infection (13,14), whereas deficiency of STAT5b results in decreased frequency and function of Tregs (15). Together, these data emphasize the essential role of IL-2/IL-2R signaling—and specifically, STAT5 activation—in peripheral tolerance mediated by Tregs.
IL-2 is a T-cell growth factor and key cytokine involved in immune regulation that is produced in a transient manner primarily by activated effector T-cells (4). Binding of IL-2 to the high-affinity IL-2R results in a wide range of biological responses including survival, differentiation, and proliferation of multiple cell types including T-cells. The IL-2R consists of a heterotrimer composed of an α-chain (CD25), a β-chain (CD122) shared with the IL-15R, and the common γ-chain (CD132) shared with the IL-7R, IL-9R, IL-15R, and IL-21R. Engagement of the IL-2R results in a cascade of signaling events initiated by phosphorylation of the tyrosine kinases Janus kinase 1 (JAK1) and JAK3 followed by phosphorylation of tyrosine residues on the IL-2R β-chain that results in phosphorylation of STAT5 and Shc. These proximal activation events lead to downstream signaling cascades, resulting in activation of IL-2–dependent genes such as FOXP3 (16). Negative regulators of the IL-2 signaling cascades, including protein tyrosine phosphatases, control the intensity and kinetics of these responses (17,18).
In NOD mice, there is a decrease in the frequency and function of Tregs at the site of inflammation (19), as well as alterations in the IL-2/IL-2R pathway (20). Recent studies have linked defects in Tregs in NOD mice to reduced availability of IL-2. These include reports of the association of the Idd3 susceptibility locus to decreased IL-2 production, resulting in impaired Treg function and proliferation at sites of inflammation (21–23). Furthermore, this Treg defect in NOD mice can be rescued by treatment with exogenous IL-2 (22). In humans, the IL-2 gene itself and genes that participate in IL-2R signaling, including CD25 and PTPN2, have been implicated in the pathogenesis of type 1 diabetes (20,24–28). However, to date, no studies have demonstrated a functional link between IL-2/IL-2R signaling and Treg deficits in humans diagnosed with type 1 diabetes.
Maintenance of FOXP3 expression is essential for the function of Tregs in vivo and has been shown in both mice and humans to be dependent on common γ-chain cytokines, with IL-2 playing a dominant role (7,9). Here, we demonstrate that maintenance of FOXP3 expression in both nTregs and iTregs is diminished in CD4+CD25+ T-cells of type 1 diabetic subjects when cultured in the presence of IL-2. This was associated with diminished IL-2R signaling in response to IL-2, as measured by decreased phosphorylation of STAT5, but not with altered IL-2R expression. Decreased STAT5 phosphorylation correlated with an increase in protein tyrosine phosphatase N2 (PTPN2) expression in CD4+ T-cells of type 1 diabetic subjects.
RESEARCH DESIGN AND METHODS
Human subjects.
Samples for this study were obtained from subjects diagnosed with type 1 diabetes and control subjects with no personal or family history of autoimmunity who are participants in the Juvenile Diabetes Research Foundation International (JDRF) Center for Translational Research protocol approved by institutional review boards at both Benaroya Research Institute and Seattle Children's Hospital. Subjects provided written informed consent prior to participation in the study. A total of 66 type 1 diabetic subjects (mean age 32 ± 13 years, range 18–58) and 125 control subjects (mean age 35 ± 12, range 18–67) were used in these studies. The number of samples used for each assay is indicated in figure legends. All experiments were performed in a blinded manner without prior knowledge of disease state.
Antibodies and reagents.
BD Pharmingen (San Jose, CA) antibodies used include fluorescein isothiocyanate–conjugated CD25; AlexaFlour488 STAT5(pY694); phycoerythrin-conjugated CD25, CD122, CD132, and STAT5(pY694); peridinin-chlorophyll-protein complex–conjugated CD4; allophycocyanin-conjugated CD4, CD45RO, and CD25; and purified anti-CD3 (UCH11) and anti-CD28 (CD28.2). Intracellular AlexaFlour647-conjugated anti-FOXP3 (clone 259D) and matching isotype control were purchased from Biolegend (San Diego, CA). For immunoblots, goat polyclonal anti-PTPN2 antibody was purchased from R&D Systems (Minneapolis, MN); polyclonal rabbit anti-JAK1, polyclonal rabbit anti-JAK3, and monoclonal rabbit anti-STAT5 were purchased from Cell Signaling Technologies. Rabbit polyclonal anti–transcription factor (TF) IIB antibody was used as a loading control (Santa Cruz Biotechnology, Santa Cruz, CA) with horseradish peroxidase–coupled rabbit anti-goat IgG (Novus Biologicals, Littleton, CO) secondary antibody. IL-2 was purchased from Chiron (Emeryville, CA). IL-7 and IL-15 were purchased from BD Pharmingen. A small molecule inhibitor of PTPN2 (compound 8) was prepared as described (29).
Flow cytometric analysis for phosphorylated STAT5.
BD Biosciences Phosphoflow staining was performed as per the manufacturer's instructions. In brief, cells were activated with different concentrations of IL-2, IL-7, or IL-15 for 10 and/or 20 min, fixed with BD Biosciences Phosflow Buffer I, and permeabilized using BD Phosflow Buffer III prior to staining with anti-pSTAT5(Y694), CD4, and CD25. For some experiments, cells were costained with CD122 and CD45RO. Initial experiments were performed using freshly isolated peripheral blood mononuclear cells (PBMCs). Similar results were obtained with thawed PBMCs. All pSTAT5 data shown here are from previously frozen PBMCs. Data were acquired using a FACSCalibur (BD Biosciences, San Diego, CA) and analyzed using FlowJo (TreeStar, Ashland, OR) or Winlist software (Verity Software House, Topsham, ME). Because CD4+CD25+ T-cells are known to be sensitive to the freeze/thaw process and fix/perm staining protocols, samples where less than 1% of CD25+ T-cells were detected were not analyzed for pSTAT5. pSTAT5(Y694) mean fluorescence intensity (MFI) data were normalized between experiments by determining the MFI fold increase (geometric MFI of the positive population/geometric MFI of the negative control) as described previously (30).
Isolation and activation of cells.
Human peripheral blood was obtained from donors, and PBMCs were prepared by centrifugation over Ficoll-Hypaque gradients. For some experiments, previously processed and frozen PBMCs were used. CD4+ T-cells and accessory cells were purified as described previously (31). For some freshly isolated samples, CD25hi (top 2–5%) were further isolated from CD4+ T-cells by sorting using a FACSVantage (BD Biosciences).
For persistence assays, sorted CD4+CD25hi T-cells (nTregs) were seeded in a 96-well plate at a concentration of 1.5 × 106/ml in a total volume of 200 μl. Cells were cultured with media alone or cytokine without stimulation through the T-cell receptor. Cells were stained for CD25, CD4, and FOXP3 expression on different days after the sort. Surface expression of CD25, CD122, and CD132 was quantified using Quantum R-PE MESF beads (Bangs Laboratories) to calculate molecules of equivalent soluble fluorochrome (MESF).
For induction assays, CD4+CD25− T-cells were activated with 5 μg/ml soluble anti-CD3 bound to irradiated (5,000 rad) accessory cells at a concentration of 1.5 × 106/ml as described previously (31). No cytokine, IL-2 (200 IU/ml), or IL-7 (10 ng/ml) was added at the initiation of culture. FOXP3 content in iTregs was determined by flow cytometry.
Western blot analysis.
Expression of IL-2 pathway proteins was analyzed in peripheral CD4+ T-cells isolated from fresh PBMCs. Whole-cell lysates were separated by denaturing SDS-PAGE (20 μg protein per lane) using NuPage 10% or 4–12% Bi-Tris gels as indicated by the manufacturer (Invitrogen, Carlsbad, CA) and transferred to Immobilon P membranes (Millipore, Billerica, MA). Immunoblots were probed with primary antibodies followed by horseradish peroxidase–coupled secondary antibody. Staining was detected by chemiluminescence (Perkin-Elmer Life Sciences). Protein expression was quantified by densitometry of films using ImageQuant Software Version 5.1 (GE Healthcare, Piscataway, NJ).
Statistics.
For analysis of experiments comparing a single variable, statistical significance was determined using two-tailed independent or paired Student t tests as noted in the figure legends. For analysis of multiple variables, an ANCOVA was performed. All analyses were performed using GraphPad Prism V4.03 and all values with P < 0.05 were considered significantly different.
RESULTS
Maintenance of FOXP3 expression in CD4+CD25+ T-cells is diminished in type 1 diabetic subjects.
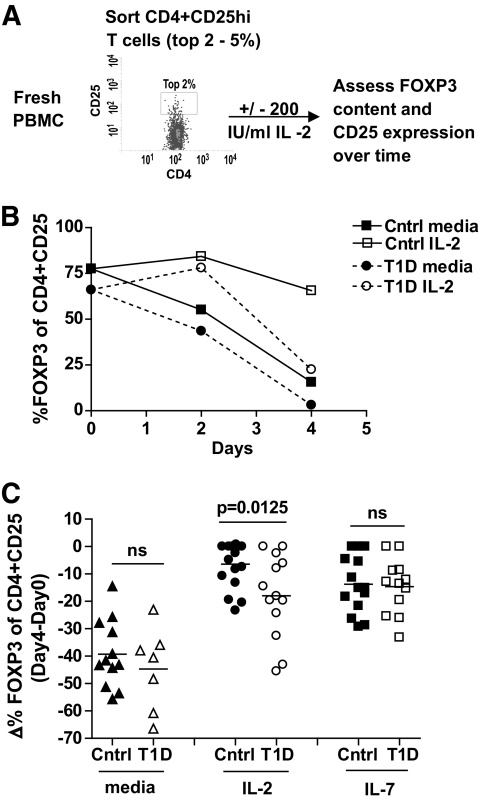
We compared persistence of FOXP3 expression in nTregs of type 1 diabetic subjects and control subjects to determine whether the maintenance of FOXP3 expression is impaired in type 1 diabetic subjects. Persistence of FOXP3 was determined by culturing nTregs (FACS-sorted CD4+CD25+ T-cells) in the presence of media or IL-2 and then measuring FOXP3 expression over time by flow cytometry, as diagramed in Figure 1A. Consistent with prior studies (7), FOXP3 expression in CD4+CD25+ T-cells of control subjects was maintained in the presence of IL-2, but not media alone. In contrast, FOXP3 persistence was significantly decreased in nTregs of type 1 diabetic subjects cultured in the presence of IL-2 as illustrated for one subject in Figure 1B and a cohort of 17 control (mean −6.42 ± 8.2 SD, Δ FOXP3 [day 4 − day 0]) and 13 type 1 diabetic (mean −17.98 ± 15.3 SD, Δ FOXP3 [day 4 − day 0]) subjects in Figure 1C. This effect was specific to IL-2 as there was no difference between the study populations when nTregs were cultured in the presence of media or IL-7.
FIG. 1.
Maintenance of FOXP3 expression in CD4+CD25+ nTregs of type 1 diabetic subjects is impaired in the presence of IL-2. As diagramed in (A), fresh CD4+CD25+ T-cells were sorted from PBMCs isolated from control and type 1 diabetic subjects, placed in culture with media alone or 200 IU/ml IL-2, and FOXP3 and CD25 expression was assessed over time by flow cytometry. B: One control and one type 1 diabetic sample are shown. C: CD4+CD25+ T-cells from multiple control (n = 17) and type 1 diabetic (n = 13) subjects were assayed as in A in the presence of media alone, 200 IU/ml IL-2, or 10 ng/ml IL-7. Bars show means and symbols represent individual subjects. Analysis was performed by gating on live CD4+CD25+ T-cells. Statistical significance was determined using an independent Student t test. Cohorts of control and type 1 diabetic subjects had mean ages of 38 (range 18–61) and 34 (range 21–46) years, respectively.
The decrease in FOXP3 persistence in type 1 diabetic subjects did not appear to be due to differences in survival of nTregs as there was no difference in viable cell counts, absolute number of FOXP3+ cells, or CD95 expression between control and type 1 diabetic subjects when cells were cultured in the presence of IL-2 (supplementary Data 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0694/DC1). Moreover, FOXP3 expression in nTregs of control subjects, but not type 1 diabetic subjects, cultured in media could be rescued by addition of IL-2 midculture (supplementary Data 2). It is possible that the composition of nTregs in control and type 1 diabetic subjects differs, thereby contributing to altered persistence of FOXP3 in the presence of IL-2. However, we found no difference in the frequency of resting CD45RA+FOXP3+ or activated CD45RA−FOXP3hi cells, two recently described subsets of nTregs (32,33), in the cohort of control and type 1 diabetic subjects assessed in Figure 1 (supplementary Data 3). In fact, demethylation of the FOXP3 gene, an additional marker of Tregs (34,35), was similar in nTregs of control and type 1 diabetic subjects (supplementary Data 4). Moreover, persistence of FOXP3 was comparable between control and type 1 diabetic subjects in the presence of IL-7. Together, these data indicate that the decrease in FOXP3 persistence was not strongly influenced by differences in cell differentiation among the CD4+CD25hi sorted populations.
nTregs of type 1 diabetic subjects display impaired IL-2R signaling.
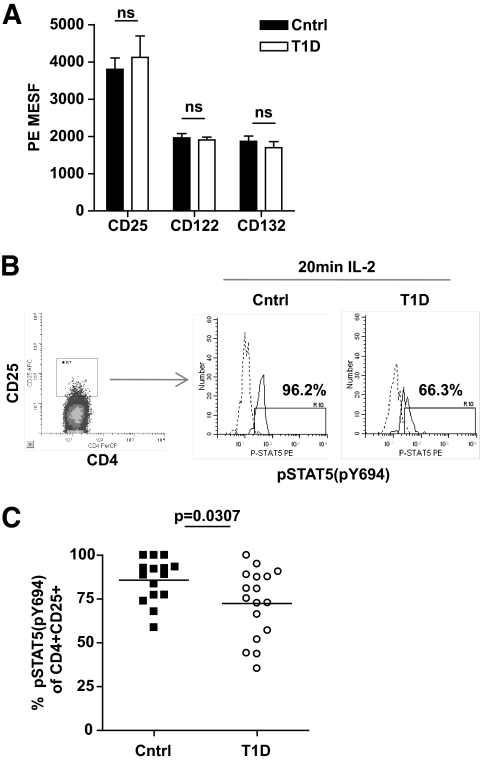
A decreased responsiveness to IL-2 in nTregs may be due to altered expression of components of the IL-2R or altered function of the IL-2R signaling cascade. Flow cytometric analysis was performed to examine the level of CD25, CD122, and CD132 expression on CD4+CD25+ T-cells. In these studies, expression of IL-2R components was comparable between control and type 1 diabetic subjects (Fig. 2A). Additionally, no differences in the level of CD25 expression in the study populations were observed after 4 days of culture (data not shown).
FIG. 2.
nTregs of type 1 diabetic subjects display impaired IL-2R signaling. A: Level of expression of CD25, CD122, and CD132 in the CD4+CD25+ population of control (n = 17) and type 1 diabetic (n = 11) subjects from Fig. 1C was determined using Quantum R-PE MESF beads. B: Thawed PBMCs from a representative control and type 1 diabetic subject were stimulated with 100 IU/ml IL-2 for 20 min prior to fixation and staining with CD4, CD25, and pSTAT5(Y694). Dashed lines are treatment with media alone and solid lines are treatment with IL-2. C: Multiple control (n = 15) and type 1 diabetic (n = 17) subjects were stimulated as in B. Bars show means and symbols represent individual subjects. Analysis of pSTAT5 was performed by gating on live CD4+CD25+ T-cells. Statistical significance was determined using an independent Student t test.
To address function of the IL-2R signaling pathway, we measured phosphorylation of STAT5 (pSTAT5) in the CD4+CD25+ population by flow cytometry after exposure to IL-2 as shown in Figure 2B for a representative control and type 1 diabetic sample. When analyzing a cohort of subjects, we found that the majority (85.83 ± 12.4% SD) of CD4+CD25+ T-cells isolated from control subjects responded to IL-2 stimulation as measured by pSTAT5. In comparison, significantly fewer (72.44 ± 15.2% SD) CD4+CD25+ T-cells of type 1 diabetic subjects responded to IL-2 (P = 0.0307) (Fig. 2C). Similar differences in pSTAT5 between Tregs of control and type 1 diabetic subjects were observed at different time points and with different doses of IL-2 (supplementary Data 5). Moreover, the level of total STAT5 protein expression did not significantly contribute to the frequency of pSTAT5 as the level of total STAT5 protein did not differ between control and type 1 diabetic subjects and the relative expression of STAT5 did not correlate with pSTAT5 frequencies for either population (supplementary Data 6). Together, these data suggest that decreased IL-2 responsiveness in nTregs of type 1 diabetes is linked to defects in IL-2R signaling.
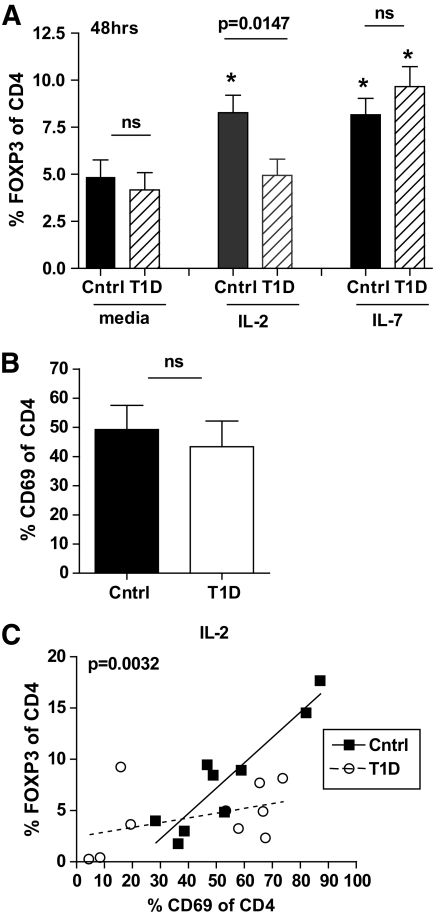
To determine whether the effects of IL-2 on FOXP3 expression altered other IL-2–dependent Treg populations, we compared induction of FOXP3 expression in control and type 1 diabetic subjects by stimulating CD4+CD25− T-cells with soluble anti-CD3 and irradiated antigen-presenting cells in the presence of media alone, IL-2, or IL-7 and measured FOXP3 expression after 48 h. Expression of FOXP3 upon activation in the presence of IL-2 was significantly lower in type 1 diabetic than control CD4+ T-cells, whereas no difference was seen between the two populations when CD4+ T-cells were activated in the presence of media alone or IL-7 (Fig. 3A). To rule out the possibility that the decrease in FOXP3 expression in CD4+ T-cells of type 1 diabetic subjects stimulated in the presence of IL-2 was due to differences in total activation between control and type 1 diabetic populations, we analyzed CD69 expression levels. We found that CD69 expression levels were comparable between the control and type 1 diabetic study populations, whereas FOXP3 expression in type 1 diabetic subjects was diminished compared with control subjects in the presence of IL-2 when analyzed with respect to CD69 (Fig. 3B and C). Comparable results were obtained using CD25 as an activation marker (supplementary Data 7). Thus, modulation of FOXP3 expression by IL-2 is diminished in both nTregs and iTregs of type 1 diabetic subjects.
FIG. 3.
FOXP3 expression in iTregs of type 1 diabetic subjects is impaired in the presence of IL-2 but not IL-7. A: CD4+CD25− T-cells were isolated from previously frozen control (n = 15) and type 1 diabetic (n = 13) subjects and activated with 5 μg/ml anti-CD3 antibody and irradiated accessory cells in the presence of media alone, 100 IU/ml IL-2, or 10 ng/ml IL-7. FOXP3 expression 48 h after activation was determined by flow cytometry by gating on live, total CD4+ T-cells. Asterisk denotes significant difference from media alone using a paired Student t test. Error bars represent means ± SEM. B: CD69 expression 48 h after activation was assessed by flow cytometry for a subset of control (n = 9) and type 1 diabetic (n = 10) subjects shown in A. C: Linear regression was performed for samples in B activated in the presence of 100 IU/ml IL-2 to determine the association between CD69 and FOXP3 expression for control (R2 = 0.8496, P = 0.0004) and type 1 diabetic (R2 = 0.1702, P = ns) subjects. The difference between the slopes of the lines was measured using an ANCOVA with the P value noted in the graph.
Diminished IL-2 responsiveness in CD4+ T-cells of type 1 diabetic subjects is a stable phenotype associated with impaired IL-2R signaling.
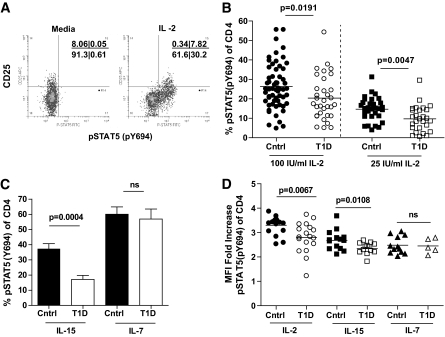
To better define the mechanism of decreased IL-2 responsiveness in T-cells of type 1 diabetic subjects, we measured STAT5 activation in total CD4+ T-cells, as diagrammed in Figure 4A after exposure to IL-2. Experimental conditions included exposure to media alone, IL-2, IL-15, or IL-7, all of which share the common γ-chain receptor component. IL-2 and IL-15 also share the IL-2R β-chain. Consistent with other reports (30), the majority of CD25+ T-cells phosphorylate STAT5 in response to IL-2, whereas a subset of CD4+CD25− T-cells also respond to IL-2. When analyzing a cohort of control and type 1 diabetic subjects, we found a significant decrease in the frequency of total CD4+ T-cells that phosphorylated STAT5 in response to higher (100 IU/ml) and lower (25 IU/ml) doses of IL-2 (Fig. 4B). A similar decrease in responsiveness of CD4+ T-cells from type 1 diabetic subjects was observed upon IL-15 exposure, but not upon exposure to IL-7, suggesting a defect in signaling associated with the IL-2R β-chain, which is shared between IL-2 and IL-15, but not IL-7 (Fig. 4C). Similarly, the levels of pSTAT5 in response to IL-2 and IL-15 as measured by MFI were significantly decreased in type 1 diabetic compared with control subjects, whereas responses to IL-7 were similar in these study populations (Fig. 4D). The decrease in pSTAT5 observed here was not due to obvious differences in the composition of the T-cell compartments of control and type 1 diabetic subjects as no difference was observed between the frequency of control and type 1 diabetic CD45RO+ and CD122+ CD4+ T-cells in this study (data not shown). Thus, CD4+ T-cells of type 1 diabetic subjects display diminished responsiveness to IL-2 that may be associated with signaling through the β-chain of the IL-2R.
FIG. 4.
CD4+ T-cells of type 1 diabetic subjects display diminished responsiveness to IL-2. Thawed PBMCs from control subjects were stimulated with 100 IU/ml IL-2 for 10 min prior to fixation and staining for CD4, CD25, and pSTAT5(Y694). Analysis was performed by gating on total live CD4+ T-cells and comparing response to media alone versus cytokine stimulation. A: Staining for one representative sample is shown. B: The frequency of CD4+ T-cells that were pSTAT5(Y694)+ in response to IL-2 was determined for control (n = 59) and type 1 diabetic (n = 33) subjects. Bars represent means and symbols represent individual subjects. C: Control (n = 12) and type 1 diabetic (n = 13) subjects were assayed for pSTAT5(Y694) in response to stimulation for 10 min with 200 pg/ml IL-15 or 40 pg/ml IL-7. Bars represent means ± SEM. D: MFI fold increase of pSTAT5(Y694) in STAT5+ CD4+ T-cells of thawed PBMCs from control (n = 12) and type 1 diabetic (n = 14) subjects was determined by comparing pSTAT5(Y694) MFI after stimulation with IL-2, IL-7, and IL-15, or media alone. Bars show means and symbols represent individual subjects. All P values were determined using an independent Student t test.
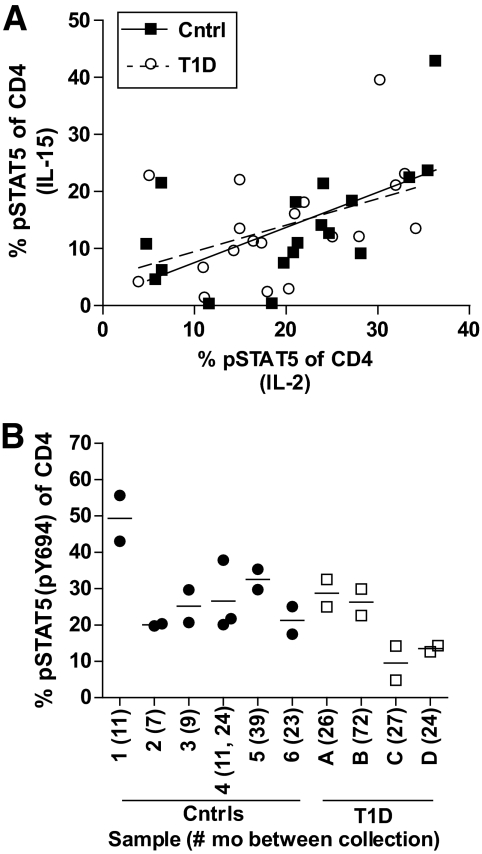
To measure whether impaired IL-2R signaling is a property of CD4+ T-cells of type 1 diabetic subjects, we compared pSTAT5 responses of CD4+ T-cells from the same individual stimulated with either IL-2 or IL-15. Overall, responsiveness to IL-2 correlated with responsiveness to IL-15 (Fig. 5A). This was a stable phenotype, as CD4+ T-cells isolated from the same individual at different times responded similarly to IL-2 stimulation (Fig. 5B). Thus, CD4+ T-cells of individual type 1 diabetic subjects consistently display impaired responsiveness to both IL-2 and IL-15, suggesting an intrinsic property of type 1 diabetic CD4+ T-cells linked to the β-chain of the IL-2R.
FIG. 5.
Diminished IL-2 responsiveness is a stable phenotype and intrinsic property of CD4+ T-cells of type 1 diabetes. A: Thawed PBMCs from control (n = 18) and type 1 diabetic (n = 20) subjects were stimulated with 100 IU/ml IL-2 or 200 pg/ml IL-15 for 10 min prior to fixation and staining for CD4, CD25, and pSTAT5(Y694). Linear regression was performed to determine the relationship between pSTAT5 responses in the same cells after stimulation with either IL-2 or IL-15. Trend lines represent linear regression of control (R2 = 0.3713, P = 0.0073) and type 1 diabetes (R2 = 0.2003, P = 0.0478) data. B: Cells isolated from the same individual but at different dates were assayed as described (in A) for response to IL-2. Sample collection dates varied from 5 months to 6 years. The number of months between sample collection did not correlate with SD or coefficient of variation for the control subjects, type 1 diabetic subjects, or all samples combined as analyzed by linear regression.
Increased expression of PTPN2 in type 1 diabetic subjects correlates with decreased STAT5 activation.
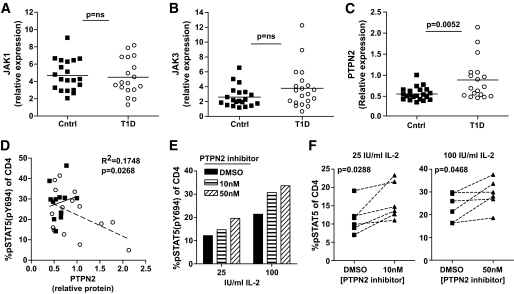
Decreased pSTAT5 in type 1 diabetic subjects may be due to alterations in the level of expression of molecules that participate in the IL-2R signaling pathway. To address this question, we measured protein expression levels of STAT5, JAK1, JAK3, and the negative regulator, PTPN2, in CD4+ T-cells of control and type 1 diabetic subjects (Fig. 6 and supplementary Data 6 and 8). We then compared these expression levels to pSTAT5 responses after exposure to IL-2. No differences were found in STAT5, JAK3, or JAK1 protein expression comparing control and type 1 diabetic subjects. By contrast, PTPN2 expression was uniform in control subjects but was increased overall in type 1 diabetic subjects, with some type 1 diabetic subjects showing a fourfold greater level of expression compared with control subjects (Fig. 6C). This increased expression of PTPN2 correlated with decreased pSTAT5 after IL-2 exposure (Fig. 6D), consistent with a down-modulatory role of PTPN2 (18). In type 1 diabetic subjects with high PTPN2 expression, decreased IL-2 response could be rescued by pretreatment with a PTPN2 inhibitor (29) (Fig. 6E and F). In fact, where PTPN2, pSTAT5, and FOXP3 persistence was measured in the same individual, we found that a type 1 diabetic subject with elevated PTPN2 expression also showed reduced pSTAT5 (4.84% of CD4, 100 IU/ml for 10 min) and diminished persistence of FOXP3 expression (−18.92, Δ FOXP3 [day 4 − day 0]). Together, these data suggest that increased expression of PTPN2 in CD4+ T-cells of some type 1 diabetic subjects may contribute to reduced levels of pSTAT5 in response to IL-2.
FIG. 6.
Altered expression of molecules in the IL-2R signaling cascade in CD4+ T-cells of type 1 diabetic subjects. CD4+ T-cells were isolated from fresh PBMCs of control and type 1 diabetic subjects, and whole-cell protein lysates were analyzed by Western blot. Immunoblots were probed with JAK1-, JAK3-, and PTPN2-specific antibodies and an anti-TFIIB antibody as a loading control. Protein expression was determined by densitometry, normalizing each sample to TFIIB and expressing total protein levels relative to a Jurkat control present on each blot. Total JAK1 (A) and JAK3 (B) protein expression was compared between control (n = 20) and type 1 diabetic (n = 18 and 20 for JAK1 and JAK3, respectively) subjects. C: PTPN2 protein expression was compared between control (n = 21) and type 1 diabetic (n = 18) subjects. Significance was determined using an independent Student t test. D: Thawed PBMCs from these same samples were assayed for pSTAT5 upon exposure with 100 IU/ml IL-2 for 10 min as in Fig. 4. Using linear regression, protein expression was compared with pSTAT5 for control (n = 13) and type 1 diabetic (n = 15) subjects. Correlation between PTPN2 protein expression in the total population (control and type 1 diabetic subjects combined) and pSTAT5 is noted in the graph. Solid trend line (R2 = 0.02, P = 0.6) and squares denote control subjects, and dashed trend line (R2 = 0.234, P = 0.067) and open circles denote type 1 diabetic subjects. E: Thawed PBMCs from the type 1 diabetic subject with the highest PTPN2 expression or (F) type 1 diabetic subject with PTPN2 expression above the mean 0.8 (n = 6) were incubated with a PTPN2 inhibitor (compound 8 in [29]) for 30 min prior to stimulation with IL-2 for 10 min as in Fig. 4.
DISCUSSION
Although the precise mechanisms by which IL-2 influences CD4+CD25+FOXP3+ Treg control of type 1 diabetes are currently being dissected in mouse models, many basic questions remain to be answered regarding FOXP3+ Tregs in humans diagnosed with type 1 diabetes. Previous studies in type 1 diabetic subjects have focused on the number and function of Tregs (36–41). Given the established role of IL-2 and IL-2R signaling in Treg maintenance (42) and the association of genes within the IL-2/IL-2R pathway with type 1 diabetes (20,24–28), we investigated whether IL-2–dependent persistence of Tregs was impaired in type 1 diabetic subjects. We found that maintenance of FOXP3 expression in the presence of IL-2 was decreased in both nTregs and iTregs of type 1 diabetic subjects. This correlated with alterations in the IL-2R β-chain signaling pathway, as opposed to expression levels of components of the IL-2R, suggesting that this diminished responsiveness to IL-2 may contribute to loss of tolerance in type 1 diabetic subjects.
The decrease in FOXP3 persistence observed here in nTregs and iTregs of type 1 diabetic subjects suggests that resistance to IL-2 may result in a decrease in the persistence of Tregs in vivo. However, the frequency of FOXP3+ T-cells in the peripheral blood does not differ between control and type 1 diabetic subjects (36). IL-2 is produced primarily by effector T-cells upon activation at the site of inflammation and, in this manner, IL-2 production controls local Treg frequency and function (22,43,44), whereas IL-7 is produced primarily by stromal cells and can also support homeostasis of lymphocyte populations including FOXP3+ Tregs (45). Thus, persistence of Tregs in type 1 diabetic subjects may be diminished at the sites of inflammation due to decreased responsiveness to IL-2 produced during inflammation, although peripheral homeostasis of the FOXP3+ Treg population may not be affected. This is consistent with the observation that the frequency of Tregs in NOD mice is impaired in the β-islets and pancreatic lymph nodes but not in peripheral sites (22). Alternatively, currently, measures of Treg frequency detected ex vivo may not be sensitive enough to measure alterations in the biology of Tregs, however, when challenged in vitro, deficiencies in the maintenance of Tregs of type 1 diabetic subjects may become more obvious. Here we show a deficit in Treg persistence in type 1 diabetic subjects, however, immune regulation by Tregs is also diminished in type 1 diabetic subjects through an additional mechanism. Effector T-cells of type 1 diabetic subjects are more resistant to regulation than effector T-cells of control subjects (40). Taken together, we suggest a model in which IL-2–dependent FOXP3 persistence may be decreased at the site of inflammation due to impaired responsiveness to IL-2, and effector T-cells may be more resistant to regulation by these less stable Tregs, together resulting in unregulated organ-specific inflammation.
Reduced IL-2 responsiveness is likely a result of impaired activation of STAT5 through the IL-2R β-chain as CD4+ T-cells of type 1 diabetic subjects displayed diminished pSTAT5 in response to both IL-2 and IL-15, cytokines with receptors containing the IL-2R β-chain, but not IL-7. Several mouse models shed light on the role of IL-2R β-chain on FOXP3+ Treg biology (reviewed in [4,20]) and, when taken into consideration with data shown here, reveal possible mechanisms of immune dysregulation in type 1 diabetic subjects. Mice lacking both IL-2 and IL-15 have reduced numbers of Tregs that can be rescued by forced expression of constitutively active STAT5 (3,8). When the active form of STAT5 was constitutively expressed (46,47) or specific tyrosine residues within the cytoplasmic domain of the IL-2R β-chain were mutated (48), a clear link was shown between IL-2 responsiveness and Treg development and homeostasis. Here, we find that FOXP3 expression in the presence of IL-2 is reduced compared with control subjects but can be rescued in the presence of IL-7. This is consistent with a requirement for STAT5 activation through the IL-2R β-chain. Together, these data suggest that impaired persistence of FOXP3 in Tregs of type 1 diabetes may be due to defects in IL-2R β-chain signaling and offer novel options for targeted therapy.
Decreased pSTAT5 in response to IL-2 was observed not only in nTregs but also in total CD4+ T-cells, suggesting an intrinsic property of CD4+ T-cells of type 1 diabetic subjects. In fact, a similar decrease in pSTAT5 upon exposure to IL-2 was observed with CD8+ T-cells (data not shown). Whether this intrinsic property of CD4+ T-cells of type 1 diabetes is due to genetic and/or environmental factors is not clear. Multiple genes in the IL-2/IL-2R pathway are associated with type 1 diabetes (20,24–28), but none to date has been found to associate with impaired Treg biology in type 1 diabetes. More studies with other autoimmune diseases, type 2 diabetic subjects, and first-degree relatives are required to better determine whether this phenotype is specific to type 1 diabetes. More broadly, this defect may have additional implications for other biological processes that require IL-2.
Increased expression of PTPN2 in CD4+ T-cells of some type 1 diabetic subjects suggests a molecular mechanism for the reduced responsiveness to IL-2 in type 1 diabetic subjects. Consistent with the down-modulatory role of PTPN2 in signal transduction (17), others have shown a role for PTPN2 in decreasing STAT1 phosphorylation in response to IFNγ stimulation of human β-islet cells (49). Here, we show for the first time that in some type 1 diabetic subjects PTPN2 protein is significantly increased in CD4+ T-cells, a cell subset of the immune system known to express high amounts of PTPN2 (50). We further demonstrate a mechanistic link between increased expression of PTPN2 in type 1 diabetic subjects and decreased pSTAT5 by rescuing IL-2 responsiveness with a PTPN2-specific inhibitor. However, some type 1 diabetic subjects have diminished pSTAT5 in response to IL-2 but the level of PTPN2 expression is comparable with control subjects. This suggests that in some type 1 diabetic subjects increased expression of PTPN2 may contribute to altered IL-2 responsiveness, whereas in others, different molecular mechanisms may be involved, both resulting in a decreased pSTAT5 phenotype. Two independent variants within introns of the PTPN2 gene are associated with type 1 diabetes (27) and may impact PTPN2 expression. This is the focus of current research in our laboratory. Interestingly, when we analyze control and type 1 diabetic subjects that do not carry risk-alleles of PTPN2, we still find a significant difference in PTPN2 expression and pSTAT5 (S.A.L., K.C., and J.B., unpublished data).
Cytokine signaling signatures are associated with autoimmune diseases. Here, we demonstrate that two phenotypes of CD4+ T-cells of type 1 diabetic subjects involve molecules in the IL-2R signaling pathway. Whether altered PTPN2 and pSTAT5 phenotypes indicate a comprehensive defect in the IL-2R signaling pathway of type 1 diabetes is not yet known. However, in conjunction with further studies, these data may suggest that CD4+ T-cells of type 1 diabetes are marked by altered IL-2R signaling. Gaining a better understanding of the mechanisms leading to these phenotypes may guide development of diagnostic assays and targeted therapies.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the JDRF (Center for Translational Research at Benaroya Research Institute [BRI]; 33-2008-398), the National Institutes of Health (NIH; R01-DK-072457, CA-69202, CA-126937), and the National Center for Research Resources (NCRR; M01-RR-00037 and 1UL1RR025014).
No potential conflicts of interest relevant to this article were reported.
We thank K. Arugamanathan for cell sorting. We acknowledge the staff of the JDRF Center for Translational Research and the Benaroya Research Institute Translational Research program for subject recruitment and sample management.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bluestone JA, Abbas AK: Natural versus adaptive regulatory T cells. Nat Rev Immunol 2003;3:253–257 [DOI] [PubMed] [Google Scholar]
- 2.Ziegler SF: FOXP3: not just for regulatory T cells anymore. Eur J Immunol 2007;37:21–23 [DOI] [PubMed] [Google Scholar]
- 3.Burchill MA, Yang J, Vang KB, Farrar MA: Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol Lett 2007;114:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malek TR: The biology of interleukin-2. Annu Rev Immunol 2008;26:453–479 [DOI] [PubMed] [Google Scholar]
- 5.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA: IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 2007;178:2018–2027 [DOI] [PubMed] [Google Scholar]
- 6.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, Frank DA, Ritz J: IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 2006;108:1571–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passerini L, Allan SE, Battaglia M, Di Nunzio S, Alstad AN, Levings MK, Roncarolo MG, Bacchetta R: STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25- effector T cells. Int Immunol 2008;20:421–431 [DOI] [PubMed] [Google Scholar]
- 8.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA: IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol 2007;178:280–290 [DOI] [PubMed] [Google Scholar]
- 9.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY: A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 2005;6:1142–1151 [DOI] [PubMed] [Google Scholar]
- 10.Sadlack B, Löhler J, Schorle H, Klebb G, Haber H, Sickel E, Noelle RJ, Horak I: Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol 1995;25:3053–3059 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Küdig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H: Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science 1995;268:1472–1476 [DOI] [PubMed] [Google Scholar]
- 12.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW: Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity 1995;3:521–530 [DOI] [PubMed] [Google Scholar]
- 13.Aoki CA, Roifman CM, Lian ZX, Bowlus CL, Norman GL, Shoenfeld Y, Mackay IR, Gershwin ME: IL-2 receptor alpha deficiency and features of primary biliary cirrhosis. J Autoimmun 2006;27:50–53 [DOI] [PubMed] [Google Scholar]
- 14.Strieder TG, Drexhage HA, Lam-Tse WK, Prummel MF, Tijssen JG, Wiersinga WM: A reduced IL2R (CD25) expression level in first and second degree female relatives of autoimmune thyroid disease patients: a sign of a poor capability to preserve tolerance? Autoimmunity 2006;39:93–98 [DOI] [PubMed] [Google Scholar]
- 15.Cohen AC, Nadeau KC, Tu W, Hwa V, Dionis K, Bezrodnik L, Teper A, Gaillard M, Heinrich J, Krensky AM, Rosenfeld RG, Lewis DB: Cutting edge: decreased accumulation and regulatory function of CD4+ CD25(high) T cells in human STAT5b deficiency. J Immunol 2006;177:2770–2774 [DOI] [PubMed] [Google Scholar]
- 16.Kovanen PE, Leonard WJ: Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev 2004;202:67–83 [DOI] [PubMed] [Google Scholar]
- 17.Doody KM, Bourdeau A, Tremblay ML: T-cell protein tyrosine phosphatase is a key regulator in immune cell signaling: lessons from the knockout mouse model and implications in human disease. Immunol Rev 2009;228:325–341 [DOI] [PubMed] [Google Scholar]
- 18.Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ: The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr Biol 2002;12:446–453 [DOI] [PubMed] [Google Scholar]
- 19.Aoki CA, Borchers AT, Ridgway WM, Keen CL, Ansari AA, Gershwin ME: NOD mice and autoimmunity. Autoimmun Rev 2005;4:373–379 [DOI] [PubMed] [Google Scholar]
- 20.Dendrou CA, Wicker LS: The IL-2/CD25 pathway determines susceptibility to T1D in humans and NOD mice. J Clin Immunol 2008;6:685–696 [DOI] [PubMed] [Google Scholar]
- 21.Sgouroudis E, Albanese A, Piccirillo CA: Impact of protective IL-2 allelic variants on CD4+ Foxp3+ regulatory T cell function in situ and resistance to autoimmune diabetes in NOD mice. J Immunol 2008;181:6283–6292 [DOI] [PubMed] [Google Scholar]
- 22.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA: Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 2008;28:687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R, Chen SL, Rosa R, Cumiskey AM, Serreze DV, Gregory S, Rogers J, Lyons PA, Healy B, Smink LJ, Todd JA, Peterson LB, Wicker LS, Santamaria P: Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet 2007;39:329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper JD, Smyth DJ, Smiles AM, Plagnol V, Walker NM, Allen JE, Downes K, Barrett JC, Healy BC, Mychaleckyj JC, Warram JH, Todd JA: Meta-analysis of genome-wide association study data identifies additional type 1 diabetes risk loci. Nat Genet 2008;40:1399–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakonarson H, Qu HQ, Bradfield JP, Marchand L, Kim CE, Glessner JT, Grabs R, Casalunovo T, Taback SP, Frackelton EC, Eckert AW, Annaiah K, Lawson ML, Otieno FG, Santa E, Shaner JL, Smith RM, Onyiah CC, Skraban R, Chiavacci RM, Robinson LJ, Stanley CA, Kirsch SE, Devoto M, Monos DS, Grant SF, Polychronakos C: A novel susceptibility locus for type 1 diabetes on Chr12q13 identified by a genome-wide association study. Diabetes 2008;57:1143–1146 [DOI] [PubMed] [Google Scholar]
- 26.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F, Lowe CE, Szeszko JS, Hafler JP, Zeitels L, Yang JH, Vella A, Nutland S, Stevens HE, Schuilenburg H, Coleman G, Maisuria M, Meadows W, Smink LJ, Healy B, Burren OS, Lam AA, Ovington NR, Allen J, Adlem E, Leung HT, Wallace C, Howson JM, Guja C, Ionescu-Tîrgovişte C, Genetics of Type 1 Diabetes in Finland. Simmonds MJ, Heward JM, Gough SC, Wellcome Trust Case Control Consortium. Dunger DB, Wicker LS, Clayton DG: Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 2007;39:857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007;447:661–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe CE, Cooper JD, Brusko T, Walker NM, Smyth DJ, Bailey R, Bourget K, Plagnol V, Field S, Atkinson M, Clayton DG, Wicker LS, Todd JA: Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet 2007;9:1074–1082 [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, Chen L, Luo Y, Gunawan A, Lawrence DS, Zhang ZY: Acquisition of a potent and selective TC-PTP inhibitor via a stepwise fluorophore-tagged combinatorial synthesis and screening strategy. J Am Chem Soc 2009;131:13072–13079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krutzik PO, Hale MB, Nolan GP: Characterization of the murine immunological signaling network with phosphospecific flow cytometry. J Immunol 2005;175:2366–2373 [DOI] [PubMed] [Google Scholar]
- 31.Long SA, Buckner JH: Combination of rapamycin and IL-2 increases de novo induction of human CD4(+)CD25(+)FOXP3(+) T cells. J Autoimmun 2008;30:293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, Edinger M: Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood 2006;108:4260–4267 [DOI] [PubMed] [Google Scholar]
- 33.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S: Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009;30:899–911 [DOI] [PubMed] [Google Scholar]
- 34.Baron U, Floess S, Wieczorek G, Baumann K, Grützkau A, Dong J, Thiel A, Boeld TJ, Hoffmann P, Edinger M, Türbachova I, Hamann A, Olek S, Huehn J: DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol 2007;37:2378–2389 [DOI] [PubMed] [Google Scholar]
- 35.Janson PC, Winerdal ME, Marits P, Thorn M, Ohlsson R, Winqvist O: FOXP3 promoter demethylation reveals the committed Treg population in humans. PLoS ONE 2008;3:e1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brusko T, Wasserfall C, McGrail K, Schatz R, Viener HL, Schatz D, Haller M, Rockell J, Gottlieb P, Clare-Salzler M, Atkinson M: No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes 2007;56:604–612 [DOI] [PubMed] [Google Scholar]
- 37.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA: Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes 2005;54:1407–1414 [DOI] [PubMed] [Google Scholar]
- 38.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI: Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes 2005;54:92–99 [DOI] [PubMed] [Google Scholar]
- 39.Long SA, Walker MR, Rieck M, James E, Kwok WW, Sanda S, Pihoker C, Greenbaum C, Nepom GT, Buckner JH: Functional islet-specific Treg can be generated from CD4(+)CD25(-) T cells of healthy and type 1 diabetic subjects. Eur J Immunol 2009;39:612–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH: The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol 2008;181:7350–7355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tritt M, Sgouroudis E, d'Hennezel E, Albanese A, Piccirillo CA: Functional waning of naturally-occurring CD4+ regulatory T cells contributes to the onset of autoimmune diabetes. Diabetes 2007;1:113–123 [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi S, Powrie F: Emerging challenges in regulatory T cell function and biology. Science 2007;317:627–629 [DOI] [PubMed] [Google Scholar]
- 43.Yu A, Malek TR: Selective availability of IL-2 is a major determinant controlling the production of CD4+CD25+Foxp3+ T regulatory cells. J Immunol 2006;177:5115–5121 [DOI] [PubMed] [Google Scholar]
- 44.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK: Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med 2005;202:1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR: A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol 2008;181:225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA: Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 2008;28:112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor DK, Walsh PT, LaRosa DF, Zhang J, Burchill MA, Farrar MA, Turka LA: Constitutive activation of STAT5 supersedes the requirement for cytokine and TCR engagement of CD4+ T cells in steady-state homeostasis. J Immunol 2006;177:2216–2223 [DOI] [PubMed] [Google Scholar]
- 48.Yu A, Zhu L, Altman NH, Malek TR: A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 2009;30:204–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore F, Colli ML, Cnop M, Igoillo EM, Cardozo AK, Cunha DA, Bugliani M, Marchetti P, Eizirik DL: PTPN2, a candidate gene for type 1 diabetes, modulates interferon-gamma-induced pancreatic beta-cell apoptosis. Diabetes 2009;58:1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cool DE, Tonks NK, Charbonneau H, Walsh KA, Fischer EH, Krebs EG: cDNA isolated from a human T-cell library encodes a member of the protein-tyrosine-phosphatase family. Proc Natl Acad Sci U S A 1989;86:5257–5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.