Abstract
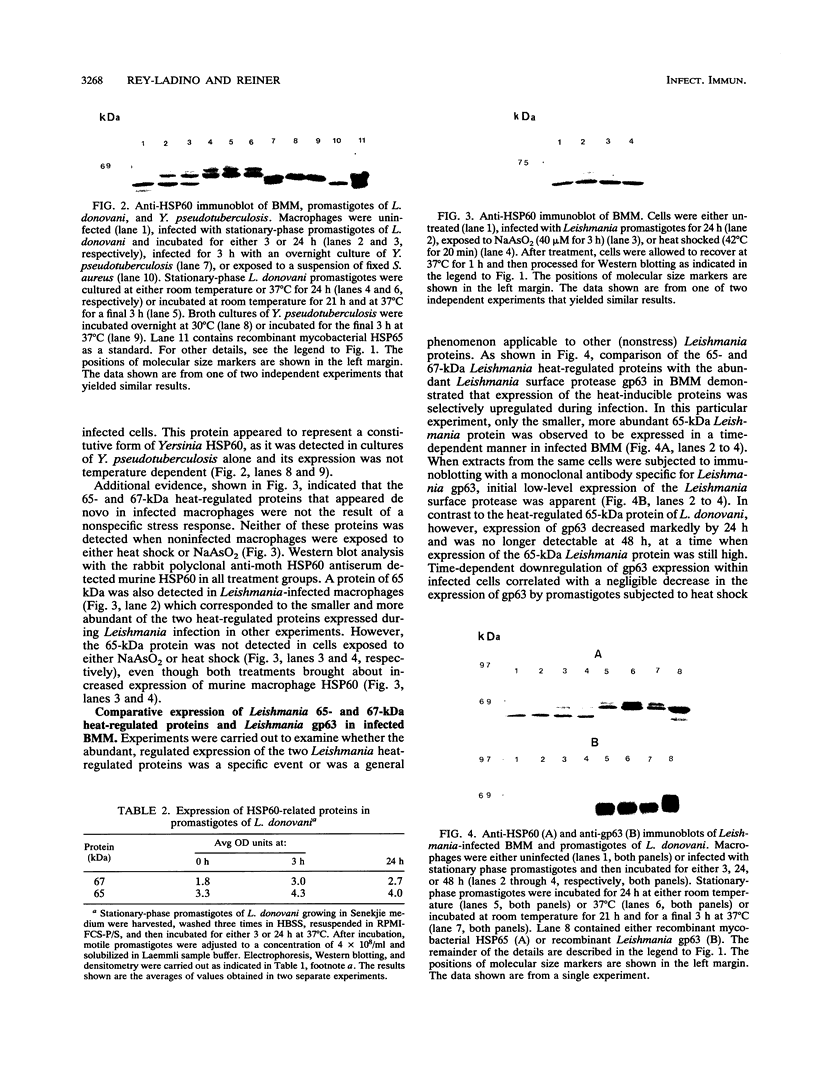
Heat shock protein (HSP) expression was examined in murine bone marrow-derived macrophages infected with stationary-phase promastigotes of Leishmania donovani. Immunoblotting performed with a rabbit polyclonal antiserum raised against HSP60 from Heliothis virescens (moth) revealed the de novo appearance of 65- and 67-kDa proteins in leishmania-infected macrophages. A third protein of 60 kDa, which represented murine HSP60, was also detected, and its expression did not change in response to infection. In contrast, expression of the novel 65- and 67-kDa proteins in infected cells was coordinately regulated and, at 24 h of infection, reached maximal levels of 52 to 100% increases above initial levels determined at 3 h. Proteins which had identical electrophoretic mobilities and were similarly regulated in response to heat were also detected in promastigotes. The appearance of these proteins in macrophages was specific to leishmania infection in that neither protein was detected in noninfected cells either in the basal state or following several treatments, including (i) infection with Yersinia pseudotuberculosis, (ii) phagocytosis of Staphylococcus aureus, (iii) NaAsO2 treatment, and (iv) heat shock. Expression of the 65- and 67-kDa heat-regulated Leishmania proteins was also observed to be selective, in that as their concentration was increasing, the abundance of the Leishmania surface protease gp63 in infected cells was noted to decrease. Murine HSP60 but not the Leishmania heat-regulated proteins was also recognized by a distinct rabbit antiserum raised against human HSP60, suggesting the presence of specific determinants within these Leishmania proteins. A monoclonal antibody that recognizes both mammalian HSP70 and HSP70 from plasmodia detected single isoforms of both Leishmania and murine HSP70 in infected cells, and the level of neither protein changed during infection. Moreover, although a murine HSP of 73 kDa was induced in response to both heat shock and NaAsO2 treatment, it was not induced to detectable levels by infection. The rapid and relatively high level of expression of inducible HSP60-related proteins of L. donovani and Leishmania HSP70 in infected macrophages suggests that these proteins are involved in pathogenesis and may be important targets of the immune response.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blisnick T., Lema F., Mazie J. C., Pereira da Silva L. P. Plasmodium falciparum: analysis of B epitopes of a polypeptide antigen expressed in Escherichia coli, using monoclonal antibodies. Exp Parasitol. 1988 Dec;67(2):247–256. doi: 10.1016/0014-4894(88)90072-0. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Coulson R. M., Smith D. F. Isolation of genes showing increased or unique expression in the infective promastigotes of Leishmania major. Mol Biochem Parasitol. 1990 Apr;40(1):63–75. doi: 10.1016/0166-6851(90)90080-6. [DOI] [PubMed] [Google Scholar]
- Descoteaux A., Matlashewski G. c-fos and tumor necrosis factor gene expression in Leishmania donovani-infected macrophages. Mol Cell Biol. 1989 Nov;9(11):5223–5227. doi: 10.1128/mcb.9.11.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudani A. K., Gupta R. S. Immunological characterization of a human homolog of the 65-kilodalton mycobacterial antigen. Infect Immun. 1989 Sep;57(9):2786–2793. doi: 10.1128/iai.57.9.2786-2793.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engman D. M., Dragon E. A., Donelson J. E. Human humoral immunity to hsp70 during Trypanosoma cruzi infection. J Immunol. 1990 May 15;144(10):3987–3991. [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Hedstrom R., Culpepper J., Harrison R. A., Agabian N., Newport G. A major immunogen in Schistosoma mansoni infections is homologous to the heat-shock protein Hsp70. J Exp Med. 1987 May 1;165(5):1430–1435. doi: 10.1084/jem.165.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K. W., Cook C. L., Hayunga E. G. Leishmanial differentiation in vitro: induction of heat shock proteins. Biochem Biophys Res Commun. 1984 Dec 14;125(2):755–760. doi: 10.1016/0006-291x(84)90603-x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Schoel B., Wand-Württenberger A., Steinhoff U., Munk M. E., Koga T. T-cells, stress proteins, and pathogenesis of mycobacterial infections. Curr Top Microbiol Immunol. 1990;155:125–141. doi: 10.1007/978-3-642-74983-4_9. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Väth U., Thole J. E., Van Embden J. D., Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987 Mar;17(3):351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- Koga T., Wand-Württenberger A., DeBruyn J., Munk M. E., Schoel B., Kaufmann S. H. T cells against a bacterial heat shock protein recognize stressed macrophages. Science. 1989 Sep 8;245(4922):1112–1115. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- Kumar N., Zhao Y., Graves P., Perez Folgar J., Maloy L., Zheng H. Human immune response directed against Plasmodium falciparum heat shock-related proteins. Infect Immun. 1990 May;58(5):1408–1414. doi: 10.1128/iai.58.5.1408-1414.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A. D., Rothbard J. B., Howland K., Young R. A., Young D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987 May;6(5):1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Young D. B. T cell recognition of stress proteins. A link between infectious and autoimmune disease. Mol Biol Med. 1990 Aug;7(4):311–321. [PubMed] [Google Scholar]
- Lawrence F., Robert-Gero M. Induction of heat shock and stress proteins in promastigotes of three Leishmania species. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4414–4417. doi: 10.1073/pnas.82.13.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Atkinson B. L., Giannini S. H., Van der Ploeg L. H. Structure and expression of the hsp 70 gene family of Leishmania major. Nucleic Acids Res. 1988 Oct 25;16(20):9567–9585. doi: 10.1093/nar/16.20.9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- MacFarlane J., Blaxter M. L., Bishop R. P., Miles M. A., Kelly J. M. Identification and characterisation of a Leishmania donovani antigen belonging to the 70-kDa heat-shock protein family. Eur J Biochem. 1990 Jun 20;190(2):377–384. doi: 10.1111/j.1432-1033.1990.tb15586.x. [DOI] [PubMed] [Google Scholar]
- Maresca B., Carratù L. The biology of the heat shock response in parasites. Parasitol Today. 1992 Aug;8(8):260–266. doi: 10.1016/0169-4758(92)90137-q. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser D., Doumbo O., Klinkert M. Q. The humoral response to heat shock protein 70 in human and murine Schistosomiasis mansoni. Parasite Immunol. 1990 Jul;12(4):341–352. doi: 10.1111/j.1365-3024.1990.tb00973.x. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Oka M., Maeda K., Jian-Guo C., Hisaeda H., Ito Y., Good R. A., Himeno K. Induction of heat shock protein closely correlates with protection against Toxoplasma gondii infection. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3155–3158. doi: 10.1073/pnas.89.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Reiner N. E. Host-parasite relationship in murine leishmaniasis: pathophysiological and immunological changes. Infect Immun. 1982 Dec;38(3):1223–1230. doi: 10.1128/iai.38.3.1223-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner N. E., Ng W., Wilson C. B., McMaster W. R., Burchett S. K. Modulation of in vitro monocyte cytokine responses to Leishmania donovani. Interferon-gamma prevents parasite-induced inhibition of interleukin 1 production and primes monocytes to respond to Leishmania by producing both tumor necrosis factor-alpha and interleukin 1. J Clin Invest. 1990 Jun;85(6):1914–1924. doi: 10.1172/JCI114654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Results of a World Health Organization-sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect Immun. 1986 Feb;51(2):718–720. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein N. M., Higashi G., Yates J., Rajan T. V. Onchocerca volvulus heat shock protein 70 is a major immunogen in amicrofilaremic individuals from a filariasis-endemic area. Mol Biochem Parasitol. 1989 Mar 15;33(3):229–235. doi: 10.1016/0166-6851(89)90084-4. [DOI] [PubMed] [Google Scholar]
- Searle S., Campos A. J., Coulson R. M., Spithill T. W., Smith D. F. A family of heat shock protein 70-related genes are expressed in the promastigotes of Leishmania major. Nucleic Acids Res. 1989 Jul 11;17(13):5081–5095. doi: 10.1093/nar/17.13.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkirk M. E., Denham D. A., Partono F., Maizels R. M. Heat shock cognate 70 is a prominent immunogen in Brugian filariasis. J Immunol. 1989 Jul 1;143(1):299–308. [PubMed] [Google Scholar]
- Shapira M., McEwen J. G., Jaffe C. L. Temperature effects on molecular processes which lead to stage differentiation in Leishmania. EMBO J. 1988 Sep;7(9):2895–2901. doi: 10.1002/j.1460-2075.1988.tb03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M., Pinelli E. Heat-shock protein 83 of Leishmania mexicana amazonensis is an abundant cytoplasmic protein with a tandemly repeated genomic arrangement. Eur J Biochem. 1989 Nov 6;185(2):231–236. doi: 10.1111/j.1432-1033.1989.tb15107.x. [DOI] [PubMed] [Google Scholar]
- Smejkal R. M., Wolff R., Olenick J. G. Leishmania braziliensis panamensis: increased infectivity resulting from heat shock. Exp Parasitol. 1988 Feb;65(1):1–9. doi: 10.1016/0014-4894(88)90101-4. [DOI] [PubMed] [Google Scholar]
- Thole J. E., van Schooten W. C., Keulen W. J., Hermans P. W., Janson A. A., de Vries R. R., Kolk A. H., van Embden J. D. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect Immun. 1988 Jun;56(6):1633–1640. doi: 10.1128/iai.56.6.1633-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toye P., Remold H. The influence of temperature and serum deprivation on the synthesis of heat-shock proteins and alpha and beta tubulin in promastigotes of Leishmania major. Mol Biochem Parasitol. 1989 Jun 1;35(1):1–10. doi: 10.1016/0166-6851(89)90136-9. [DOI] [PubMed] [Google Scholar]
- Ul'masov Kh A., Ovezmukhammedov A., Karaev K. K., Evgen'ev M. B. Molekuliarnye mekhanizmy adaptatsii k gipertermii u vysshikh organizmov. III. Induktsiia belkov teplovogo shoka u dvukh vidov leishmanii. Mol Biol (Mosk) 1988 Nov-Dec;22(6):1583–1589. [PubMed] [Google Scholar]
- Van der Ploeg L. H., Giannini S. H., Cantor C. R. Heat shock genes: regulatory role for differentiation in parasitic protozoa. Science. 1985 Jun 21;228(4706):1443–1446. doi: 10.1126/science.4012301. [DOI] [PubMed] [Google Scholar]
- Young R. A. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]