Abstract
Large-scale studies agree that the observed decline in prostate cancer mortality that began in the early 1990s, shortly after prostate-specific antigen testing was introduced in the United States, is most likely explained by more widespread treatment of prostate cancer, including hormonal therapy. Practitioners should be aware of the risk of the development of osteoporosis and of skeletal side effects related to hormonal therapy to optimize the care of men with prostate cancer.
Key words: Hormone deprivation therapy, Androgen deprivation therapy, Bone destruction, Osteoporosis, Osteopenia
Prostate cancer is the most commonly diagnosed cancer among men in the United States, with approximately 192,280 cases anticipated in 2009.1 It also remains a common cause of cancer death, with 27,360 deaths anticipated in 2009. Moreover, the declining US death rates from cardiovascular and smoking-related disease coupled with the aging of the population associated with the Baby Boom generation may beget an anticipated increase in prostate cancer diagnoses in the coming years. It has been estimated that about 10% of the US population was over the age of 65 years in 2000 and that this proportion will approximately double by 2030.2 As a condition of aging men, prostate cancer is apt to remain a significant, if not growing, public health problem.
Current efforts to reduce the mortality burden of prostate cancer have included prostate-specific antigen (PSA)-based screening, but its effect on mortality as assessed in randomized trials, particularly during the first 10 years of follow-up, is controversial.3,4 But these large-scale studies agree that the observed decline in prostate cancer mortality that began in the early 1990s, shortly after PSA testing was introduced in the United States, is most likely explained by more widespread treatment of prostate cancer, including hormonal therapy.5 Given these considerations, it is quite likely that hormone deprivation therapy will remain an important treatment for men with prostate cancer. Therefore, a thorough understanding of its long-term side effects is necessary if we are to optimize the care of men with prostate cancer.
Androgen Deprivation Therapy for Prostate Cancer
Androgen deprivation therapy, the elimination of testosterone by medical (eg, estrogens or luteinizing hormone-releasing hormone agonists and antagonists) or surgical castration, has been used to treat prostate cancer since the 1940s.6 This therapy has been most commonly recommended on the basis of randomized, prospective trial results for men with lymph node metastases identified at the time of radical prostatectomy and as an adjunct to radiation for patients with advanced prostate cancer.7,8 In these settings, use of hormone therapy improves biochemical and clinical response rates, as well as diseasespecific survival. However, hormone therapy has also been commonly used among many patients with localized prostate cancer, for which there are no prospective, randomized trial data demonstrating improved outcomes.9 The same considerations-widespread use without prospective, randomized data to support improved results-apply to hormone therapy for men with biochemical failure after primary surgical or radiation therapy for clinically localized disease.10 The use of androgen deprivation therapy has steadily increased among men with localized prostate cancer irrespective of whether it is low or high risk.11 Thus, even though there are limited prospective, randomized data supporting its use for many stages of prostate cancer, a considerable proportion of men diagnosed with prostate cancer ultimately receive androgen deprivation therapy. Moreover, due to the protracted natural history of prostate cancer, many men are apt to receive hormonal therapy for a prolonged period.
It has become increasingly recognized that androgen deprivation therapy is associated with long-term, adverse side effects that impact quality of life; these include hot flashes, depression, diabetes, coronary artery disease, obesity, and skeletal complications, including osteoporosis and an increased risk of fractures.12–14 The mechanism by which androgen deprivation therapy predisposes to osteoporosis and an increased risk of fracture is related to osteoclast-induced bone resorption. Several studies have measured bone mineral density (BMD) of men receiving hormonal therapy and have observed consistent and pronounced decreases in BMD within 1 year (Table 1). This reduction in bone density places men at considerable risk for fractures,15 most commonly of the vertebrae but also of the wrist and hip.
Table 1.
Androgen Deprivation Therapy Effects on Bone Mineral Density in Men With Prostate Cancer: Pronounced Decreases Are a Consistent Finding
| Study | Treatment | BMD (% decrease at 12 mo) | |
| Eriksson1 | Orchiectomy | Hip: −9.6% | Radius: −4.5% |
| Maillefert2 | GnRH agonist | Hip: −3.9% | L spine: −4.5% |
| Daniell3 | Orchiectomy or GnRH agonist | Hip: −2.4% | |
| Berrutti4 | GnRH agonist | Hip: −0.6% | L spine: −2.3% |
| Higano5 | LHRH agonist plus antiandrogen | Hip: −2.7% | L spine: −4.7% |
| Mittan6 | GnRH agonist | Hip: −3.3% | Radius: −5.3% |
1. Eriksson et al. Calcif Tissue int. 1995;57:97–99; 2. Maillefert et al. J Urol. 1999;161:1219–1222; 3. Daniell et al. J Urol. 2000;163:181–186; 4. Berrutti et al. J Urol. 2002;167:2361–2367; 5. Higano et al. Proc Am Soc Clin Oncol. 1998;18:314a; 6. Mittan et al. J Clin Endocrinol Metab. 2002;87:3656–3661.
Although bone loss may occur as a consequence of aging in healthy men, it is accelerated by the use of hormonal therapy. A prospective study of serial BMD measurements in 152 men with prostate cancer receiving hormonal therapy showed they lost BMD at multiple skeletal sites at a rate of approximately 2% per year, which is a 5- to 10-fold increased rate in comparison with healthy men and men with prostate cancer who are not receiving androgen deprivation therapy.16 Markers of bone formation and resorption were elevated in men receiving hormone therapy, and men with the highest levels had the greatest loss of BMD at 1 year.
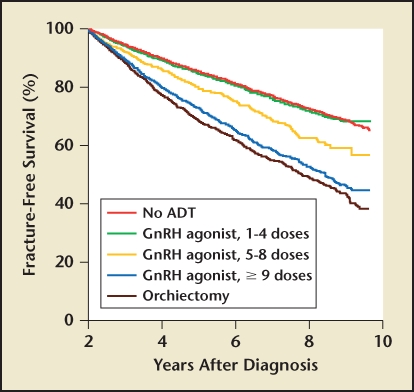
Bone loss is usually most dramatic during the first year following the institution of hormonal therapy, and it increases with the duration of hormone therapy. After a decade of hormone therapy, nearly every patient will have BMD levels that fall into the ranges of osteopenia and osteoporosis, which is a predisposing risk factor for fractures.17 As might be expected, the risk of fractures increases with the duration of androgen deprivation therapy (Figure 1).18 Moreover, bone fractures are a statistically significant negative predictor of survival for men with prostate cancer.19,20
Figure 1.
Risk of fractures increases with longer duration of androgen deprivation therapy. N = 50,613 men listed in the Surveillance, Epidemiology and End Results database and Medicare as having received a diagnosis of prostate cancer in the period from 1992 through 1997 and with follow-up through 2001. Reproduced with permission from Shahinian VB et al.18
Because the substantial skeletal side effects from androgen deprivation therapy, screening via dual-energy x-ray absorptiometry (DXA) at 1 and 3 years after initiation of therapy has been recommended, as well as 1000 to 1200 mg of calcium and 400 to 800 IU of vitamin D per day. Patients at risk may also receive oral bisphosphonate therapy if their T score is below −2.5 or if they are at higher-than-average risk for fractures due to an increased risk of falling, overall frail medical condition, immobility, a history of prior skeletal fractures, or a concomitant steroid use. However, compliance with this therapy is less than ideal, as most patients discontinue therapy within the first year.21
The Vicious Cycle Hypothesis of Bone Destruction and Metastatic Prostate Cancer
Normal bone physiology requires balance between osteoclast-mediated bone resorption and new formation by osteoblasts. An important mediator of osteoclast activation, differentiation, and survival is RANK ligand.22 When prostate cancer metastasizes to bone, it initiates a vicious cycle of accelerated bone destruction.23 Although men with prostate cancer are often found to have predominately osteoblastic lesions, there is significant associated osteolytic activity, as measured by increased serum and urine markers of bone resorption (see below), which is comparable to, and in some cases higher than, that seen among patients with purely osteolytic lesions from breast cancer or multiple myeloma.24 Factors produced by the tumor cells stimulate osteoblasts to express RANK ligand (Figure 2). RANK ligand promotes osteoclastic activity that increases bone resorption, which results in release of local factors from the bone microenvironment that can promote further growth of tumor cells in the bone. The presence of bone metastases irrespective of the simultaneous use of hormonal therapy predisposes men to more frequent and more severe skeletal-related events, including pathologic fractures, in comparison with men receiving hormonal therapy alone. This occurs because of the substantial loss of bone density due to the osteolysis associated with the metastasis. It has been estimated that about 49% of patients with metastatic prostate cancer experience a skeletal-related event within 2 years25; the types of skeletal-related events anticipated in the presence of metastatic prostate cancer are shown in Figure 3.26
Figure 2.
The Vicious Cycle Hypothesis of bone destruction in metastatic cancer. Adapted with permission from Roodman GD.23
Figure 3.
Skeletal morbidity in hormone-refractory metastatic prostate cancer patients encompasses a range of bone complications. Data from Saad F et al.26
Management of bone metastasis to prevent skeletal-related events includes bisphosphonate therapy and will likely expand in the near future as other treatment modalities are evaluated. An important component of managing men at risk for skeletal-related events is risk stratification. Urinary and serum markers of bone turnover include N-telopeptide (NTx) and bone alkaline phosphatase (BALP). The ratio of NTx to creatinine has been shown to correlate with outcomes in men with prostate cancer.27 The ratio of posttreatment NTx to creatinine and BALP levels are independent predictors of overall skeletalrelated events, time to a skeletal-related event, and mortality in patients with prostate cancer.28 Treatment with bisphosphonates has been shown to reduce NTx to creatinine and BALP levels in patients with bone metastases, translating into a reduced risk of skeletal-related events and presumptively of prolonged survival.27,28 Stabilization of NTx to creatinine levels in patients at risk for skeletalrelated events suggests a good prognosis.26
Summary
Bone health is an important consideration for men with prostate cancer, and hormonal therapy may induce osteoporosis. Practitioners should be aware of the risk of the development of osteoporosis and of skeletal side effects related to hormonal therapy. Practitioners should screen for this using DXA scan and implement preventive strategies, including calcium replacement and use of vitamin D. According to current National Comprehensive Cancer Network guidelines, patients at very high risk, that is, those with T scores −2.5, should consider additional therapy such as bisphosphonates.29 Men with prostate cancer metastatic to the bone are particularly at high risk for skeletal-related events that include pathologic fractures, spinal cord compression, and the need for surgical and radiation therapy; these men should be treated with intravenous BP zoledronic acid. DXA scans and other imaging procedures, such as radiographs, computed tomography, magnetic resonance imaging, and urinary NTx levels put physicians in the best position to take preemptive steps to avoid skeletal-related risks in men receiving hormonal therapy for prostate cancer.
Main Points.
The use of androgen deprivation therapy has steadily increased among men with localized prostate cancer.
It has become increasingly recognized that androgen deprivation therapy is associated with long-term, adverse side effects that impact quality of life; these include hot flashes, depression, diabetes, coronary artery disease, obesity, and skeletal complications, including osteoporosis and an increased risk of fractures.
The presence of bone metastases irrespective of the simultaneous use of hormonal therapy predisposes men to more frequent and more severe skeletal-related events.
Management of bone metastasis to prevent skeletal-related events includes bisphosphonate therapy and will likely expand in the near future as other treatment modalities are evaluated.
Footnotes
This article was conceived of and fully funded by Amgen, and Amgen provided background direction for the article.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.National Institute on Aging, US Department of Commerce, Bureau of the Census, authors. Aging in the United States-Past, Present, and Future. Washington, DC: National Institute on Aging, US Department of Commerce, Bureau of the Census; [Accessed November 3, 2009]. http://www.census.gov/ipc/prod/97agewc.pdf. [Google Scholar]
- 3.Andriole GL, Crawford ED, Grubb RL, III, et al. PLCO Project Team, authors. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schröder FH, Hugosson J, Roobol MJ, et al. ERSPC Investigators. Screening and prostatecancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 5.Etzioni R, Feuer E. Studies of prostate cancer mortality: caution advised. Lancet Oncol. 2008;9:407–409. doi: 10.1016/S1470-2045(08)70112-8. [DOI] [PubMed] [Google Scholar]
- 6.Huggins C, Hodges CV. Studies on prostate cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. [Google Scholar]
- 7.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomized trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 8.Messing EM, Manola J, Sarosdy M, et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–1788. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 9.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooperberg MR, Lubeck DP, Meng MV, et al. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22:2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 13.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 14.D’Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 15.Daniell HW. Osteoporosis after orchiectomy for prostate cancer. J Urol. 1997;157:439–444. [PubMed] [Google Scholar]
- 16.Greenspan SL, Coates P, Sereika SM, et al. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90:6410–6417. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 17.Morote J, Morin JP, Orsola A, et al. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology. 2007;69:500–504. doi: 10.1016/j.urology.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 19.Oefelein MG, Ricchiuti V, Conrad W, Resnick MI. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol. 2002;168:1005–1007. doi: 10.1016/S0022-5347(05)64561-2. [DOI] [PubMed] [Google Scholar]
- 20.Lau E, Ong K, Kurtz S, et al. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am. 2008;90:1479–1486. doi: 10.2106/JBJS.G.00675. [DOI] [PubMed] [Google Scholar]
- 21.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437–443. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 23.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 24.Brown JE, Cook RJ, Major P, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 25.Saad F, Gleason DM, Murray R, et al. for the Zoledronic Acid Prostate Cancer Study Group, authors. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–892. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 26.Saad F, Gleason DM, Murray R, et al. for the Zoledronic Acid Prostate Cancer Study Group, authors. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 27.Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 28.Dearnaley DP, Mason MD, Parmar MKB, et al. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10:872–876. doi: 10.1016/S1470-2045(09)70201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Comprehensive Cancer Network (NCCN), authors NCCN Clinical Practice Guidelines in Oncology™. Prostate Cancer. Fort Washington, PA: NCCN; 2009. [Google Scholar]