Abstract
Procedural and surgical site infections create difficult and complex clinical scenarios. A source for pathogens is often thought to be the skin surface, making skin preparation at the time of the procedure critical. The most common skin preparation agents used today include products containing iodophors or chlorhexidine gluconate. Agents are further classified by whether they are aqueous-based or alcohol-based solutions. Traditional aqueous-based iodophors, such as povidone-iodine, are one of the few products that can be safely used on mucous membrane surfaces. Alcohol-based solutions are quick, sustained, and durable, with broader spectrum antimicrobial activity. These agents seem ideal for longer open surgeries with the potential for irrigation or surgical spillage, such as cystoprostatectomy, radical prostatectomy, and retroperitoneal lymph node dissection.
Key words: Skin pathogens, Procedural and surgical site infection, Skin preparation solutions
Surgical site infection (SSI) complicates an estimated 5% of all clean-contaminated operations performed annually in US hospitals and accounts for the most common nosocomial infection in surgical patients.1 Patients who develop SSI have longer and costlier hospitalizations and are more likely to spend time in an intensive care unit (ICU), are 5 times more likely to be readmitted, and are twice as likely to die.2
Recognizing this substantial morbidity and economic burden, in 1999 the Centers for Disease Control (CDC) issued standardized guidelines for the prevention of surgical infections. These included making specific evidence-based recommendations for modifying patient factors that may predispose to infection, for the use of antimicrobial prophylaxis, for optimizing sterility in the operating room, and for the use of antiseptic agents for skin preparation.
The choice of which specific agent to use for skin preparation was not addressed due to the diversity of sites and approaches in surgery, as well as the absence of data on SSI risk in well-controlled, operation-specific studies.1 Therefore, the choice of agent should be based primarily on the surgeon’s knowledge of the product’s efficacy, cost, and ease of use. Urologic surgeons have the additional challenge of choosing the best agent for the variety of procedures that they perform, including intraperitoneal and extraperitoneal surgery; scrotal, perineal, and vaginal operations; endoscopy; and percutaneous renal surgery. Each of these operative sites has different endogenous flora, body contours, and skin types, all factors that influence the risk of SSI and, therefore, the best type of antiseptic skin agent to use. This article focuses on skin preparation for the prevention of SSI with an assessment of currently available antiseptic products and their application to urologic surgery.
History
The first use of an antiseptic skin agent in surgery is credited to the English surgeon Joseph Lister (1827–1912). Prior to the mid-19th century, limb amputation was associated with an alarming 50% postoperative mortality from sepsis. Following Louis Pasteur’s discovery that tissue decay was caused by microscopic organisms, Lister theorized that the spread of these microbes through surgical wounds was responsible for death in the postoperative period. Lister began treating wounds with carbolic acid (phenol) in an effort to prevent tissue decay and the resultant infectious complications. As a result, the incidence of surgical sepsis fell dramatically, catalyzing the adoption of modern antiseptic techniques, including instrument sterilization, the use of surgical scrub and rubber gloves, and sterile patient preparation.3
Modern Surgical Skin Preparation
The most common skin preparation agents used today include products containing iodophors or chlorhexidine gluconate (CHG). Agents are further classified by whether they are aqueous or alcohol-based solutions (Table 1).1
Table 1.
Characteristics of Antiseptic Solutions
| Antiseptic | Mechanism of Action | Antimicrobial Coverage | Onset | Duration | Application | Examples |
| Aqueous-iodophor | Free iodine − protein, DNA damage | Excellent for gram + bacteria, good for gram −, fungi, virus, Mtb | Intermediate | 2 hours19 | 2-step scrub and paint | Betadine* Scrub Care† |
| Aqueous-CHG | Disrupts membranes | Excellent for gram +, good for gram − and virus, fair for fungus, poor for Mtb | Intermediate | 6 hours20 | 2-step scrub and dry, repeat | Hibiclens‡ |
| Alcohol-iodophor | Denatures protein, free iodine − protein, DNA damage | Improved gram −, Mtb activity | Rapid | 48 hours (DuraPrep)11 96 hours (Prevail-FX)21 | 1-step paint Dry time, minimum of 3 min on hairless surface | DuraPrep solution§ Prevail-FX† |
| Alcohol-CHG | Denatures protein, disrupts membranes | Improved gram −, Mtb, fungal activity | Rapid | 48 hours22,23 | Dry site: 30-sec scrub Moist site: 2-min scrub Dry time, minimum of 3 min on hairless surface | ChloraPrep |
Mtb, Mycobacterium tuberculosis; CHG, chlorhexidine gluconate.
Betadine® is from Purdue Products, LP (Stamford, CT).
ScrubCare® and Prevail-FX® are from Cardinal Health (Dublin, OH).
Hibiclens® is from Mölnlycke Health Care US LLC (Norcross, GA).
3M™ DuraPrep™ Surgical Solution (Iodine Povacrylex [0.7% available iodine] and Isopropyl Alcohol, 74% w/w) Patient Preoperative Skin Preparation is from 3M Health Care (St. Paul, MN).
ChloraPrep® is from CareFusion, Inc. (Leawood, KS).
Based on data from Mangram AJ et al.1
Aqueous-Based Solutions
Aqueous-based iodophors such as povidone-iodine (PVP-I) contain iodine complexed with a solubilizing agent that allows for the release of free iodine when in solution. Iodine acts in an antiseptic fashion by destroying microbial proteins and DNA. Iodophor-containing products enjoy widespread use because of their broad-spectrum antimicrobial properties, efficacy, and safety on nearly all skin surfaces in patients regardless of age. In the aqueous form, most commercially available iodophors require a 2-step application in a scrub-and-paint technique, and their activity is limited by the amount of time the agent is in contact with the skin.4 A second product, aqueous-based chlorhexidine gluconate (CHG), works by disrupting bacterial cell membranes. CHG has more sustained antimicrobial activity and is more resistant to neutralization by blood products than the iodophors. CHG is applied in a similar manner to PVP-I, but should not be used in the genital region. This agent has gained popularity as a hand-scrubbing and showering antiseptic prior to surgery, but also continues to be used as a patient skin preparation agent.5
Alcohol-Based Solutions
Ethyl and isopropyl alcohol are 2 of the most effective antiseptic agents available. When used alone, alcohol is fast and short acting, has broad-spectrum antimicrobial activity, and is relatively inexpensive.1 Alcohol-based solutions that contain CHG or iodophors have sustained and durable antimicrobial activity that lasts long after alcohol evaporation.6 Because alcohol dries on exposed skin within moments of application, these can be applied with a 1-step preparation as opposed to a scrub-and-paint technique.
A limitation to the use of alcohol in the operating room is its flammability on skin surfaces prior to evaporation. There have been a few reports of operating room fires originating from alcohol-based skin preparation resulting in significant injury to patients and staff.7 Flammability can be avoided by allowing skin to completely dry and avoiding preparation of areas with excessive body hair that can delay alcohol vaporization.
Additionally, alcohol-based solutions should not be applied to mucous membranes and therefore have limited utility as antiseptic agents prior to transurethral or transvaginal surgery. Nevertheless, combination solutions with alcohol and CHG or iodophors have gained popularity among general, cardiac, and orthopedic surgeons and may have additional utility in certain urologic procedures. Recent studies suggest that these products may have greater efficacy, easier application, improved durability, and a superior cost profile when compared with traditional aqueous-based solutions.
One such product, 3M™ DuraPrep™ Surgical Solution (Iodine Povacrylex [0.7% available iodine] and Isopropyl Alcohol, 74% w/w) Patient Preoperative Skin Preparation (3M Health Care, St. Paul, MN), is an antiseptic skin solution that contains iodine povacrylex and isopropyl alcohol. It is applied in 1 step, has a dry time of a minimum of 3 minutes on hairless skin, leaves a water-insoluble film on the skin surface that maintains antimicrobial activity for up to 48 hours, and resists wash-off by saline and blood products (Figure 1). In vitro studies have demonstrated that DuraPrep solution is effective against a broad range of microorganisms, including those most commonly encountered in genitourinary (GU) surgery, including gram-negative rods, Staphylococcus species, and Enterococcus, as well as multidrug-resistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus epidermidis (MRSE), and vancomycin-resistant enterococci (VRE).8 Furthermore, DuraPrep solution accomplishes a 6-fold bacterial log reduction within 1 minute of contact with a greater percentage release of free iodine when compared with the leading aqueous iodophors.8
Figure 1.
Application of DuraPrep™ Surgical Solution (3M Health Care, St. Paul, MN). Adapted with permission from 3M Health Care.
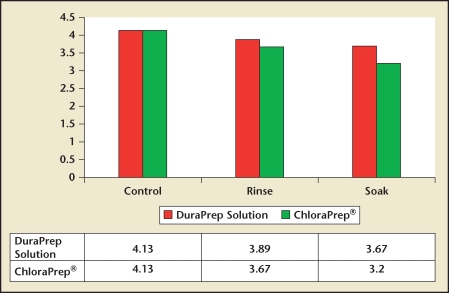
Another potential advantage of this product is its durability in the surgical environment. In a prospective, randomized surgical simulation study, DuraPrep solution demonstrated better antimicrobial activity after saline soak when compared with the leading CHG alcohol-based solution (ChloraPrep®; CareFusion, Inc., Leawood, KS), suggesting that it is particularly suitable for use in “wet” surgical environments (Figure 2).9 Another unique feature of DuraPrep solution is that it enhances adhesion between surgical drapes and the prepared skin surface, theoretically limiting the spread of organisms onto the surgical field. In a randomized, prospective study comparing drape adhesion in patients undergoing total joint replacement, patients prepared with DuraPrep solution had significantly less area of drape lift than those prepared with PVP-I, 1.5 cm2 versus 9.9 cm2, respectively (P < .0001).10 This adhesive property may be particularly advantageous for long, open procedures with the potential for fluid spillage on the surgical field, such as cystoprostatectomy, radical prostatectomy, and retroperitoneal lymph node dissection (RPLND).
Figure 2.
Bacterial log reduction after saline challenge. *P < .003 for soak condition. Reproduced with permission from Stahl JB et al.9
Although there have been no studies in the urologic literature addressing the effect of this product on SSI, clinical studies have been conducted in general, cardiac, and orthopedic surgery, as well as in patients undergoing anesthesia procedures. In a prospective, randomized study of general surgery patients undergoing operations 3 hours or longer, the use of DuraPrep solution resulted in a 3-fold decrease in SSI when compared with tincture of iodine.11 Another study of 3209 general surgical procedures compared the use of 3 skin preparations: a povidone-iodine scrub-paint combination (Betadine®, Purdue Products, LP, Stamford, CT) (with an isopropyl alcohol application between the steps), ChloraPrep, and DuraPrep solution.12 This study employed a sequential implementation design, and each agent was used for a 6-month period for all general surgery cases. PSSIs were tracked for 30 days.
DuraPrep solution was associated with the lowest infection rate (3.9%, compared with 6.4% for Betadine and 7.1% for ChloraPrep [P = .002]). In subgroup analysis, no difference in outcomes was seen between patients prepared with Betadine/alcohol and those prepared with DuraPrep solution, but patients in both these groups had significantly lower PSSI rates compared with patients prepared with ChloraPrep (4.8% vs 8.2% [P = .001]).
In the cardiac literature, a comparison of DuraPrep solution with the leading PVP-I in patients at high risk for SSI undergoing open heart surgery, 4 of 101 patients in the DuraPrep solution group developed wound infections compared with 14 of 108 in the PVP-I group.13 At another center, the introduction of DuraPrep solution in a cardiac surgery service was associated with a more than 50% reduction in overall SSI, sternal wound infection, and repeat surgical intervention for infection.14
In a study of patients undergoing epidural catheter placement on an obstetrics ward, DuraPrep solution was prospectively compared with PVP-I. The DuraPrep solution group showed a significant decrease in the number of positive skin cultures obtained immediately after disinfection and immediately prior to catheter removal. In addition, bacteria was cultured from 2 epidural catheter tips in patients treated with DuraPrep solution compared with 13 positive cultures from catheter tips in the PVP-I group.15 This finding suggests that DuraPrep solution may be particularly suitable for percutaneous renal access procedures where catheters are frequently left in place postoperatively, thus serving as a potential entry point for infection.
Finally, a prospective clinical study in the orthopedic literature suggests that alcohol-based solutions with iodophor or CHG may have improved efficacy at reducing bacterial counts in “moist” surgical sites or body regions with increased endogenous bacterial colonization. One hundred twenty-five patients undergoing foot and ankle surgery were randomized to receive preparation with 3M™ DuraPrep™ Surgical Solution (Iodine Povacrylex [0.7% available iodine] and Isopropyl Alcohol, 74% w/w) Patient Preoperative Skin Preparation, ChloraPrep, or Techni-Care® Surgical Scrub (Care-Tech Laboratories, Inc., St. Louis, MO). The sites treated with alcohol-based solutions had on average a 50% reduction in positive cultures compared with those treated with traditional antiseptic agents. Overall, ChloraPrep performed 2-fold better than DuraPrep solution, but the findings of this study have been criticized because no neutralization agent was used prior to obtaining cultures from the surface of the treated areas. Because ChloraPrep is a non-film forming antiseptic, without the use of a neutralizer, it is likely that in this group, sampling contaminated with antiseptic led to ongoing bacterial death and exaggerated efficacy. Additionally, no patients developed SSI in the DuraPrep solution group.16
These findings can be generalized to other “moist” surgical sites, suggesting that alcohol-based solutions may be efficacious for use in groin, scrotal, or perineal urologic surgery, especially for implantation of foreign devices such as penile prostheses or artificial urinary sphincters where minimizing bacterial counts is critical.
In addition to an analysis of efficacy, ease of use, cost, and user satisfaction are important considerations when choosing a skin preparation agent. In a prospective comparison of alcohol-based iodophors with traditional PVP-I preparation, the alcohol-based solutions had shorter application and drying times. Taking into consideration operating room time and product expenses, the alcohol-containing products had lower overall costs.17 Other studies have confirmed this finding, showing that the use of DuraPrep solution has potential savings of $78 per patient (Table 2).18 Despite these advantages, operating room personnel preferred PVP-I scrub-and-paint to the alcohol preparations, citing concern over flammability as the most important overall deciding factor. Familiarity with PVP-I scrub-and-paint, however, may have introduced bias into the assessment of user satisfaction because personnel had little to no experience with alcohol-based solutions prior to the study.19 With safe use and proper instruction, alcohol-based antiseptics may save valuable time and operating room resources.
Table 2.
Comparison of Antiseptic Products
| OR Durability | Incidence of Surgical Site Infection | Ease of Use | |||||
| Antiseptic | Soak | Drape lift | General | General | Cardiac | Application | Cost |
| Stahl et al9 N = 36 P = .006 | Jacobson et al10 N = 171 P < .0001 | Pinheiro et al11 N = 214 P < .05 | Swenson et al12 N = 3209*P < .002 | Segal and Anderson13 N = 209 P = .02 | Armstrong et al17 N = 25 | Roberts et al18 N = 200 P = .0001 | |
| DuraPrep solution† | 3.7 bacterial log reduction | 1.5 cm2 | 4.8% | 3.9% | 4% | 82.8 sec With drape application | $56.96 |
| Tincture of iodine | – | – | 14.7% | – | – | – | |
| Prevail‡ | – | – | – | – | 42.2 sec | – | |
| ChloraPrep§ | 3.2 bacterial log reduction | – | – | 7.1% | – | – | – |
| PVP-I | – | 9.9 cm2 | – | 6.4% | 13% | 228 sec | $135.28 |
OR, operating room; PVP-I, povidone-iodine.
Number of procedures.
DuraPrep™ solution is from 3M Health Care (St. Paul, MN).
Prevail-FX® is from Cardinal Health (Dublin, OH).
ChloraPrep® is from CareFusion, Inc. (Leawood, KS).
Adapted with permission from Roberts AJ et al.18
Conclusion
The goal of preoperative skin preparation is to reduce the incidence of SSI in a safe, user-friendly, and cost-effective manner. Because urologists perform a breadth of different operations accessing numerous surgical sites, a standard antiseptic agent is unlikely to be uniformly optimal. Traditional aqueous-based iodophors such as PVP-I are ideal for transvaginal and transurethral surgery and are one of the few products that can be safely used on mucous membrane surfaces. Likewise, alcoholbased solutions such as DuraPrep solution are quick, sustained, and durable with broader spectrum antimicrobial activity. These seem ideal for longer open surgeries with the potential for irrigation or surgical spillage, for percutaneous procedures with indwelling catheters, and for prosthesis implantation when minimizing skin colony counts is critical to prevent hardware infection. Because alcohol is flammable, when using these products care must be taken to allow adequate drying time and to remove excessive hair from the prepared field that may delay alcohol vaporization.
Main Points.
Aqueous-based iodophors, such as povidone-iodine, contain iodine complexed with a solubilizing agent, allowing for the release of free iodine when in a solution. Iodine acts in an antiseptic manner by destroying microbial proteins and DNA. Iodophor-containing products enjoy widespread use because of their broad-spectrum antimicrobial properties, efficacy, and safety on nearly all skin surfaces regardless of the patient’s age.
Ethyl and isopropyl alcohol are 2 of the most effective antiseptic agents available. When used alone, alcohol is fast and short acting, has broad-spectrum antimicrobial activity, and is relatively inexpensive. Flammability can be avoided by allowing skin to completely dry and by avoiding preparation of areas with excessive body hair that can delay alcohol vaporization.
Recent studies suggest that alcohol-based solutions may have greater efficacy, easier application, improved durability, and a superior cost profile when compared with traditional aqueous-based solutions.
DuraPrep solution, an antiseptic skin solution that contains iodine povacrylex in isopropyl alcohol, shows durability in the surgical/procedural environment and enhances adhesion between surgical drapes and the prepared skin surface, theoretically limiting the spread of organisms onto the surgical field.
Alcohol-based solutions are quick, sustained, and durable, with broader spectrum antimicrobial activity. These agents seem ideal for longer open surgeries with the potential for irrigation or surgical spillage.
Footnotes
Dr. Micah L. Hemani and Dr. Herbert Lepor have been reimbursed by 3M Company for their contributions.
References
- 1.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–278. doi: 10.1086/501620. quiz 279–280. [DOI] [PubMed] [Google Scholar]
- 2.Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–730. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 3.Newsom BD. Surgical wound infections: a historical review. Int J Infect Control. 2008;4:1. [Google Scholar]
- 4.Digison MB. A review of antiseptic agents for pre-operative skin preparation. Plast Surg Nurs. 2007;27:185–189. doi: 10.1097/01.PSN.0000306182.50071.e2. quiz 190–191. [DOI] [PubMed] [Google Scholar]
- 5.Hibbard JS. Analyses comparing the antimicrobial activity and safety of current antiseptic agents: a review. J Infus Nurs. 2005;28:194–207. doi: 10.1097/00129804-200505000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Beck WX. After 2000 years, alcohol still excels. Infections in Surgery. 1985 [Google Scholar]
- 7.Weber SM, Hargunani CA, Wax MK. DuraPrep and the risk of fire during tracheostomy. Head Neck. 2006;28:649–652. doi: 10.1002/hed.20396. [DOI] [PubMed] [Google Scholar]
- 8.Data on file. St. Paul, MN;: 3M Health Care; [Google Scholar]
- 9.Stahl JB, Morse D, Parks PJ. Resistance of antimicrobial skin preparations to saline rinse using a seeded bacteria model. Am J Infect Control. 2007;35:367–373. doi: 10.1016/j.ajic.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson C, Osmon DR, Hanssen A, et al. Prevention of wound contamination using DuraPrep Solution plus loban 2 drapes. Clin Orthop Relat Res. 2005;439:32–37. doi: 10.1097/01.blo.0000182245.29830.bc. [DOI] [PubMed] [Google Scholar]
- 11.Pinheiro SM, Couto BR, Pimenta JP, et al. Effects of iodophor skin preparation in reducing surgical site infection. Abstract presented at: 14th Annual Meeting of the Society for Healthcare Epidemiology of America; April 17–20, 2004; Philadelphia, PA. Abstract 329. [Google Scholar]
- 12.Swenson BR, Hedrick TL, Metzger R, et al. Effects of preoperative skin preparation on postoperative wound infection rates: a prospective study of 3 skin preparation protocols. Infect Control Hosp Epidemiol. 2009;30:964–971. doi: 10.1086/605926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segal CG, Anderson JJ. Preoperative skin preparation of cardiac patients. AORN J. 2002;76:821–828. doi: 10.1016/s0001-2092(06)61035-1. [DOI] [PubMed] [Google Scholar]
- 14.Squier C, Miller T, DiLucia B, et al. Cardiac bypass surgery: intervention to decrease surgical site infections. Abstract presented at: 4th Decennial International conference on Nosocomial and Health Care-Associated Infections in conjunction with the 10th Annual Meeting of the Society for Healthcare Epidemiology of America; March 5–9, 2009; Atlanta, GA. [Google Scholar]
- 15.Birnbach DJ, Meadows W, Stein DJ, et al. Comparison of povidone iodine and DuraPrep, an iodophor-in-isopropyl alcohol solution, for skin disinfection prior to epidural catheter insertion in parturients. Anesthesiology. 2003;98:164–169. doi: 10.1097/00000542-200301000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Ostrander RV, Botte MJ, Brage ME. Efficacy of surgical preparation solutions in foot and ankle surgery. J Bone Joint Surg (Am) 2005;87:980–985. doi: 10.2106/JBJS.D.01977. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong EP, Patrick KL, Erstad BL. Comparison of preoperative skin preparation products. Pharmacotherapy. 2001;21:345–350. doi: 10.1592/phco.21.3.345.34196. [DOI] [PubMed] [Google Scholar]
- 18.Roberts AJ, Wilcox K, Devineni R, et al. Skin preparations in CABG surgery: a prospective randomized trial. Comp Surg. 1995;14:741–748. [Google Scholar]
- 19.Khera SY, Kostyal DA, Deshmukh N. A comparison of chlorhexidine and povidone-iodine skin preparation for surgical operations. Curr Surg. 1999;56:341–343. [Google Scholar]
- 20.Data on file. Norcross, GA: Mölnlycke Health Care, Inc.; [Google Scholar]
- 21.Data on file. Dublin, OH: Cardinal Health; [Google Scholar]
- 22.Garcia R, Mulberry G, Brady A, Hibbard JS. Comparison of ChloraPrep and Betadine as preoperative skin preparation antiseptics. Poster presented at: 40th Annual Meeting of the Infectious Disease Society of America; October 25, 2002; Chicago, IL. [Google Scholar]
- 23.Data on file. San Diego, CA: CareFusion, Inc.; [Google Scholar]