Abstract
Focal therapy has been proposed in recent years as a means of bridging the gap between radical prostatectomy and active surveillance for treatment of prostate cancer. The rationale for focal therapy comes from its success in treating other malignancies. One of the challenges in applying such an approach to the treatment of prostate cancer has been the multifocal nature of the disease. This review addresses the selection of potentially ideal candidates for focal therapy and discusses which modalities are currently being used and proposed for focal therapy. Setting and meeting guidelines for oncologic efficacy is a challenge we must embrace to safely deliver this potentially revolutionary approach to treating men with prostate cancer.
Key words: Focal therapy; Photodynamic therapy; Prostatic neoplasms; Prostate-specific antigen; Prostatectomy; Ultrasound, high-intensity focused, transrectal; Cryosurgery
With the advent of prostate-specific antigen (PSA) screening there has been a stage migration, with radical prostatectomy (RP) being performed with increasing frequency in men with low-risk disease.1 Whole gland treatment of prostate cancer carries a significant risk of incontinence and sexual dysfunction. Even in the most experienced centers, the rate of potency following RP is approximately 60%.2–4 Stage migration has led many to recommend active surveillance (AS) as a means to decrease the number of men who may be overtreated; however, AS has been slow to gain acceptance in the United States.
An analysis of over 5300 men from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) National Prostate Cancer Registry5 showed that only 7% of men with clinically localized prostate cancer chose AS as an initial option. Aside from the anxiety that stems from not treating a diagnosed cancer, the greater difficulty with AS lies in selection of candidates and appropriate parameters for surveillance, allowing prompt intervention without compromising cure rates.
Focal therapy has been proposed in recent years as a means of bridging the gap between whole gland treatment and AS. Many believe that for patients with low-risk disease, focal therapy is the ideal option for maximizing quality of life by avoiding the effects of whole gland radiation or surgery while alleviating the anxiety and uncertainty of AS. The definition of focal therapy itself is not well established and includes lesion-targeted therapy (LAT), hemiablative therapy (HAT), or subtotal gland therapy (STAT), sparing at least 1 neurovascular bundle.6
The rationale for focal therapy comes from its success in treating other malignancies. In breast cancer treatment, for example, radical mastectomy has been replaced in many instances by local excision and Mohs surgery has led to less radical surgery for the treatment of melanoma.7 In our own field, the push for nephron-sparing surgery has led to the favoring of partial nephrectomy in tumors less than 7 cm, with oncologic outcomes similar to those of radical nephrectomy.8
The challenge in applying such an approach to the treatment of prostate cancer has been the multifocal nature of prostate cancer and the fact that most cancers are detected without identifying a lesion on palpation or imaging studies.9,10
In this review, we revisit the current status of focal therapy in the treatment of prostate cancer. We discuss whether there are ideal candidates for focal therapy; we then discuss how these candidates should be selected. We review which modalities are currently being used and proposed for focal therapy. Finally, we discuss potential definitions of successful treatment. As this article shows, there are still many aspects of focal therapy that are yet to be defined, that warrant a great need for further research.
Candidate Selection for Focal Therapy
It is estimated that between 20% to 38% of men with prostate cancer have unilateral disease.9,11,12 Given the challenges of imaging and mapping tumors in the prostate, focal therapy via HAT has been proposed as a means of identifying and treating men with low-stage prostate cancer. Given the surgical constraints of performing a “partial prostatectomy,” surgically ablative techniques are ideally suited to carrying out hemiablation. Such a therapy would ideally treat the side of disease, while sparing the neurovascular bundle on the contralateral side.
We recently reviewed our own RP series of more than 1400 men over the past 7 years and found that 21% of men had unilateral disease. When we further stratified these men with unilateral disease to include only those with very low-risk features (clinical stage T2a or lower, Gleason score < 7, PSA < 10 ng/mL, and tumor involvement < 10%), only 11% of the overall cohort had pathologically unilateral disease. This very select group would represent an ideal cohort for HAT.13
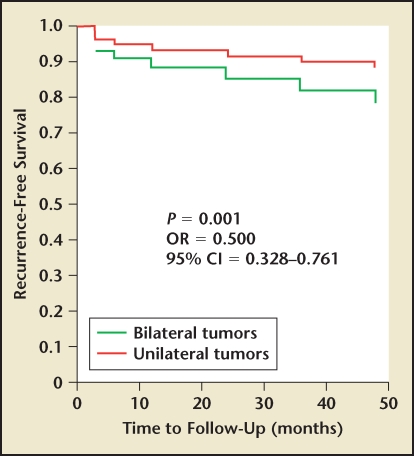
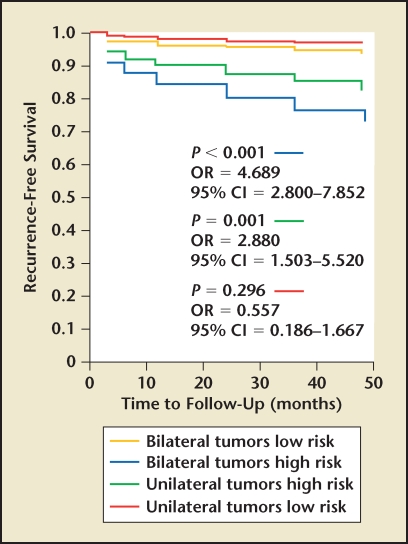
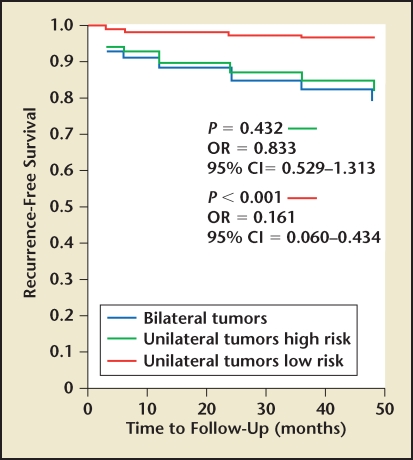
When evaluating our cohort of patients with unilateral prostate cancer with respect to pathologic and oncologic outcomes, we found that men with unilateral disease had lower rates of extracapsular extension, seminal vesicle invasion, and Gleason scores when compared with their bilateral counterparts (Table 1). When we evaluated PSA relapse, we found that men with unilateral disease had lower overall rates of PSA relapse (8.3% vs 16.7%) as well as slower median time to relapse (6 mo vs 12 mo). In our own series, PSA relapse was defined as PSA greater than 0.1 ng/mL at least 3 months following RP. Upon further analysis, however, it appeared that this effect was secondary to risk stratification and not laterality. When stratifying men into low-risk (PSA < 10 ng/mL, biopsy tumor volume < 10%, Gleason score < 7) and high-risk categories, we found no difference between unilateral or bilateral low-risk groups14 (Figures 1–3). Similarly, men with high-risk disease had no significant difference in PSA relapse whether they were unilateral or bilateral. This led us to conclude that with improvements in focal ablation delivery methods, men with unilateral or bilateral low-risk prostate cancer may be ideal candidates for focal therapy.
Table 1.
Pathologic and Oncologic Outcomes in Men Undergoing Radical Prostatectomy
| Pathologic Factor | Unilateral (n = 311) | Bilateral (n = 1147) | P |
| Extracapsular extension | 40/301 (13%) | 225/1083 (21%) | < .01 |
| Seminal vesicle invasion | 5/298 (2%) | 71/1078 (7%) | < .01 |
| Gleason score ≥ 7 | 125/300 (42%) | 552/1080 (51%) | < .01 |
| Biochemical recurrence | 25/300 (8.3%) | 166/990 (16.7%) | < .001 |
| Time to PSA recurrence | 12 mo (n = 25) | 6 mo (n = 166) | < .001 |
PSA, prostate-specific antigen.
Figure 1.
Prostate-specific antigen recurrence-free survival in men with unilateral or bilateral prostate cancer. CI, confidence interval; OR, odds ratio. Adapted with permission from Tareen B et al.14
Figure 3.
Prostate-specific antigen recurrence-free survival in men with low- and high-risk unilateral or bilateral prostate cancer. CI, confidence interval; OR, odds ratio. Adapted with permission from Tareen B et al.14
In addition to treating unilateral disease, many have proposed that those patients with a dominant focus of cancer on 1 side of the gland with insignificant disease on the other would also be adequate candidates for HAT. Supporting that idea, Ohori and colleagues15 examined extent and localization of disease in a review of pathologic prostatectomy specimens and found that 80% of total tumor volume arose from the dominant focus. In addition to the volume, Gleason grade is an important predictor of oncologic outcomes. Noguchi and coworkers10 evaluated 222 men with stage T1c prostate cancer and found that secondary cancers in multifocal prostate tumors did not adversely differ from preoperative parameters, including PSA and biopsy findings. They did, however, find that presence and percentage of Gleason score 4/5 in biopsy and prostatectomy specimens was a predictor of biochemical failure after RP.
The concept of treating only the index tumor in selected men is based on a number of pathologic studies. Villers and coauthors16 showed that 80% of secondary tumors are less than 0.5 mL-a definition that has often been used to denote clinically indolent prostate cancers. Similarly, Rukstalis and associates17 found that the median size of ancillary lesions was 0.3 cc and proposed that 79% of men would likely have insignificant residual cancer if the index tumor was ablated.
Although treatment of the index tumor has been suggested, at this time there is no existing technology to allow for reliable disease mapping. Without this, there will always be the uncertainty of leaving a significant tumor untreated. Much like active surveillance, we would still be left with the lack of defined follow-up protocols for the remainder of the “normal” prostate that was not ablated. What remains to be answered is whether the residual disease following focal treatment would compromise long-term disease control.
It can safely be said that men who are not ideal for unifocal ablation are men with large, multifocal tumor burden, or those men with high-risk disease. A recent Task Force on Prostate Cancer and the Focal Lesion Paradigm proposed that men with clinical stage greater than T2a, PSA greater than 10 ng/mL, PSA density greater than 0.15 ng, or PSA velocity less than 2 ng/mL/y within the year prior to diagnosis, and no Gleason 4 or 5, are not suited for focal therapy.18
Identification of Candidates: How Do We Map the Prostate?
The major obstacle in appropriately administering focal therapy lies in proper identification of the ideal patient based on clinical and imaging parameters. Although numerous studies have allowed us to gain insight into the pathologic nature of prostate cancer, predictions of tumor volume currently must be made using a combination of biopsy and imaging.
Biopsy
The most common modality for diagnosing prostate cancer is office transrectal ultrasound (TRUS)-guided prostate biopsy. Despite advances and increased experience in use of ultrasound, biopsy remains a poor predictor of the extent of tumor on final pathologic specimen. In a review of 289 patients undergoing at least a sextant biopsy, Gregori and colleagues19 found that upgrading occurred in 40% and downgrading in at least 15%.
Sextant biopsy has a false-negative rate of approximately 30%.20 Even with extended 10- to 12-core biopsies, with additional lateral and apical sampling, there is significant upgrading when compared with final RP specimen.21,22
If patients with unilateral cancers are indeed ideal candidates for focal therapy, then we must question the possibility of using standard TRUS biopsy to predict laterality. Scales and coworkers12 recently undertook such a study evaluating 261 patients from the Shared Equal Access Regional Cancer Hospital (SEARCH) database with stage T1c Gleason 6 disease and found only a 35% positive predictive value of a unilateral biopsy when compared with final RP pathologic specimen.
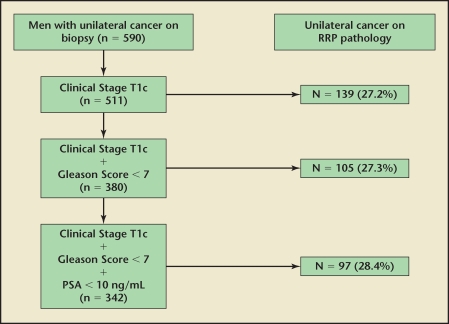
We found similar results when evaluating 590 patients from our database with unilateral cancer on biopsy. We found that only 26% of patients had true unilateral disease on final RP specimen.23 When we attempted to further stratify these patients on the basis of low risk (PSA < 10 ng/mL, clinical stage T1c, Gleason score < 7), we found that no clinical characteristic increased the accuracy of detecting unilateral cancer.
The next obvious question would be whether increasing the number of cores in a TRUS biopsy would increase the accuracy of tumor location prediction. Grossklaus and associates24 examined 135 patients undergoing RP and compared patients who had 6 or fewer biopsy cores with those who had more than 6 cores. They found no difference in the number of cores taken at time of biopsy in predicting pathologic stage or tumor laterality. In our own series, we found similar results and showed that the accuracy, whether it was 6, 8, or 12 cores, remained consistently 25% (Figure 4).
Figure 4.
Likelihood of unilateral disease on final radical prostatectomy specimen when unilateral prostate cancer is present on biopsy. PSA, prostate-specific antigen; RRP, radical retropubic prostatectomy. Adapted from Tareen B et al.23
Transperineal biopsies have traditionally been used in men with high index of suspicion (either from a rising PSA or rectal examination) and yet negative biopsy. Many have performed a perineal saturation biopsy using the classic brachytherapy template.25 Crawford and associates25 reported a 95% accuracy of disease laterality when using a perineal brachytherapy template using 5-mm sections. Computer-generated models were used to compare the accuracy to whole mount sections following prostatectomy. Similar results were found by Furuno and coauthors,26 who performed a similar template biopsy and found an 87% accuracy in comparison with surgical pathology.
Barzell and Melamed27 recently reported on their 4-year experience with transperineal 3-dimensional (3D) pathologic mapping of the prostate for selecting men for focal therapy. Of 80 patients undergoing extensive template-guided transperineal pathologic mapping of the prostate in conjunction with repeat TRUS-guided biopsies, they found 43% of patients unsuitable for focal therapy. They found that 3D mapping biopsy in comparison with repeat TRUS-guided biopsies found a false-negative rate of 47%, sensitivity of 54%, and negative predictive value of 49%. No pretreatment variables (eg, age, PSA, percentage free PSA, prostate volume, transition zone volume, Gleason score, TNM stage, number of positive cores, and maximum percentage of positive cores) correlated with patient suitability for focal therapy.27 This study was recently updated and in 100 patients referred for unilateral HAT, extended transperineal biopsy with an average of 46 cores showed a 23% upstaging and 55% bilateral disease compared with standard TRUS biopsy.28
Another model of 3-D prostate modeling is the TargetScan® (Envisioneering Medical Technologies, St. Louis, MO) system. Early results suggest that such a system has several benefits, including (1) reproducibility of biopsy technique and results; (2) the ability to accurately record biopsy sites and, therefore, cancer regions in a 2-coordinate system; (3) improved cancer detection and localization; and (4) better correlation of Gleason score with prostatectomy specimens than conventional TRUS-guided biopsy. In a study of RP specimens, TargetScan found 88% of cancers and correctly determined the presence or absence of cancer in 88% of prostate regions.29 A recent multi-institution retrospective review of the TargetScan system demonstrated a 47.6% cancer detection rate among patients undergoing first-time biopsy with a higher degree of concordance with prostatectomy specimens when compared with contemporary extended core TRUS biopsy.30 A similar device recently approved for use, the Artemis® (Eigen Corporation, Grass Valley, CA) biopsy system, converts 2-dimensional ultrasound into multiple dimensions and allows for individualized biopsy templates based on age, PSA, and ethnicity. Although the data are in the early stages, such technology in combination with further advancements in imaging may help to improve planning for focal therapy and allow for more accurate monitoring of cancer progression over time.
Even though templated mapping biopsies may prove to provide the accuracy needed to confidently administer focal therapy, they have several limitations. There is the theoretical risk of increased infection and 2 studies have reported a 10% increase in the rate of acute urinary retention following perineal biopsy.31,32 Another major concern stemming from saturation biopsy is the potentially increased morbidity of RP in patients who may exhibit increased scarring and abnormal tissue planes at the time of surgery.
Saturation template biopsy would likely require anesthesia because the average 20- to 40-mL gland would require at least 40 biopsy cores, which would be poorly tolerated in the office setting. Just as the surgeon administering brachytherapy must adjust for prostate manipulation from the needle, similar adjustments would need to be made for mapping.
Finally, the cost of the procedure is another important factor to be taken into consideration. Although templated biopsies may prove to ultimately be the method of choice to select patients, guidelines would have to establish which patients would be best suited for template biopsy, because the cost of performing such a procedure on all patients with low-risk disease would be significant.
Image-Guided Biopsy
Magnetic resonance imaging (MRI) has been proposed as an alternative imaging modality to ultrasound for guiding prostate biopsy. In 1 small series, studying a cohort of patients with prior negative TRUS biopsy, cancer was detected in 55.5% of patients undergoing MRI-guided transrectal biopsy.33 However, a more recent study suggests that the yield of MRI-guided biopsy is similar to repeat systematic TRUS-guided biopsy in this patient population.34 Despite these contrary early results, MR prostate imaging has a relatively high sensitivity for localized prostate cancer and is likely to have a growing role in active surveillance protocols and in identifying candidates for focal therapy.
Imaging
The use of MRI in prostate cancer has increased substantially over the past several years. Proposed uses include preoperative tumor staging and assessment of men with negative biopsy and high PSA for detection of occult cancers. Negative predictive value of 80% to 90% have been reported with this methodology.35 Other indications include AS and postoperative detection of recurrence.36,37
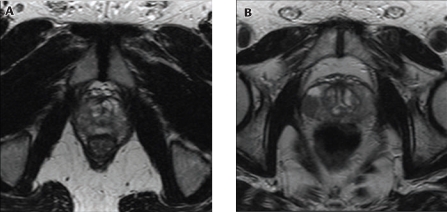
One of the most promising future uses of MRI may be in the area of focal therapy. Whereas template biopsy appears to have a high degree of accuracy for mapping prostate cancer, MRI provides a noninvasive means of potentially providing similar information without the side effects of saturation biopsies and a general anesthesia (Figure 5).
Figure 5.
(A) T2-weighted magnetic resonance imaging (MRI) scan showing bilateral prostate cancer. (B) Axial T2-weighted MRI shows unilateral right-sided prostate cancer in the peripheral zone mid-gland, in low T2 signal (arrow).
Primary reports for accuracy of endorectal MRI (using conventional T2-weighted imaging) in imaging prostate cancer show sensitivity up to 85% depending on the study design.38–40 Evaluation of the MRI literature must critically appraise not only type of MRI technique used, but also the definition of significant tumor, whether peripheral tumors only were included, and whether imaging findings were correlated with pathologic examination of the surgical specimen.
One of the limitations of MRI is the difficulty of diagnosing central gland cancer, due to overlapping appearances with benign prostate hyperplasia. Additionally, men who have had recent biopsies often present with hemorrhage in the peripheral zone, which limits MRI accuracy for tumor detection. Therefore, patients should be imaged at least 3 weeks after biopsy.
Real-time MRI/ultrasound fusion has recently been used to guide prostate biopsies. A pilot study of 2 National Cancer Institute trials was used recently to set up daily external radiation therapy.41 It is believed that this would provide a rapid way to facilitate MRI-guided prostate biopsies without the cost or equipment issues required for MRI-guided interventions in the MRI suite.41 Two other promising technologies using MRI include dynamic contrast-enhanced MRI and diffusion-weighted MRI.
We recently undertook a pilot study to evaluate whether MRI would improve the selection of men for unilateral HAT when combined with clinical and biopsy data. Our evaluation of 40 patients who underwent MRI prior to RP found that the negative predictive value of the “normal” lobe in men with suspected unilateral disease on final RP specimen could be improved by combining MRI findings with biopsy data.42
Methods of Focal Ablation of the Prostate
Cryotherapy has been used for over 2 decades for whole gland treatment of the prostate. Cryotherapy works by 3 processes: (1) direct cell damage from freeze-thaw cycle, (2) coagulation necrosis in the days following treatment, and (3) apoptosis (gene-regulated programmed cell death). The main complication with cryotherapy for whole gland ablation has been the high rates of impotence, ranging from 80% to 100%.43,44
Short-term efficacy has been reported in 1 multicenter trial of whole gland therapy showing 78% biochemical progression-free rates at 1 year. These results must be interpreted cautiously because 37% of these men received androgen deprivation.44 Bahn and colleagues43 have reported results at 7 years with progression-free probability between 61% and 92% in patients at low risk. They also reported that over 95% of these men were impotent following treatment. Biochemical-free survival has been difficult to define for cryotherapy with so many authors choosing to use radiation therapy definitions such as the American Society for Therapeutic Radiology and Oncology (ASTRO) and Phoenix criteria.
Early results of focal therapy via nerve-sparing HAT have been reported by a number of groups with encouraging outcomes.45–47 Onik and coworkers45 reported results of focal therapy on 48 men undergoing unilateral cryotherapy with at least 2 years of follow-up (mean follow-up, 4.5 years). They report a 94% PSA progression-free rate using ASTRO criteria48; 36 of 40 men with preoperative potency remained potent, whereas all 48 retained continence.28,45 Similar results were reported by Bahn and coworkers46 in 31 patients with mean follow-up of 70 months. They reported a 96% negative biopsy rate following treatment and 89% potency if men using (phosphodiesterase type 5 [PDE5]) inhibitors were included in their results (Table 2).
Table 2.
Results for Focal Therapy for Prostate Cancer
| Study | Patients (N) | Follow-Up (mo) | PSA-Free Progression | Potency (%) |
| Onik G et al45 | 48 | 24 mo minimum (4.5 mean) | 94% (ASTRO) | 90 |
| Bahn DK et al46 | 31 | 70 mo | 96% negative biopsy rate | 89 (including PDE5 inhibitors) |
| Lambert EH et al47 | 25 | Median 28 mo | PSA nadir < 50% baseline | |
| Ellis DS et al56 | 60 | Mean 15.2 ± 7.4 mo | 80.4% (ASTRO) | 70.6 at 12 mo |
| Muto S et al57 (HIFU) | 70 (29 focal) | Median 32 mo |
ASTRO, American Society for Therapeutic Radiology and Oncology; HIFU, high-intensity focused ultrasound; PDE5, phosphodiesterase type 5; PSA, prostate-specific antigen.
Definitions of biochemical recurrence-free progression are not well established and for men undergoing unilateral HAT this remains a challenging dilemma. Lambert and colleagues47 use a definition of PSA nadir < 50% from baseline in their report of 25 men undergoing unilateral cryotherapy with a median of 28 months follow-up. Longer follow-up and validation through other mature series will be needed to define the role of PSA and follow-up schemes during the postprocedure monitoring period.
High-Intensity Focused Ultrasound
High-intensity focused ultrasound (HIFU) has been used for treating malignancy in the kidney, liver, pancreas, and breast. In Europe it has been widely used for whole gland therapy for prostate cancer. Currently 2 systems are used: the Ablatherm® (EDAP TMS S.A., Vaulx-en-Velin, France) and the Sonablate® (Focus Surgery Inc., Indianapolis, IN). Both systems work by generating and focusing high-energy ultrasound waves at greater than 60°C. HIFU is truly a minimally invasive treatment in that there is no breach of skin or mucosa and among its benefits is real time ultrasound feedback of tissue destruction.
One of the major limitations of HIFU using the Ablatherm device for whole gland treatment of prostate cancer is the high rate of urinary retention following treatment. This has led many centers to routinely use concomitant transurethral resection of the prostate (TURP) to prevent this complication.49,50
The largest series of whole gland treatment with HIFU is reported by Poissonnier and associates,49 who report results in 227 patients and observed an 86% negative rate of biopsy at 3 months. PSA nadir typically occurred between 8 weeks and 4 months, ranging between 0.2 and 0.33 ng/mL. They reported a stricture rate of 12% and urinary incontinence rate of 13%. Results are somewhat difficult to interpret because the protocol was changed during the duration of the 9th year review. There was a significant rate of urinary retention and so they proposed concomitant TURP with the Ablatherm. Loss of potency was reported in 25% to 33%.
Photodynamic Therapy
Photodynamic therapy (PDT) is currently being explored for whole gland treatment in phase II trials for preliminary treatment of localized disease. PDT was first used to treat skin lesions and subsequently used for cancers involving central nervous system, head and neck, breast, and esophagus.
The principle behind PDT involves ablation using 3 elements: a photo-sensitizer, light source, and oxygen.51
The photosensitizer for prostate cancer is administered by intravenous infusion and is activated at the site of action by fiberoptic illumination at wavelengths that activate the photosensitizer.52 Numerous photosensitizers are currently under investigation and include Photofrin (Axcan Pharma, Birmingham, AL), Foscan (Biolitec Pharma, Dublin, Ireland), PhotoPoint (Miravant Medical Technologies, Santa Barbara, CA), LuTex (Pharmacyclics, Sunnyvale, CA), Visudyne (Novartis, East Hanover, NJ), and palladium-bacteriopheophorbide, or WST-11 (Stakel; Steba Biotech, Toussus-Le-Noble, France).
PDT using Tookad (WST-09; Negma-Lerads, Magny-Les-Hameaux, France) recently completed a phase I trial in 24 patients.53 Initial toxicity profiles were encouraging. Based on this early experience, a phase II trial enrolling 24 subjects is now approved and underway.
PDT was also reported in 15 patients following failure of radiation using Foscan as a photosensitizer. Nathan and coauthors54 reported that PSA decreased in 9 patients, but almost all eventually required androgen deprivation. There was a high rate of erectile dysfunction and urethral damage was reported in 4 patients, with 1 patient developing a rectourethral fistula.
There are currently 2 major trials underway using PDT: 1 in the United Kingdom for primary therapy for localized disease, and 1 in Canada for retreatment after failed external beam radiation.
Radiotherapy
Of all potential therapies for focal therapy, radiation therapy has the best-known biologic basis for tumor ablation. With improvements in intensity-modulated radiation therapy (IMRT) 3D imaging, radiation may provide an ideal means to deliver focal therapy to a precise target. At this point, this is very much experimental and future efforts would need to focus on defining dosing and the possibility of retreatment in the event of future development of cancer in the contralateral lobe.
Similarly, brachytherapy has been proposed as a means to apply focal radiation. Although brachytherapy has been touted as a modality with decreased rates of potency, studies have shown that in as early as 2 years, the potency from treatment equals that of RP.55
Challenges and Future Research
Focal therapy for low-risk prostate cancer is still in its infancy. Currently, we do have the basic technology to carry out focal therapy. The challenge lies in accurately, safely, and economically identifying men who are ideal candidates based on biopsy, clinical, and imaging data. Advances in biopsy technique, imaging, and molecular risk stratification will enhance our ability to select patients for focal therapy.
The important next steps should be to use an evidence-based approach to study the selection of ideal candidates and subsequently define successful oncologic outcomes of focal therapy. Attempting to set and meet guidelines for oncologic efficacy is a challenge we must embrace to safely deliver this potentially revolutionary approach to treating men with prostate cancer.
Main Points.
Focal therapy has been proposed in recent years as a means of bridging the gap between radical prostatectomy and active surveillance for treatment of prostate cancer. The rationale for focal therapy comes from its success in treating other malignancies.
Given the challenges of imaging and mapping tumors in the prostate, focal therapy via hemiablative therapy has been proposed as a means of identifying and treating men with low-stage prostate cancer.
With improvements in focal ablation delivery methods, men with unilateral or bilateral low-risk prostate cancer may be ideal candidates for focal therapy.
Despite advances and increased experience in the use of ultrasound, biopsy remains a poor predictor of the extent of tumor on final pathologic specimen. If patients with unilateral cancers are indeed ideal candidates for focal therapy, then we must question the possibility of using standard transrectal ultrasound biopsy to predict laterality.
Magnetic resonance imaging (MRI) has been proposed as an alternative imaging modality to ultrasound for guiding prostate biopsy. MR prostate imaging has a relatively high sensitivity for localized prostate cancer and is likely to play a growing role in active surveillance protocols and in identifying candidates for focal therapy.
High-intensity focused ultrasound is truly a minimally invasive treatment in that there is no breach of skin or mucosa and among its benefits is real time ultrasound feedback of tissue destruction.
Photodynamic therapy (PDT) is currently being explored for whole gland treatment in phase II trials for preliminary treatment of localized disease. The principle behind PDT involves ablation using 3 elements: a photosensitizer, light source, and oxygen.
Of all potential therapies for focal therapy, radiation therapy has the best-known biologic basis for tumor ablation. With improvements in intensity-modulated radiation therapy 3D imaging, radiation may provide an ideal means to deliver focal therapy to a precise target.
Figure 2.
Prostate-specific antigen recurrence-free survival in men with bilateral cancer and unilateral low- and high-risk disease. CI, confidence interval; OR, odds ratio. Adapted with permission from Tareen B et al.14
References
- 1.Loeb S, Gonzalez CM, Roehl KA, et al. Pathological characteristics of prostate cancer detected through prostate specific antigen based screening. J Urol. 2006;175:902–906. doi: 10.1016/S0022-5347(05)00327-7. [DOI] [PubMed] [Google Scholar]
- 2.Catalona WJ, Basler JW. Return of erections and urinary continence following nerve sparing radical retropubic prostatectomy. J Urol. 1993;150:905–907. doi: 10.1016/s0022-5347(17)35645-8. [DOI] [PubMed] [Google Scholar]
- 3.Menon M, Shrivastava A, Kaul S, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol. 2007;51:648–657. doi: 10.1016/j.eururo.2006.10.055. discussion 657–658. [DOI] [PubMed] [Google Scholar]
- 4.Marien T, Sankin A, Lepor H. Factors predicting preservation of erectile function in men undergoing open radical retropubic prostatectomy. J Urol. 2009;181:1817–1822. doi: 10.1016/j.juro.2008.11.105. [DOI] [PubMed] [Google Scholar]
- 5.Harlan SR, Cooperberg MR, Elkin EP, et al. Time trends and characteristics of men choosing watchful waiting for initial treatment of localized prostate cancer: results from CaPSURE. J Urol. 2003;170:1804–1807. doi: 10.1097/01.ju.0000091641.34674.11. [DOI] [PubMed] [Google Scholar]
- 6.Jones JS. Focal or subtotal therapy for early stage prostate cancer. Curr Treat Options Oncol. 2007;8:165–172. doi: 10.1007/s11864-007-0033-1. [DOI] [PubMed] [Google Scholar]
- 7.Morris AD, Morris RD, Wilson JF, et al. Breast-conserving therapy vs mastectomy in early-stage breast cancer: a meta-analysis of 10-year survival. Cancer J Sci Am. 1997;3:6–12. [PubMed] [Google Scholar]
- 8.Leibovich BC, Blute ML, Cheville JC, et al. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066–1070. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 9.Djavan B, Susani M, Bursa B, et al. Predictability and significance of multifocal prostate cancer in the radical prostatectomy specimen. Tech Urol. 1999;5:139–142. [PubMed] [Google Scholar]
- 10.Noguchi M, Stamey TA, McNeal JE, Nolley R. Prognostic factors for multifocal prostate cancer in radical prostatectomy specimens: lack of significance of secondary cancers. J Urol. 2003;170:459–463. doi: 10.1097/01.ju.0000070928.49986.04. [DOI] [PubMed] [Google Scholar]
- 11.Mouraviev V, Mayes JM, Sun L, et al. Prostate cancer laterality as a rationale of focal ablative therapy for the treatment of clinically localized prostate cancer. Cancer. 2007;110:906–910. doi: 10.1002/cncr.22858. [DOI] [PubMed] [Google Scholar]
- 12.Scales CD , Jr, Presti JC , Jr, Kane CJ, et al. Predicting unilateral prostate cancer based on biopsy features: implications for focal ablative therapy-results from the SEARCH database. J Urol. 2007;178:1249–1252. doi: 10.1016/j.juro.2007.05.151. [DOI] [PubMed] [Google Scholar]
- 13.Tareen B, Sankin A, Godoy G, et al. Appropriate candidates for hemiablative focal therapy are infrequently encountered among men selected for radical prostatectomy in contemporary cohort. Urology. 2009;73:351–354. doi: 10.1016/j.urology.2008.08.504. discussion 354–355. [DOI] [PubMed] [Google Scholar]
- 14.Tareen B, Godoy G, Sankin A, et al. Laterality alone should not drive selection of candidates for hemi-ablative focal therapy. J Urol. 2009;181:1082–1089. doi: 10.1016/j.juro.2008.10.155. discussion 1089–1090. [DOI] [PubMed] [Google Scholar]
- 15.Ohori M, Eastham J, Koh H, et al. Is focal therapy reasonable in patients with early stage prostate cancer (CaP)-an analysis of radical prostatectomy (RP) specimens. J Urol. 2006;75:507. Abstract 1574. [Google Scholar]
- 16.Villers A, McNeal JE, Freiha FS, Stamey TA. Multiple cancers in the prostate. Morphologic features of clinically recognized versus incidental tumors. Cancer. 1992;70:2313–2318. doi: 10.1002/1097-0142(19921101)70:9<2313::aid-cncr2820700917>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Rukstalis DB, Goldknopf JL, Crowley EM, Garcia FU. Prostate cryoablation: a scientific rationale for future modifications. Urology. 2002;60(suppl 1):19–25. doi: 10.1016/s0090-4295(02)01680-1. [DOI] [PubMed] [Google Scholar]
- 18.Eggener SE, Zelefsky MJ, Sartor O, et al. Focal therapy for localized prostate cancer: a critical appraisal of rationale and modalities. J Urol. 2007;178:2260–2267. doi: 10.1016/j.juro.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 19.Gregori A, Vieweg J, Dahm P, Paulson DF. Comparison of ultrasound-guided biopsies and prostatectomy specimens: predictive accuracy of Gleason score and tumor site. Urol Int. 2001;66:66–71. doi: 10.1159/000056573. [DOI] [PubMed] [Google Scholar]
- 20.Applewhite JC, Matlaga BR, McCullough DL. Results of the 5 region prostate biopsy method: the repeat biopsy population. J Urol. 2002;168:500–503. [PubMed] [Google Scholar]
- 21.Walz J, Graefen M, Chun FKH, et al. High incidence of prostate cancer detected by saturation biopsy after previous negative biopsy series. Eur Urol. 2006;50:498–505. doi: 10.1016/j.eururo.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Keetch DW, Catalona WJ, Smith DS. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol. 1994;151:1571–1574. doi: 10.1016/s0022-5347(17)35304-1. [DOI] [PubMed] [Google Scholar]
- 23.Tareen B, Godoy G, Sankin A, et al. Can contemporary transrectal prostate biopsy accurately select candidates for hemi-ablative focal therapy of prostate cancer? Br J Urol. 2009;104:195–199. doi: 10.1111/j.1464-410X.2009.08347.x. [DOI] [PubMed] [Google Scholar]
- 24.Grossklaus DJ, Coffey CS, Shappelle SB, et al. Prediction of tumour volume and pathological stage in radical prostatectomy specimens is not improved by taking more prostate needle-biopsy cores. BJU Int. 2001;88:722–726. doi: 10.1046/j.1464-4096.2001.02413.x. [DOI] [PubMed] [Google Scholar]
- 25.Crawford ED, Wilson SS, Torkko KC, et al. Clinical staging of prostate cancer: a computer-simulated study of transperineal prostate biopsy. BJU Int. 2005;96:999–1004. doi: 10.1111/j.1464-410X.2005.05801.x. [DOI] [PubMed] [Google Scholar]
- 26.Furuno T, Demura T, Kaneta T, et al. Difference of cancer core distribution between first and repeat biopsy in patients diagnosed by extensive transperineal ultrasound guided template prostate biopsy. Prostate. 2004;58:76–81. doi: 10.1002/pros.10298. [DOI] [PubMed] [Google Scholar]
- 27.Barzell WE, Melamed MR. Appropriate patient selection in the focal treatment of prostate cancer: the role of transperineal 3-dimensional pathologic mapping of the prostate-a 4-year experience. Urology. 2007;70(suppl 1):S27–S35. doi: 10.1016/j.urology.2007.06.1126. [DOI] [PubMed] [Google Scholar]
- 28.Onik G, Barzell W. Transperineal 3D mapping biopsy of the prostate: an essential tool in selecting patients for focal prostate cancer therapy. Urol Oncol. 2008;26:506–510. doi: 10.1016/j.urolonc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Andriole GL, Bullock TL, Belani JS, et al. Is there a better way to biopsy the prostate? Prospects for a novel transrectal systematic biopsy approach. Urology. 2007;70(suppl 1):S22–S26. doi: 10.1016/j.urology.2007.06.1128. [DOI] [PubMed] [Google Scholar]
- 30.Megwalu II, Ferguson GG, Wei JT, et al. Evaluation of a novel precision template-guided biopsy system for detecting prostate cancer. BJU Int. 2008;102:546–550. doi: 10.1111/j.1464-410X.2008.07832.x. [DOI] [PubMed] [Google Scholar]
- 31.Miller J, Perumalla C, Heap G. Complications of transrectal versus transperineal prostate biopsy. ANZ J Surg. 2005;75:48–50. doi: 10.1111/j.1445-2197.2005.03284.x. [DOI] [PubMed] [Google Scholar]
- 32.Pinkstaff DM, Igel TC, Petrou SP, et al. Systematic transperineal ultrasound-guided template biopsy of the prostate: three-year experience. Urology. 2005;65:735–739. doi: 10.1016/j.urology.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 33.Anastasiadis AG, Lichy MP, Nagele U, et al. MRI-guided biopsy of the prostate increases diagnostic performance in men with elevated or increasing PSA levels after previous negative TRUS biopsies. Eur Urol. 2006;50:738–748. doi: 10.1016/j.eururo.2006.03.007. discussion 748–749. [DOI] [PubMed] [Google Scholar]
- 34.Singh AK, Krieger A, Lattouf JB, et al. Patient selection determines the prostate cancer yield of dynamic contrast-enhanced magnetic resonance imaging-guided transrectal biopsies in a closed 3-Tesla scanner. BJU Int. 2008;101:181–185. doi: 10.1111/j.1464-410X.2007.07219.x. [DOI] [PubMed] [Google Scholar]
- 35.Noworolski SM, Henry RG, Vigneron DB, Kurhanewicz J. Dynamic contrast-enhanced MRI in normal and abnormal prostate tissues as defined by biopsy, MRI, and 3D MRSI. Magn Reson Med. 2005;53:249–255. doi: 10.1002/mrm.20374. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Mullerad M, Chen H-N, et al. Prostate cancer: incremental value of endorectal MR imaging findings for prediction of extracapsular extension. Radiology. 2004;232:133–139. doi: 10.1148/radiol.2321031086. [DOI] [PubMed] [Google Scholar]
- 37.Pucar D, Koutcher JA, Shah A, et al. Preliminary assessment of magnetic resonance spectroscopic imaging in predicting treatment outcome in patients with prostate cancer at high risk for relapse. Clin Prostate Cancer. 2004;3:174–181. doi: 10.3816/cgc.2004.n.028. [DOI] [PubMed] [Google Scholar]
- 38.Carter HB, Brem RF, Tempany CM, et al. Nonpalpable prostate cancer: detection with MR imaging. Radiology. 1991;178:523–525. doi: 10.1148/radiology.178.2.1987620. [DOI] [PubMed] [Google Scholar]
- 39.Hricak H, White S, Vigneron D, et al. Carcinoma of the prostate gland: MR imaging with pelvic phased-array coils versus integrated endorectal-pelvic phased-array coils. Radiology. 1994;193:703–709. doi: 10.1148/radiology.193.3.7972810. [DOI] [PubMed] [Google Scholar]
- 40.Jager GJ, Ruijter ET, van de Kaa CA, et al. Local staging of prostate cancer with endorectal MR imaging: correlation with histopathology. AJR Am J Roentgenol. 1996;166:845–852. doi: 10.2214/ajr.166.4.8610561. [DOI] [PubMed] [Google Scholar]
- 41.Singh AK, Kruecker J, Xu S, et al. Initial clinical experience with real-time transrectal ultrasonography-magnetic resonance imaging fusion-guided prostate biopsy. BJU Int. 2008;101:841–845. doi: 10.1111/j.1464-410X.2007.07348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tareen B, Ito T, Godoy G, et al. Can MRI improve the predictive ability of biopsy in selecting men for unilateral hemiablative focal therapy? J Urol. 2009;181(suppl):175–176. [Google Scholar]
- 43.Bahn DK, Lee F, Badalament R, et al. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urology. 2002;60(suppl 1):3–11. doi: 10.1016/s0090-4295(02)01678-3. [DOI] [PubMed] [Google Scholar]
- 44.Han KR, Cohen JK, Miller RJ, et al. Treatment of organ confined prostate cancer with third generation cryosurgery: preliminary multicenter experience. J Urol. 2003;170:1126–1130. doi: 10.1097/01.ju.0000087860.52991.a8. [DOI] [PubMed] [Google Scholar]
- 45.Onik G, Vaughan D, Lotenfoe R, et al. “Male lumpectomy”: focal therapy for prostate cancer using cryoablation. Urology. 2007;70(suppl 1):S16–S21. doi: 10.1016/j.urology.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Bahn DK, Silverman P, Lee F, et al. Focal prostate cryoablation: initial results show cancer control and potency preservation. J Endourol. 2006;20:688–692. doi: 10.1089/end.2006.20.688. [DOI] [PubMed] [Google Scholar]
- 47.Lambert EH, Bolte K, Masson P, Katz AE. Focal cryosurgery: encouraging health outcomes for unifocal prostate cancer. Urology. 2007;69:1117–1120. doi: 10.1016/j.urology.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 48.Consensus statement: guidelines for PSA following radiation therapy, authors. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 49.Poissonnier L, Chapelon J-Y, Rouvière O, et al. Control of prostate cancer by transrectal HIFU in 227 patients. Eur Urol. 2007;51:381–387. doi: 10.1016/j.eururo.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Uchida T, Ohkusa H, Nagata Y, et al. Treatment of localized prostate cancer using high-intensity focused ultrasound. BJU Int. 2006;97:56–61. doi: 10.1111/j.1464-410X.2006.05864.x. [DOI] [PubMed] [Google Scholar]
- 51.Lepor H. Vascular targeted photodynamic therapy for localized prostate cancer. Rev Urol. 2008;10:254–261. [PMC free article] [PubMed] [Google Scholar]
- 52.Eggener SE, Coleman JA. Focal treatment of prostate cancer with vascular-targeted photodynamic therapy. ScientificWorldJournal. 2008;8:963–973. doi: 10.1100/tsw.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trachtenberg J, Weersink RA, Davidson SRH, et al. Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: a study of escalating light doses. BJU Int. 2008;102:556–562. doi: 10.1111/j.1464-410X.2008.07753.x. [DOI] [PubMed] [Google Scholar]
- 54.Nathan TR, Whitelaw DE, Chang SC, et al. Photodynamic therapy for prostate cancer recurrence after radiotherapy: a phase I study. J Urol. 2002;168:1427–1432. doi: 10.1016/S0022-5347(05)64466-7. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez-Ortiz RF, Broderick GA, Rovner ES, et al. Erectile function and quality of life after interstitial radiation therapy for prostate cancer. Int J Impot Res. 2000;12(suppl 3):S18–S24. doi: 10.1038/sj.ijir.3900557. [DOI] [PubMed] [Google Scholar]
- 56.Ellis DS, Manny TB , Jr, Rewcastle JC. Focal cryosurgery followed by penile rehabilitation as primary treatment for localized prostate cancer: initial results. Urology. 2007;70(suppl 1):S9–S15. doi: 10.1016/j.urology.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 57.Muto S, Yoshii T, Saito K, et al. Focal therapy with high-intensity-focused ultrasound in the treatment of localized prostate cancer. Jpn J Clin Oncol. 2008;2008;(38):192–199. doi: 10.1093/jjco/hym173. [DOI] [PubMed] [Google Scholar]