Abstract
Bone is the most preferred site for metastatic dissemination in breast cancer. The purpose of this study was to examine the expression of a set of antibodies that could serve as predictive biomarkers associated with breast cancer metastasis in a subset of sixteen (16) breast cancer patients who developed bone metastasis. The clinical and pathologic data were obtained, and tissue microarrays were constructed. Tissue microarray slides were stained for TFF-1, CXRC4, MMP1, PTHrP, HER2, CD44, FGFR3 and IL-11. The expression rates were compared between the metastatic breast cancer to bone (MBC-B) group and a group of sixty-four (64) primary breast cancer (PBC). The results demonstrated that MBC-B group patients were more likely to be HER2 positive (P = 0.016). There was no significant difference on estrogen receptor or progesterone receptor expression between MBC-B group and PBC group (P > 0.05). There was a high expression of CXCR4, MMP-1, CD44, TFF-1, PTHrP, FGFR3 and IL-11, in both, PBC and MBC-B, and no significant differences between the groups were identified. We found that tumors associated with bone metastasis tended to be larger than 2 cm. The high morbidity associated to metastatic breast cancer prompts the identification of predictive biomarkers of relapse of breast tumors to categorize patients at high risk of bone metastasis and serve as targeted therapy.
Keywords: Breast cancer, bone metastasis, immunohistochemistry, HER2, ER, TFF-1
Introduction
Breast cancer is the most common female cancer in the world and the bone is its most preferred metastatic site [1-2]. Overall, 6575% of patients with advanced disease will develop bone relapse [1], the cause of highly devastating conditions including pain, pathological bone fracture and spinal cord compression, that ultimately decrease the quality of life. The surveillance and prompt detection of early bone metastasis (BM) might prevent the development of such complications, but the heterogeneity of the breast cancer disease has been the main obstacle in the understanding of the metastatic process, and the biology of the distant site to which the breast tumor preferentially relapses [3-4].
Metastasis is a multistep process requiring the coordinated expression of many protein products. The “seed and soil” theory described by Paget showed that different carcinomas have distinct patterns of metastasis [5]; Paget's theory has served researchers in the study of metastatic disease [6]. The growing of tumor cells in remote sites requires of multiple interactions between the cancer cells and the specific organ, and recent studies using animal models and human breast cancer cell lines have shown that bone metastasis may not occur unless specific genes are expressed [2]. However, many of these genes are still unknown [3], and perhaps, the discovery of molecular gene signatures may be able to predict in the near future, tumors with high metastatic potential, and secondarily, identify targeted therapies.
The purpose of this study was to examine the expression of a set of immunohistochemical markers (CXCR4, TFF-1, CD44, IL-11, HER2, MMP-1 and PTHrP) that were chosen from previously published studies, by using cohorts of primary and metastatic breast cancer tumors to bone [3,7-9] and are summarized in Table 1. We hypothesized that this group of markers can identify breast cancer patients at high risk of metastatic bone disease. We identified that some of these markers may be useful as predictors of high risk of bone relapse in breast cancer metastatic disease and are applicable in the routine pathology practice.
Table 1.
Selected studies
| Author | Antibody | Result |
|---|---|---|
| Smid et al [3] | TFF1 | TFF1 expression is positively correlated with tumor relapse to bone by RT-PCR. |
| Guise TA [7] | PTHrP | PTHrP may have a role in osteolytic bone lesions in breast cancer |
| Kang et al [8] | IL-11 CXCR4 MMP-1 | Overexpression of bone metastasis gene set in osteolytic metastasis formation by microarray analysis of breast cancer cells |
| Muller et al [9] | CXCR4 | High expression of CXCR4 in breast cancer cell lines, malignant breast tumors andmetastasis by flow cytometry, IHC and mRNA expression |
IHC indicates immunohistochemistry; RT-PCR reverse transcriptase polymerase chain.
Materials and methods
Patients and tissue specimens
Sixteen (16) metastatic breast cancers to bone (MBC-B) formalin-fixed paraffin embedded tissue (FFPET) blocks were randomly obtained from the Magee-Women's Hospital (MWH) tumor registry at the University of Pittsburgh Medical center in Pittsburgh, Pennsylvania. Sixty-four (64) primary breast cancers (PBC) without bone metastatic disease FFPET blocks where used as controls. Clinical data and pathological variables were obtained following a medical record review. Areas of invasive adenocarcinoma were identified on corresponding hematoxylin and eosin stained slides. A tissue microarray with 3-fold redundancy was created for these cases, consisting of cores with a diameter of 0.6 mm. The data from MBC-B tumors was compared against data from PBC tumors from patients who remained free of metastases.
Immunohistochemistry
The protein expression was assessed by using serial 5-um thick sections of each TMA containing specimens obtained from MBC-B and PBC tumors. Slides were deparaffinized using two xylene exchanges followed by rehydration through an ethanol gradient. All cases were immunostained with rabbit polyclonal PAX2 antibody (clone Z-RX2, Invitrogen; Carlsbad, CA) on the BenchMark XT automated stainer (Ventana Medical Systems, Inc; Tucson, AZ). Primary antibodies and dilutions were as follows: Trefoil Factor 1 (TFF-1) (a.k.a. Estrogen inducible protein pS2) (Abcam Inc, clone SPM313, predilute), Collagenase 1 (MMP-1) (Lab Vision, RB-1536, rabbit polyclonal, 1:40), CXCR4 (Abcam Inc, ab2074, rabbit polyclonal, 1:100), Parathyroid hormone-related protein (PTHrP) (Cosmo Bio Co., LTD/YII-Y201-EX, rabbit polyclonal, 1:100), CD44 (Dako/M7082, clone DF1485, 1:20), c-erbB2 (Ventana Medical, 4B5, predilute), IL-11 (Santa Cruz/ Sc7924, rabbit polyclonal, 1:40), FGFR3 (Lab Vision/RB-10248, rabbit polyclonal, 1:40). For CXCR4, PTHrP, IL-11, CD44 and FGFR3, heat-induced epitope retrieval was carried out by placing tissue sections in Dako Target retrieval solution (TRS, pH 6.0), and maintaining heat in a steamer 96C for 20 minutes. After cooling down, the slides were incubated in the primary antibodies. The retrieval methods for c-erbB2 (CC1/ pH 8.0), MMP-1 (Steamer 20'/Trilogy; Cell Marque) and TFF-1 (Protease 1, 4 minutes) were applied. Positive and negative controls were used. Immunohistochemical stains were reviewed by one pathologist (M.C.), and stains were considered positive if more than 1% of the tumor cells showed reactivity. The staining pattern was evaluated qualitatively (positive or negative), according to the specific antibody as follows: PTHrP (cytoplasmic), CD44 (membrane), TFF-1 (cytoplasmic and nuclear), MMP1 (cytoplasmic), CXCR4 (cytoplasmic and membrane), IL-11 (nuclear), HER2 (membrane) and FGFR3 (nuclear). Staining for HER2 was scored on 0/1+ (negative), 2+ (equivocal) and 3+ (positive) according to manufacturer's recommendations. The results were compared with clinical and pathological parameters.
Statistical analysis
Fisher's exact test was used to compare two proportions and generate the statistical significance (P- values smaller than 0.05) for clinical features and immunohistochemical markers that were differentially expressed between the groups.
Results
Clinical and pathologic data
A summary of the clinical and pathologic findings of the bone metastasis and control patients without metastasis, including time of bone relapse (>1 year or < 1year), estrogen receptor (ER) status, progesterone receptor (PgR) status, histological type of tumor, tumor size and lymph node status were recorded and are shown in Table 2.
Table 2.
Clinical and pathologic data
| Clinical Features | MBCB | PBC | |
|---|---|---|---|
| (n =16) | (n = 64) | ||
| ER | Negative | 6.25% (1/16) | 14% (9/64) |
| Positive | 93.75% (15/16) | 86% (55/64) | |
| PgR | Negative | 12.5% (2/16) | 22% (14/64) |
| Positive | 81.25% (13/16) | 78% (50/64) | |
| Unknown | 6.25% (1/16) | 0% (0/64) | |
| HER2 | Negative | 50% (8/16) | 91% (58/64) |
| Positive | 18.75% (3/16) | 9% (6/64) | |
| Equivocal | 18.75% (3/16) | 0% (0/64) | |
| Unknown | 12.5% (2/16) | 0% (0/64) | |
| BR | Rapid | 43.75% (7/16) | N/A |
| Slow | 56.25% (9/16) | N/A | |
| Type | Ductal | 87.5% (14/16) | 81.25% (52/64) |
| Lobular | 6.25% (1/16) | 14% (9/64) | |
| Mixed | 6.25% (1/16) | 3% (2/64) | |
| Metaplastic | 0% (0/16) | 1.55% (1/64) | |
| Node status | Positive | 50% (8/16) | 40.6% (26/64) |
| Negative | 50% (8/16) | 53.2% (34/64) | |
| Unknown | 0% (0/16) | 6.2% (4/64) | |
| Tumor size | <2 cm | 25% (4/16) | 57.8% (37/64) |
| >2 cm | 50% (8/16) | 29.7% (19/64) | |
| >5 cm | 18.75% (3/16) | 10.9% ('7/64) | |
| Unknown | 6.25% (1/16) | 1.6% (1/64) | |
ER indicates estrogen receptor; PgR progesterone receptor; BR bone relapse.
In the MCB-B group, 14 (87.5%) tumors had invasive ductal carcinoma (IDC). The average of tumor size was 2.6 cm (ranging from 0.4 to 8 cm); axillary node metastasis was found in the 50% of the cases. All patients received standard chemotherapy and hormonal treatment. Seven (43.75%) out of 16 tumors were considered to have rapid bone metastasis progression, with a relapse to bone occurring in less than 1 year. Similarly, in nine (56.25%) of the cases, the recurrence occurred in more than 1 year. In the PBC group, 52 (81.25%) had IDC. The average of tumor size was 2.6 cm (ranging from 0.8 cm to 10 cm); axillary lymph node metastasis was identified in 40.6% (26/64) cases. Sixty-six (66%, 42/64) patients were Stage 1; 24% (15/64) were stage 2, 8% (5/64) were stage 3; 1% (1/64) were stage 4 tumors and 1% (1/64) the stage was unknown. Most patients received standard chemotherapy/hormonal Treatment.
Expression rates of ER and PgR alone showed no significant difference between MBC-B and PBC groups (P > 0.05). Breast cancers with BM were more likely to be HER2 positive compared with PBC tumors (18.5% vs 9%, P =0.016). Patients with tumors larger than 2 cm were more likely found in the MBC-B group than PBC (68.75% and 40.6%, P = 0.042). MBC-B tumors were more frequently seen with nodal metastasis than primary tumors without BM (50% and 40.6%), even though there was no significant difference (P = 0.779). Histological type was not associated to BM (P = 0.578).
Immunohistochemical findings
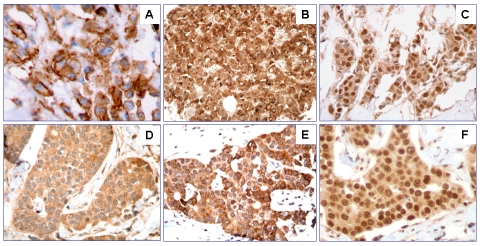
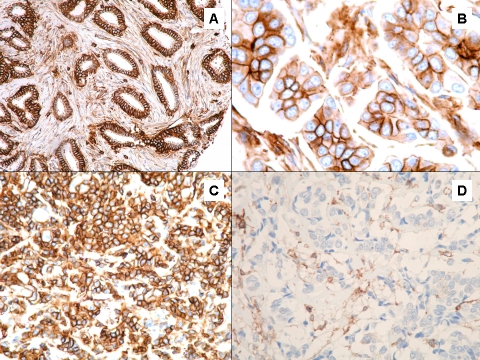
The immunohistochemical results for PBC and MBC-B for slow and rapid progression are summarized in Table 3. CD44 expression was present in 86% of MBC-B rapid progression tumors and in the 100% of the MBC-B slow progression tumors (Figure 1A). Overall, MBC-B tumors showed higher expression of CD44 (94%) compared with patients who did not develop BM (89%) (Figure 2). In addition, the tumors from patients who developed bone metastasis also showed higher expression of TFF-1 compared with patients with no relapsing disease to bone (88% and 83%) (Figure 1B). However, the differences in expression of CD44 and TFF-1 were no statistically significant (P > 0.05). Expression rates of CXCR4 (Figure 1C), MMP-1 (Figure 1D), PTHrP (Figure 1E), FGFR3 and IL-11 (Figure 1F) showed no significant difference between metastatic tumors to bone and primary tumors.
Table 3.
Immunoprofile of MBC-B versus PBC
| n | TFF1 (%) | MMP1 (%) | CXCR4 (%) | PTHrP (%) | CD44 (%) | FGFR-3 (%) | IL-11 (%) | |
|---|---|---|---|---|---|---|---|---|
| MBC-B RAPID | 7 | 86 | 100 | 100 | 100 | 86 | 100 | 100 |
| MBC-B SLOW | 9 | 89 | 100 | 100 | 100 | 100 | 100 | 100 |
| PBC | 64 | 83 | 100 | 100 | 100 | 89 | 100 | 100 |
MBC-B indicates metastatic breast cancer to bone; PBC primary breast
Figure 1.
CD44 protein expression in metastatic breast cancer (A), TFF-1 protein expression (B), CXCR4 protein expression (C), MMP-1 protein expression (D), PTHrP protein expression (E), IL-11 protein expression (F).
Figure 2.
CD44 expression in metastatic breast cancer (A-B), CD44 expression in primary breast cancer (C-D).
Discussion
Breast cancer heterogeneity is the main obstacle to successful identification of breast cancer with metastatic potential [2]. In this study, our strategy was to identify if a group of immunohistochemical markers, chosen based on review of previously published studies, could predict which patients are going to develop bone relapse [3,7-9].
Metastatic breast cancer is a complex, nonrandom [10], sequential multi-step process that requires the expression of specific genes that act in concert [8, 11]. In addition, cooperation of numerous molecules has been observed and the mechanisms of metastasis are not fully understood.
Recent data from gene expression profiling studies using patient's samples have provided breast to bone gene signatures able to identify patients with high risk of distant recurrence [12]. Although the identification of those signatures has been of a great value providing insight for detection of potential biomarkers, the limitations of microarray studies are mainly due to the lack of overlapping genes between signatures and similarly, the lack of reproducibility.
Gene molecular profiling studies have provided information suggestive of ER status as a stronger predictor of MBC, indicating that probably, the biology of MBC is intrinsically related to the biology of the ER. Smid et al [3, 13] found that five genes are highly expresses in samples from patients with relapse to bone. TFF-1 (pS2) was one of such genes, a partner in the ERα pathway, which has been found co-expressed with GATA3 and ERα [14]. Herein, we showed that MBC-B had higher expression of ER than PBC (93.75% versus 86%), a result similar to that of Wei et al (85% vs 59%) [15]. Furthermore, in agreement with previously published studies, we found that MBC-B tumors have a higher expression of TFF-1 in comparison to PBC tumors (88% versus 83%). The co-expression of TFF-1 and ER in MBC-B confirms its close relationship and perhaps, its predictive significance; however, in this study, when comparing the MBC-B and PBC groups, no statistically significant differences were seen.
The pattern of metastatic involvement and dissemination is distinctive for each organ, and the establishment and growth of metastasis depend on interactions between tumor cells and the microenvironment. Breast cancer tumor cells can disseminate very early, as detected previously in bone marrow of patients with early-stage disease [16]. The organ-spe-cific metastasis of breast cancer cells requires the expression of distinctive molecules and receptors [8, 11]. In a recent study, Hicks et al showed that breast cancer tumors metastatic to brain are more likely to be ER-negative and express basal cytokeratin, HER2 or EGFR [17].
In contrast, Wei et al reported that majority of breast cancer tumors that tend to relapse to bone are ER-positive, and found no differences in expression rates of HER2 in breast cancer without BM and those with BM [15]. Similarly, chemokine receptors are involved in cancer metastasis [9], and a high expression of CXCR4 has been implicated in non small cell cancer, melanoma, colorectal cancer, breast cancer and oral squamous cell carcinoma [10, 18-20]; the aberrant nuclear expression of CXCR4 has been observed by Na et al [21] in a group of non-small cell lung cancer, and considered as a factor associated with lymph node metastasis. We did not see a significant difference in nuclear and cytoplasmic CXCR4 expression in PBC and MBC-B, and nearly all tumors showed strong immunoreactivity. Possible explanations to this finding could be related to the specific molecular mechanisms responsible of nodal metastases, the intrinsic tumor biology and the differences related to the antibody.
Breast metastatic bone disease have the proclivity to cause osteolytic lesions; growth in the bone requires the ability to promote bone resorption by inducing osteoclasts to secrete PTHrP, tumor necrosis factor α (TNFα) and IL11 [16]. PTHrP is recognized as responsible agent for increased bone resorption in the hypercalcemia of the malignancy. PTHrP expression has been reported in the 60% of PBC and in a subset of patient who developed BM. In fact, in our study PTHrP expression was present in all MBC-B and PBC tumors, and no differences in between the two groups were identified.
Kang et al generated a gene signature, and found that IL-11 and MMP-1 and CXCR4 were highly overexpressed in metastatic cells to bone, functionally cooperating to form osteolytic lesions in athymic nude mice [8]. Although in our study we found diffuse and strong expression of these markers, we were not able to identify a distinctive expression and all of the MCB-B and PBC tumors showed immunoreactivity for such immunomarkers. Perhaps, the explanation to the discrepancy in the results might be attributed to the type of approach and methodology (e.g. gene versus protein expression) and the use of animal models and human breast cell lines instead of human breast tissue tumor.
High levels of CD44 in breast cancer cells, a hyaluronan (HA) receptor, have been linked to breast cancer invasion. Some studies have suggested that binding of HA to CD44 is assoalso found in this study that the proportion of HER2-positive cases in the PBC group is smaller than the published in previous studies which show that HER2 expression is identified in approximately 20 to 30% of invasive breast cancer [24-25]. Possible explanations to this issue include the small number of patients in our MBC-B group or a selection bias in PBC ciated to activation of the HER2/neu receptor [22]. Wobus et al found that CD44-EGFR-erbB2 protein complexes occur in a high proportion of metastatic mammary carcinomas [23]. We demonstrated a higher expression of CD44 in MBC-B in comparison to PBC (94% vs 89%). The immunohistochemical expression was strong and diffuse in MBC-B and variable in PBC (Figure 2), but this finding was not statistically significant (P = 0.49). Similarly, we found that HER2 overexpression was more likely seen in MBC-B group (18.75%) than PBC group (9%). Although we identified a higher expression of CD44 and HER2 in MBC-B than PBC, we were not able to identify a significant association in between those biomarkers. We group; however, the results of the present study are in agreement with the pathobiology of the disease and the proportion of HER2 positive tumors is higher among tumors with higher grade. The expression of HER2 has been associated with more aggressive tumors, increased relapse and shorter survival [24-25], and in addition, several studies have showed increased risk of brain metastatic disease inpatient with HER2 overexpressed/amplified tumors [25-26].
In summary, we identified a high protein expression of CXCR4, MMP-1, PTHrP, FGFR3, CD44, TFF-1 and IL-11 in both, MBC-B and PBC tumors using immunohistochemical staining on TMA sections. Furthermore, the results of our study have suggested that a group of HER2-positive tumors have an increased risk for bone metastasis. The significant difference in expression indicates that its identification can serve as a tool to guide therapeutic decision-making. We are aware that the limitations of this study are due to the small sample number of metastatic tumors to bone; however, we believe that this study supports the importance of ER and ER-related genes in the bone metastatic process, and its is likely that a group of immunohistochemical markers including HER2/ER/TFF-1/CD44 may serve as a tool to identify patients with increased risk for bone metastasis. These results need to be confirmed in larger studies.
References
- 1.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Kominsky SL, Davidson NE. A “bone” fide predictor of metastasis? Predicting breast cancer metastasis to bone. J Clin Oncol. 2006;24:2227–2229. doi: 10.1200/JCO.2005.05.5319. [DOI] [PubMed] [Google Scholar]
- 3.Smid M, Wang Y, Klijn JG, Sieuwerts AM, Zhang Y, Atkins D, Martens JW, Foekens JA. Genes associated with breast cancer metastatic to bone. J Clin Oncol. 2006;24:2261–2267. doi: 10.1200/JCO.2005.03.8802. [DOI] [PubMed] [Google Scholar]
- 4.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 5.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 6.Rose AA, Siegel PM. Breast cancer-derived factors facilitate osteolytic bone metastasis. Bull Cancer. 2006;93:931–943. [PubMed] [Google Scholar]
- 7.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98:1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 9.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25:345–356. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 11.Gupta GP, Minn AJ, Kang Y, Siegel PM, Serganova I, Cordon-Cardo C, Olshen AB, Gerald WL, Massague J. Identifying site-specific metastasis genes and functions. Cold Spring Harb Symp Quant Biol. 2005;70:149–158. doi: 10.1101/sqb.2005.70.018. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 13.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 14.Wilson BJ, Giguere V. Meta-analysis of human cancer microarrays reveals GATA3 is integral to the estrogen receptor alpha pathway. Mol Cancer. 2008;7:49. doi: 10.1186/1476-4598-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei B, Wang J, Bourne P, Yang Q, Hicks D, Bu H, Tang P. Bone metastasis is strongly associated with estrogen receptor-positive/progesterone receptor-negative breast carcinomas. Hum Pathol. 2008;39:1809–1815. doi: 10.1016/j.humpath.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hicks DG, Short SM, Prescott NL, Tarr SM, Coleman KA, Yoder BJ, Crowe JP, Choueiri TK, Dawson AE, Budd GT, Tubbs RR, Casey G, Weil RJ. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 18.Uchida D, Begum NM, Tomizuka Y, Bando T, Almofti A, Yoshida H, Sato M. Acquisition of lymph node, but not distant metastatic potentials, by the overexpression of CXCR4 in human oral squamous cell carcinoma. Lab Invest. 2004;84:1538–1546. doi: 10.1038/labinvest.3700190. [DOI] [PubMed] [Google Scholar]
- 19.Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gonner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–1750. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 20.Murakami T, Maki W, Cardones AR, Fang H, Tun KA, Nestle FO, Hwang ST. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- 21.Na IK, Scheibenbogen C, Adam C, Stroux A, Ghadjar P, Thiel E, Keilholz U, Coupland SE. Nuclear expression of CXCR4 in tumor cells of non-small cell lung cancer is correlated with lymph node metastasis. Hum Pathol. 2008;39:1751–1755. doi: 10.1016/j.humpath.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Rose AA, Siegel PM. Breast cancer-derived factors facilitate osteolytic bone metastasis. Bull Cancer. 2006;93:931–943. [PubMed] [Google Scholar]
- 23.Wobus M, Rangwala R, Sheyn I, Hennigan R, Coila B, Lower EE, Yassin RS, Sherman LS. CD44 associates with EGFR and erbB2 in metastasizing mammary carcinoma cells. Appl Immunohistochem Mol Morphol. 2002;10:34–39. doi: 10.1097/00129039-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Kallioniemi OP, Holli K, Visakorpi T, Koivula T, Helin HH, Isola JJ. Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int J Cancer. 1991;49:650–655. doi: 10.1002/ijc.2910490504. [DOI] [PubMed] [Google Scholar]
- 25.Duchnowska R, Dziadziuszko R, Czartoryska-Arlukowicz B, Radecka B, Szostakiewicz B, Sosinska-Mielcarek K, Karpinska A, Staroslawska E, Kubiatowski T, Szczylik C. Risk factors for brain relapse in HER2-positive metastatic breast cancer patients. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-008-0275-z. Jan 7[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Altaha R, Crowell E, Hobbs G, Higa G, Abraham J. Increased risk of brain metastases in patients with HER-2/neu-positive breast carcinoma. Cancer. 2005;103:442–443. doi: 10.1002/cncr.20813. [DOI] [PubMed] [Google Scholar]