Abstract
Human aldo-keto reductase (AKR) 1C3 is a monomeric cytoplasmic multifunctional enzyme that reduces ketosteroids, ketoprostaglandins, and lipid aldehydes. AKR1C3 was initially identified as an enzyme involved in steroid metabolism. However, immunohistochemistry has demonstrated AKR1C3 in normal adult kidneys with expression in Bowman' capsule, the mesangial cells, proximal and distal tubules, as well as mature urothelial epithelium. The significance of its spatial distribution and metabolic activities in the kidney remains undefined. In addition to its ability to catalyze steroid hormones (including androgen, desoxycorticosterone, and progesterone) and involvement in prostaglandins metabolism, we suspect that AKR1C3 may function as a chemical barrier in the renal tubules for normal function in mature kidneys. Moreover, AKR1C3 may represent a developmental marker for some urological epithelial tissues. In this study, we demonstrate widespread expression of AKR1C3 in renal neoplasms with a phenotype recapitulating mature kidney (i.e., renal cell carcinoma) and urothelium also known as transitional epithelium (i.e., papillary urothelial carcinoma), but noted limited AKR1C3 expression in renal neoplasms with a phenotype recapitulating embryonic kidneys (i.e., Wilms' tumor). Our results suggest that AKR1C3 may represent a developmental marker that is related to renal epithelium maturity.
Keywords: Aldo-keto reductase, renal cell carcinoma, papillary urothelial carcinoma, Wilms' tumor, kidney cancer
Introduction
The aldo-keto reductases (AKRs) comprise a functionally diverse 15 gene family [1]. Members of the AKR superfamily are generally monomeric (37 kD) cytosolic NAD(P)(H)-de-pendent oxidoreductases that share a common (α/β)8-barrel structural motif and convert carbonyl groups to primary or secondary alcohols (visit: www.med.upenn.edu/akr) [2]. Members of the AKR1C family are involved in the formation and removal of lipophilic hormones.
Natural substrates for the family enzymes include steroids, prostaglandins (PGs), and lipid aldehydes [3]. In humans, at least four AKR1C isoforms exist; namely AKR1C1 [20α(3α)-hydroxysteroid dehydrogenase (HSD)] [4], AKR1C2 (type 3 3α-HSD) [5, 6], AKR1C3 (type 2 3α/type 5 17β-HSD) [7, 8], and AKR1C4 (type 1 3α-HSD) [6].
Originally cloned from human prostate [8] and placental cDNA libraries [9], AKR1C3 is known to catalyze androgen, estrogen, and PG metabolism by the enzyme's 3α-hydroxysteroid dehydrogenase (HSD), 17β-HSD, and 11-keto-prostaglandin reductase activities, respectively [8, 10, 11]. As a result, AKR1C3 is capable of governing ligand access to various nuclear receptors [12]. Deregulated expression of AKR1C3 has been demonstrated in multiple types of cancers, including myelodysplastic syndrome (MDS, refractory anemia) [13], breast cancer [14], endometrial cancer [15], lung cancer [16], as well as localized [17, 18] and advanced [19, 20] prostate cancer.
Renal neoplasms comprise a heterogenous group of neoplasms with different phenotypic profiles. Renal cell carcinoma (RCC) is a tumor that recapitulates the phenotypic features of mature renal tubules. It represents over 90% of malignant renal neoplasms in both men and women and is associated with several genetic aberrations [21]. While RCC occurs only rarely in children, Wilms' tumor (WT) is the most common childhood renal neoplasm that rarely occurs in adults. WTs accounts for 90% of childhood renal malignancy and 6% of all pediatric cancers [22]. Phenotypically, WTs closely recapitulate features of the embryonic kidney. Papillary urothelial carcinoma (PUC) phenotypically recapitulate features of mature urotheliuim [23] and is the most common malignant urinary bladder cancer in adults.
We have demonstrated AKR1C3 expression in normal adult kidney [24]. AKR1C3 expression is noted in Bowman's capsule, mesangial cells, convoluted tubules, and pelvic urothelial epithelium. In this report, we demonstrate positive AKR1C3 immunoreactivity in tumors that recapitulate mature urogenital tissues, namely RCC and PUC. In contrast, AKR1C3 immunoreactivity in WTs, a tumor that recapitulates the embryonic kidney, varies from sporadic to widespread. In particular, components with tubule formation indicative of mature differentiation in WTs demonstrate the strongest AKR1C3 immunoreactivity. These results suggest that AKR1C3 might be involved in embryonic development and maturation of kidney and potentially represents a cell maturation marker.
Materials and methods
Materials
Mouse anti-human AKR1C3 monoclonal antibody was produced as described [25]. Goat serum and stable diaminobenzidine tetra-hydrochloride (DAB) were purchased from Invitrogen (Carlsbad, CA). Biotinylated goat anti-mouse IgG antibody and horseradish peroxidase (HRP)-conjugated streptavidin were obtained from Vector Laboratories (Burlingame, CA). Hematoxylin and permount mounting media were obtained from Sigma-Aldrich (St. Louis, MO).
Human Tissues
Archival, formalin fixed, paraffin embedded human normal kidney, kidney cancer, and WT samples were obtained from the Departments of Pathology and Urology and the protocol was approved by the University of Oklahoma Health Sciences Center Institutional Review Board (IRB).
Immunohistochemistry of Tissue Sections
Immunohistochemistry of human tissue sections followed our previously reported procedures [18]. Briefly, tissue sections cut about 46 μm were mounted and baked at 60°C for 1 hr. Sections were de-paraffinized with xylene and re-hydrated in graded ethanol followed by rinses with 0.1 M Tris-HCl (pH 7.6). Endogenous peroxidase activity was blocked by incubating the sections with 1.6% H2O2 in methanol for 30 min. Antigen retrieval was performed with 0.01 M sodium citric acid buffer (pH 6.0) at 95°C for 1 hr. Non-specific binding was blocked by incubating the tissue sections with 0.1 M Tris-HCl containing 10% goat serum for 2 hr. AKR1C3 was then detected by incubated the sections with the mouse anti-AKR1C3 monoclonal antibody (clone NP6G6.A6) at a 1:200 dilution in the above blocking solution, and incubated in a moist chamber at 4°C overnight. Negative controls were performed in parallel in the absence of the primary antibody. After washes with 0.1 M Tris-HCl, the slides were incubated with 1:400 dilution of biotinylated horse anti-mouse secondary antibody and incubated at room temperature for 2 hr. Following another rinses with 0.1 M Tris-HCl, antibody binding was detected by incubating the sectioins with HRP-conjugated streptavidin at room temperature for 30 min. DAB-H2O2 substrate was then added to the slides and incubated at room temperature for an additional 4 min. Tissue sections were counter stained lightly with hematoxylin, dehydrated in graded alcohol, cleared in xylene, and mounted with Permount Mounting Media for visualization by light-microscopy.
Results
Expression of AKR1C3 in Adult RCC and PUC
Medium to strong immunoreactivity for AKR1C3 was widely detected (over 75% of tumor) in 86.6% (13 out of 15 cases) of the RCC cases (Table 1). All except two (86.6%) cases have medium to strong intensity of staining. In the remaining two cases, 20% and 5% expression were demonstrated respectively. Both cytoplasmic and nuclear immunoreactivity were present in these cases (Figure 1A-C). The extent and intensity of immunoreactivity did not appear to be correlated with the Fuhrman nuclear grade.
Table 1.
Expression of AKR1C3 in renal cell carcinoma
| Case | Sex | Age (year) | Subtype | Fuhrman Nuclear Grade | Positive (%) | Intensity |
|---|---|---|---|---|---|---|
| 1 | M | 79 | Chromophobe | 3 | 100% | Medium |
| 2 | F | 50 | Clear cell | 2 | 100% | Strong |
| 3 | M | 51 | Papillary | 3 | 100% | Medium |
| 4 | M | 64 | Clear cell | 3 | 100% | Medium |
| 5 | M | 58 | Papillary | 4 | 100% | Medium |
| 6 | F | 67 | Clear cell | 4 | 100% | Strong |
| 7 | M | 83 | Clear cell | 4 | 100% | Medium |
| 8 | M | 56 | Clear cell | 3 | 100% | Strong |
| 9 | M | 40 | Clear cell | 2 | 100% | Strong |
| 10 | M | 64 | Clear cell | 4 | 100% | Medium |
| 11 | M | 63 | Clear cell | 2 | 100% | Strong |
| 12 | M | 60 | Clear cell | 2 | 80% | Weak |
| 13 | M | 59 | Clear cell | 2 | 75% | Strong |
| 14 | M | 63 | Clear cell | 2 | 20% | Weak |
| 15 | M | 80 | Clear cell | 2 | 5% | Medium |
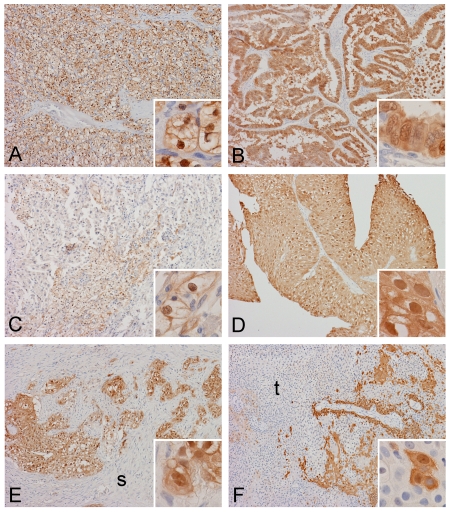
Figure 1.
Spatial distribution of AKR1C3 in adult renal neoplasms. Strong immunoreactivity is demonstrated in this clear cell renal cell carcinoma (A) and papillary renal cell carcinoma (B). In a minority of renal cell carcinoma, the immunoreactivity is less strong and extensive (C). Immunoreactivities in non-invasive papillary urothelial carcinoma (D) and invasive urothelial carcinoma (E) are also strong. Note that the stroma (s) invaded by the tumor is negative. In a small number of urothelial carcinoma (F), negative areas are present in some tumor cells (t) (Original magnification of images: 10×, inset: 60×).
Similarly, 85.7% (6 out of 7) cases of PUC cases demonstrated predominantly strong (5 out of the 6 positive cases) immunoreactivity in practically all tumor cells (100% immunoreactive). In the remaining case, 30% of the areas demonstrated strong immunoreactivity (Table 2). Similar to RCC, both cytoplasmic and nuclear immunoreactivity were present (Figure 1D-F). The extent and intensity of immunoreactivity did not appear to be correlated with the pathological grade.
Table 2.
Expression of AKR1C3 in papillary urothelial carcinoma
| Case | Sex | Age (year) | Grade | Invasion | Positive (%) | Intensity |
|---|---|---|---|---|---|---|
| 1 | M | 51 | Low | No | 100% | Strong |
| 2 | M | 58 | Low | No | 100% | Strong |
| 3 | M | 53 | High | Focally invasive | 100% | Strong |
| 4 | M | 60 | High | Invasive | 100% | Medium |
| 5 | M | 78 | Low | No | 100% | Strong |
| 6 | F | 56 | High | Invasive | 100% | Strong |
| 7 | F | 72 | High | No | 30% | Strong |
Expression of AKR1C3 in Pediatric WT
In contrast to RCC and PUC, the immunoreactivity of AKR1C3 of WTs spanned a spectrum from less than 5% to 100% positive immunoreactivity; and the staining intensity varied from weak to strong (Table 3) in our 14 cases. In contrast to RCC and PUC cases where most tumors demonstrated strong and widespread immunoreactivity, 35.7% (5 cases) of the WTs are negative or possessed an insignificant amount (less than 5%) of staining. The distribution of immunoreactivity varied from an entire area of solid staining (Figure 2A), to areas with patchy staining (Figure 2B), to areas with only occasional (Figure 2C), or sporadic staining (Figure 2D). The strongest immunoreactivity was encountered in areas that mimic mature renal tubules (differentiation) formation. Paradoxically, some neoplastic tubules with weak or negative immunoreactivity could be found adjacent to neoplastic tubules with strong immunoreactivity (Figure 2B). In tumors with very limited immunoreactivity, the small spindle cells are often the only positive cells (Figure 2C D). Similar to RCC and PUC, both nuclear and cytoplasmic staining patterns were demonstrated (Figure 2A-D).
Table 3.
Expression of AKR1C3 in WT
| Case | Age (Year/Month/Day) | Sex | Positive (%) | Intensity |
|---|---|---|---|---|
| 1 | 6Y | F | 100% | Strong |
| 2 | 8M 10D | F | 100% | Moderate |
| 3 | 3Y 10M | F | 50% | Weak |
| 4 | 1Y 6M | F | 50% | Strong |
| 5 | 2Y 11M | F | 50% | Weak |
| 6 | 3Y 6M | F | 20% | Strong |
| 7 | 4Y 5M | F | 20% | Strong |
| 8 | 3Y 10M | F | 10% | Weak |
| 9 | 4Y 6M | F | 10% | Strong |
| 10 | 6Y | F | 5% | Weak |
| 11 | 2Y 4M | F | 0% | Negative |
| 12 | 6Y | M | 0% | Negative |
| 13 | 3Y 11M | F | 0% | Negative |
| 14 | 7Y | F | 0% | Negative |
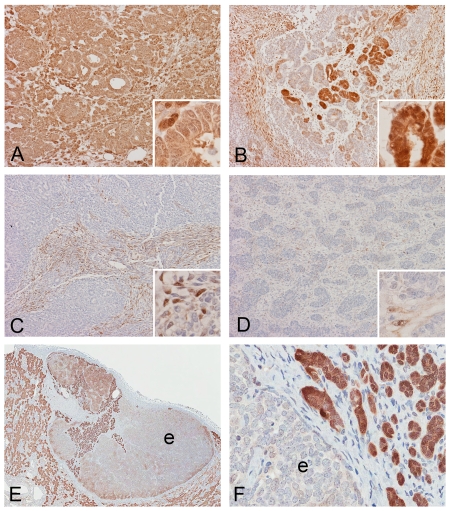
Figure 2.
Spatial distribution of AKR1C3 in pediatric WTs. Immunoreactivity in Wilm's tumor varies from diffusely and strongly positive (A) to patchy positivity (B) to occasional positivity (C) to scant single cell reactivity (D) and totally negative (not shown). Strongest immunoreactivity is demonstrated in some areas with tubule formation (B, inset). In the only case of hyperplastic nephrogenic rest (E and F), components with tubule differentiation (t) are strongly reactive in contrast to the epithelioid component (e) without tubule formation which is weakly positive to negative. The immunoreactivity in non-neoplastic parenchyma (np) is similar to our previously described distribution (Original magnification of images: 10×, inset: 60×).
Expression of AKR1C3 in hyperplastic nephrogenic rest
In one of the WT with less than 5% positive immunoreactivity with only weak intensity, a hyperplastic perilobar nephrogenic rest separated from the main tumor was noted in the residual non-neoplastic renal parenchyma. Strong immunoreactivity was noted in cells with tubule formation within the nephrogenic rest, but the primitive blastemal cells within the rest with epithelioid morphology varied from positive to negative (Figure 2E, F). In contrast to the WTs, all neoplastic tubules in this hyperplastic nephrogenic rest are strongly positive.
In the residual non-neoplastic renal parenchymal tissue in our pediatric cases, we did not observe any difference in immunoreactivity pattern from the normal adult kidney samples (Figure 2E).
Discussion
This study demonstrated widespread immunoreactivity of AKR1C3 in neoplasms with phenotypic features of mature urogenital tissues, namely renal tubules for RCC and urothelial epithelium for PUC. In contrast, the expression of AKR1C3 in WT, a tumor that recapitulates features of embryonic kidneys varied from less than 5% with weak reactivity to 100% with strong reactivity. In WTs cases, the strongest positive immunoreactivity was demonstrated in neoplastic areas with tubule formation (differentiation). Similarly, in the only case of hyperplastic perilobar nephrogenic rest that was included in this study, strongest immunoreacity appears in areas with tubule formation (differentiation).
The monoclonal antibody used in this study has been shown to possess monospecificity against human AKR1C3 (clone #NP6.G6.A6) [25]. There is a rabbit polyclonal antibody prepared against a synthetic peptide corresponding to the C-terminal (297-320 amino acids) of human AKR1C3 [26]. However, the polyclonal antibody has not been shown to be monospecific for AKR1C3 by testing its immunoreactivity against all known human AKR1C isozymes. Human kidney expresses AKR1C isoforms; and a difference in the specificity of the antibody may result in different patterns in immunohistochemical staining.
Human kidney is capable of producing and metabolizing steroid hormones [27] and PGs [28], and steroid hormones [29, 30] and PGs [31-33] are important modulators for normal kidney development. As a multifunctional enzyme, AKR1C3 is capable of producing various steroid and PG metabolites in the kidney. For example, AKR1C3, via its 20-ketosteroid reductase activity, will metabolize the potent mineralocorticoid desoxycorticosterone to its 20α-hydroxy inactive metabolite [34]. AKR1C3 has been shown by immunohistochemistry to be expressed in the mineralocorticoid-responsive epithelial cells of the renal cortical and medullary ducts. The enzyme is thus localized to protect the mineralocorticoid receptor from desoxycorticosterone in the kidney. In addition, AKR1C3 converts progesterone to 20α-hydroxy-progesterone within the kidney [27], and the progesterone receptor (PR) has been detected in fetal kidney between 11 and 21 gestational weeks [35]. Enzymes capable of catalyzing steroid hormones have also been shown to change with age and maturation of the kidney of several animal models [36, 37]. Both the rat and mouse kidney show changes in related, though not identical, steroid metabolizing enzymes [36, 37]. It is possible that the maturing human kidney requires AKR1C3-mediated steroid and/or PG metabolites for proper development. This could account for the variable expression in WT as it recapitulates the immature kidney. It is also possible that AKR1C3 provides the kidney a physical barrier in addition to the chemical barriers. The physical barrier within the kidney, particularly within the proximal and distal tubules, is critical for proper water and solute reabsorption as well as exclusion of proteins from the urine. While currently no data exists to demonstrate a direct involvement of AKR1C3 in this capacity, its consistent immunoreactive pattern leads to this possibility. Of particular note, is the absence of AKR1C3 in the glomeruli, the most open portion of the nephron. In contrast, within the proximal and distal tubules, with selective water and ion transport, AKR1C3 expression is exceptionally strong; however, there is no experimental data currently available to support this concept.
As we have shown in our prior study, AKR1C3 is widely expressed in renal tubules and urothelial epithelium [24]. As RCC and PUC are neoplasms that recapitulate the phenotypic features of these two types of tissue respectively, it is not surprising to find a high level of expression of AKR1C3 in these tissues. In contrast, WTs demonstrate a wide spectrum of differentiation of immature kidney from blastemal to tubule differentiation. While variable levels of AKR1C3 expression are present in WTs with a spectrum of morphologic differentiation, the strongest AKR1C3 immunoreactivities are observed in neoplastic tissue with tubule differentiation which represents the most advanced differentiation. AKR1C3 immunoreactivity is relatively weak in some blastemal regions which represent the less differentiated areas. Similar observations are also noted in the only hyperplastic nephrogenic rest in this study. Although the pathological relationship between lack of AKR1C3 expression and WT remains unclear, our results suggest that AKR1C3 may represent a protein predominantly expressed in mature kidney tissue, and may be a marker of maturation. Though impossible ethically, the ideal approach to address this hypothesis is to study human embryonic kidneys at different stages of development, since AKR1C3 homolog has not been identified in animal models [24].
Acknowledgments
This work was supported in part by grants 1R01-CA90744 and P30-ES013508 awarded to TMP and Department of Veterans Affairs Merit Review Award to HKL.
References
- 1.Jez JM, Flynn TG, Penning TM. A new nomenclature for the aldo-keto reductase superfamily. Biochem Pharmacol. 1997;54:639–647. doi: 10.1016/s0006-2952(97)84253-0. [DOI] [PubMed] [Google Scholar]
- 2.Jez JM, Bennett MJ, Schlegel BP, Lewis M, Penning TM. Comparative anatomy of the aldo-keto reductase superfamily. Biochem J. 1997;326(Pt 3):625–636. doi: 10.1042/bj3260625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyndman D, Bauman DR, Heredia VV, Penning TM. The aldo-keto reductase superfamily homepage. Chem Biol Interact. 2003;143-144:621–631. doi: 10.1016/s0009-2797(02)00193-x. [DOI] [PubMed] [Google Scholar]
- 4.Hara A, Matsuura K, Tamada Y, Sato K, Miyabe Y, Deyashiki Y, Ishida N. Relationship of human liver dihydrodiol dehydrogenases to hepatic bile-acid-binding protein and an oxidoreductase of human colon cells. Biochem J. 1996;313:373–376. doi: 10.1042/bj3130373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dufort I, Soucy P, Labrie F, Luu-The V. Molecular cloning of human type 3 3ahydroxysteroid dehydrogenase that differs from 20a-hydroxysteroid dehydrogenase by seven amino acids. Biochem Biophy Res Communication. 1996;228:474–479. doi: 10.1006/bbrc.1996.1684. [DOI] [PubMed] [Google Scholar]
- 6.Deyashiki Y, Ogasawara A, Nakayama T, Nakanishi M, Miyabe Y, Sato K, Hara A. Molecular cloning of two human liver 3a-hydroxysteroid/dihydrodiol dehydrogenase isoenzymes that are identical with chlordecone reductase and bile-acid binder. Biochem J. 1994;299:545–552. doi: 10.1042/bj2990545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna M, Qin KN, Wang RW, Cheng KC. Substrate specificity, gene structure, and tissue-specific distribution of multiple human 3a-hydroxysteroid dehydrogenases. J Biol Chem. 1995;270:20162–20168. doi: 10.1074/jbc.270.34.20162. [DOI] [PubMed] [Google Scholar]
- 8.Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3ahydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3a/17b-HSD activity and cellular distribution. Mol Endocrinol. 1997;11:1971–1984. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- 9.Dufort I, Rheault P, Huang XF, Soucy P, Luu-The V. Characteristics of a highly labile human type 5 17b-hydroxysteroid dehydrogenase. Endocrinology. 1999;140:568–574. doi: 10.1210/endo.140.2.6531. [DOI] [PubMed] [Google Scholar]
- 10.Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K. Human 3a-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuura K, Shiraishi H, Hara A, Sato K, Deyashiki Y, Ninomiya M, Sakai S. Identification of a principal mRNA species for human 3a-hydroxysteroid dehydrogenase isoform (AKR1C3) that exhibits high prostaglandin D2 11-ketoreductase activity. J Biochem (Tokyo) 1998;124:940–946. doi: 10.1093/oxfordjournals.jbchem.a022211. [DOI] [PubMed] [Google Scholar]
- 12.Penning TM, Steckelbroeck S, Bauman DR, Miller MW, Jin Y, Peehl DM, Fung KM, Lin HK. Aldo-keto reductase (AKR) 1C3: Role in prostate disease and the development of specific inhibitors. Mol Cell Endocrinol. 2006;248:182–191. doi: 10.1016/j.mce.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Mahadevan D, DiMento J, Croce KD, Riley C, George B, Fuchs D, Mathews T, Wilson C, Lobell M. Transcriptosome and serum cytokine profiling of an atypical case of myelodysplastic syndrome with progression to acute myelogenous leukemia. Am J Hematol. 2006;81:779–786. doi: 10.1002/ajh.20690. [DOI] [PubMed] [Google Scholar]
- 14.Lewis MJ, Wiebe JP, Heathcote JG. Expression of progesterone metabolizing enzyme genes (AKR1C1, AKR1C2, AKR1C3, SRD5A1, SRD5A2) is altered in human breast carcinoma. BMC Cancer. 2004;4:27. doi: 10.1186/1471-2407-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizner TL, Smuc T, Rupreht R, Sinkovec J, Penning TM. AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol Cell Endocrinol. 2006;248:126–135. doi: 10.1016/j.mce.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Lan Q, Mumford JL, Shen M, Demarini DM, Bonner MR, He X, Yeager M, Welch R, Chanock S, Tian L, Chapman RS, Zheng T, Keohavong P, Caporaso N, Rothman N. Oxidative damage-related genes AKR1C3 and OGG1 modulate risks for lung cancer due to exposure to PAH-rich coal combustion emissions. Carcinogenesis. 2004;25:2177–2181. doi: 10.1093/carcin/bgh240. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura Y, Suzuki T, Nakabayashi M, Endoh M, Sakamoto K, Mikami Y, Moriya T, Ito A, Takahashi S, Yamada S, Arai Y, Sasano H. In situ androgen producing enzymes in human prostate cancer. Endocr Relat Cancer. 2005;12:101–107. doi: 10.1677/erc.1.00914. [DOI] [PubMed] [Google Scholar]
- 18.Fung KM, Samara ENS, Wong C, Metwalli A, Krlin R, Bane B, Liu CZ, Yang JT, Pitha JT, Culkin DJ, Kropp BP, Penning TM, Lin HK. Increased expression of type 2 3ahydroxysteroid dehydrogenase/type 5 17bhydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr Relat Cancer. 2006;13:169–180. doi: 10.1677/erc.1.01048. [DOI] [PubMed] [Google Scholar]
- 19.Stanbrough M, Bubley G, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 20.Wako K, Kawasaki T, Yamana K, Suzuki K, Jiang S, Umezu H, Nishiyama T, Takahashi K, Hamakubo T, Kodama T, Naito M. Expression of androgen receptor through androgen-converting enzymes is associated with biological aggressiveness in prostate cancer. J Clin Pathol. 2008;61:448–454. doi: 10.1136/jcp.2007.050906. [DOI] [PubMed] [Google Scholar]
- 21.Eble JN, Togashi K, Pisani P. Renal cell carcinoma. In: Eble JN, Sauter G, Epstein JI, Sesterhenn I, editors. World Health Organization Classification of Tumours. Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. pp. 12–14. [Google Scholar]
- 22.Grovas A, Fremgen A, Rauck A, Ruymann FB, Hutchinson CL, Winchester DP, Menck HR. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80:2321–2332. doi: 10.1002/(sici)1097-0142(19971215)80:12<2321::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Beltran A, Sauter G, Gasser T, Hartmann A, Scmitz-Drager BJ, Helpap B, Ayala AG, Tamboli P. Infiltrating urothelial carcinoma. In: Eble JN, Sauter G, Epstein JI, Sesterhenn I, editors. World Health Organization Classification of Tumours. Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. pp. 93–109. [Google Scholar]
- 24.Azzarello J, Fung KM, Lin HK. Tissue distribution of human AKR1C3 and rat homolog in adult genitourinary system. J Histochem Cytochem. 2008;56:853–861. doi: 10.1369/jhc.2008.951384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin HK, Steckelbroeck S, Fung KM, Jones AN, Penning TM. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3a-hydroxysteroid dehydrogenase/type 5 17b-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69:795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 26.El-Alfy M, Luu-The V, Huang XF, Berger L, Labrie F, Pelletier G. Localization of type 5 17bhydroxysteroid dehydrogenase, 3ahydroxysteroid dehydrogenase, and androgen receptor in the human prostate by in situ hybridization and immunocytochemistry. Endocrinol. 1999;140:1481–1491. doi: 10.1210/endo.140.3.6585. [DOI] [PubMed] [Google Scholar]
- 27.Quinkler M, Bumke-Vogt C, Meyer B, Bahr V, Oelkers W, Diederich S. The human kidney is a progesterone-metabolizing and androgen-producing organ. J Clin Endocrinol Metab. 2003;88:2803–2809. doi: 10.1210/jc.2002-021970. [DOI] [PubMed] [Google Scholar]
- 28.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 29.Condon J, Gosden C, Gardener D, Nickson P, Hewison M, Howie AJ, Stewart PM. Expression of type 2 11beta-hydroxysteroid dehydrogenase and corticosteroid hormone receptors in early human fetal life. J Clin Endocrinol Metab. 1998;83:4490–4497. doi: 10.1210/jcem.83.12.5302. [DOI] [PubMed] [Google Scholar]
- 30.Abdelgadir SE, Connolly PB, Resko JA. Androgen binding in peripheral tissues of fetal rhesus macaques: effects of androgen metabolism in liver. J Steroid Biochem Mol Biol. 1990;37:545–551. doi: 10.1016/0960-0760(90)90399-6. [DOI] [PubMed] [Google Scholar]
- 31.Gleason CA. Prostaglandins and the developing kidney. Semin Perinatol. 1987;11:12–21. [PubMed] [Google Scholar]
- 32.van der Heijden BJ, Carlus C, Narcy F, Bavoux F, Delezoide AL, Gubler MC. Persistent anuria, neonatal death, and renal microcystic lesions after prenatal exposure to indomethacin. Am J Obstet Gynecol. 1994;171:617–623. doi: 10.1016/0002-9378(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 33.Komhoff M, Wang JL, Cheng HF, Langenbach R, McKanna JA, Harris RC, Breyer MD. Cyclooxygenase-2-selective inhibitors impair glomerulogenesis and renal cortical development. Kidney Int. 2000;57:414–422. doi: 10.1046/j.1523-1755.2000.00861.x. [DOI] [PubMed] [Google Scholar]
- 34.Sharma KK, Lindqvist A, Zhou XJ, Auchus RJ, Penning TM, Andersson S. Deoxycorticosterone inactivation by AKR1C3 in human mineralocorticoid target tissues. Mol Cell Endocrinol. 2006;248:79–86. doi: 10.1016/j.mce.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 35.Inoue T, Akahira JI, Takeyama J, Suzuki T, Darnel AD, Kaneko C, Kurokawa Y, Satomi S, Sasano H. Spatial and topological distribution of progesterone receptor A and B isoforms during human development. Mol Cell Endocrinol. 2001;182:83–89. doi: 10.1016/s0303-7207(01)00549-4. [DOI] [PubMed] [Google Scholar]
- 36.Dalla Valle L, Toffolo V, Vianello S, Belvedere P, Colombo L. Expression of cytochrome P450c17 and other steroid-converting enzymes in the rat kidney throughout the lifespan. J Steroid Biochem Mol Biol. 2004;91:49–58. doi: 10.1016/j.jsbmb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa M, Tsukada F, Nakayama T, Matsuura K, Hara A, Sawada H. Identification of two dihydrodiol dehydrogenases associated with 3(17)a-hydroxysteroid dehydrogenase activity in mouse kidney. J Biochem. 1989;106:633–638. doi: 10.1093/oxfordjournals.jbchem.a122908. [DOI] [PubMed] [Google Scholar]