Abstract
The author herein reports histopathologic features of 31 surgical cases of gastrointestinal stromal tumor (GIST) of the digestive organs. The 31 cases of GIST were diagnosed in our pathology laboratory. They consisted of 24 cases of gastric GIST, 1 case of hepatic GIST, 1 case of small intestinal GIST, 4 cases of colon GIST, and 1 case of rectal GIST. The age of the patients ranged from 56 year to 84 years with a mean of 71 years. Male to female ratio was 21:10. The presenting symptoms were gastrointestinal bleeding in 13 cases, abdominal pain and discomfort in 13 cases, and asymptomatic in 5 cases. Endoscopy and imaging modalities including US, CT and MRI were useful to detect the tumors in all cases, and biopsies confirmed the GIST diagnosis in 21 cases. The size of GIST ranged from 1 cm to 12 cm with a mean of 4.3 cm. Grossly, 23 cases were submucosal tumors, 6 serosa-side tumors, 1 solid tumor in the liver, and 1 rectal polyp. Histologically, 28 cases were of spindle cell type and 3 of epithelioid type. According to mitotic counts and tumor size, the malignant risk was very low in 4 cases, low in 14 cases, intermediate in 9 cases, and high in 4 cases. Immunohistochemically, all cases were positive for KIT and vimentin, 30 cases for CD34, and 4 cases for α-smooth muscle actin. None were positive for desmin and S100 protein. Ki-67 labeling ranged from 2% to 18%. P53 protein was negative in all cases. PDGFRA was positive in 20 cases among 24 cases examined. Genetic analysis using PCR-direct sequencing method was performed in 5 GISTs; all the 5 GISTs showed point mutations or deletions in KIT gene, but did not in PDGFRA gene. The 5 cases of GIST were positive for PDGFRA protein, suggesting that PDGFRA overexpression is not associated with PDGFRA gene mutations. Four of the 31 cases showed metastases. The chemotherapy was imatinib mesylate in 6 cases, and none in 25 cases. Four cases of high risk died of GIST, and 27 cases are alive now without tumors.
Keywords: GIST, desmin, KIT, PDGFRA, clinicopathology, immunohisology, genetics
Introduction
Gastrointestinal stromal tumor (GIST) was once considered as smooth muscle or neurogenic tumors, but recent advances in KIT gene and platelet derived growth factor receptor-α (PDGFRA) gene have shown that GISTs are associated with gain-of-function mutations of KIT gene and less frequently of PDGFRA gene [1, 2]. GIST is believed to be derived from interstitial cell of Cajal (ICC) (pacemaker cell) which is present in the muscular layer of the gastrointestinal walls (3). ICC expresses KIT protein (CD117) and CD34. In practice, immunohistochemical demonstration of KIT and/or CD34 is a hallmark in the diagnosis of GIST [3-5]. Many studies of GIST have been published in the English literature. All types of GIST are considered to have malignant potential. The author also published three cases of extra-gastrointestinal stromal tumors of the uterus, omentum, and transverse mesocolon [6-8]. Herein, the author reports 31 cases of GIST, which were present in the archival pathologic specimens of our pathology laboratory in the last 15 years.
Materials and methods
The author retrieved 31 surgical cases of GIST of digestive organs from the archival pathology files of our pathology laboratory in the last 15 years. Clinical records of the 31 cases were also reviewed. Extra-gastrointestinal stromal tumors except for hepatic GIST were excluded from this study. The HE sections of the 31 cases of GIST were reviewed under a microscopy.
An immunohistochemical studies were performed by Dako's envision method (Dako Corp. Glostrup, Denmark), as previously described [9, 10]. In brief, the sections were treated by microwave oven heating, and then primary antibodies were applied. Second, the Dako's envison kits were applied to the sections. Diaminobenzidine was used as chromogen. The primary antibodies used were KIT (polyclonal, Dako), CD34 (QBEND10, Dako), vimentin (Vim 3B4, Dako), desmin (D33, Dako), α-smooth muscle actin (1A4, Dako), S100 protein (polyclonal, Dako), p53 protein (DO7, Dako), and Ki-67 antigen (MIB1, Dako). In 24 cases of GIST, immunostaining of PDGFRA was performed, using anti-PDGFRA antibody (polyclonal, Santa Cruz, CA, USA).
A genetic analysis for KIT gene (exons 9, 11, 13, and 17) and PDGFRA gene (exons 12 and 18) was performed in 5 cases with GIST. The exons of both genes were selected because they are frequent mutation sites in GIST [3-5]. In DNA was extracted from the paraffin sections of the GIST and analyzed by the PCR-direct sequencing method, as previously described [6-8]. The primers are shown in Table 1. In brief, genomic DNA was extracted from paraffin blocks with proteinase K digestion and phenol/chloroform extraction, and subjected to PCR for 40 cycles (94°C for one minute, 52°C for one minute, 72°C for one minute), using a thermal cycler (GeneAmp PCR system 9700, Applied Biosystems, ABI, CA). The annealing temperature was 53°C. PCR products were extracted, and subjected to a computed automatic DNA sequencer (ABI PRIZM 3100 Genetic Analyzer, Applied Biosystems, ABI, CA).
Table 1.
Primer sequence
| Forward | Reverse | |
|---|---|---|
| KIT exon 9 | 5′-TCC TAG AGT AAG CCA GGG CTT-3′ | 5′-TGG TAG ACA GAG CCT AAA CAT CC-3′ |
| KIT exon11 | 5′-GAT CTA TTT TTC CCT TTC TC-3′ | 5′AGC CCC TGT TTC ATA CTG AC-3′ |
| KIT exon 13 | 5′-GCT TGA CAT CAG TTT GCC AG -3′ | 5′-AAA GGC AGC TTG GAC ACG GCT TTA-3′ |
| KIT exon 17 | 5′-CTC CTC CAA CCT AAT AGT GT-3′ | 5′-GTC AAG CAG AGA ATG GGT AC-3′ |
| PDGFRA exon12 | 5′-TTG GAT ATT CAC CAG TTA CCT GTC-3′ | 5′-CAA GGG AAA AGC TCT TGG-3′ |
| PDGFRA exon 18 | 5′-ACC ATG GAT CAG CCA GTC TT-3′ | 5′-TGA AGG AGG ATG AGC CTG ACC-3′ |
The malignant potential of GIST was assessed by mitotic counts and tumor size, as described by Fletcher et al. [11].
Results
The 31 cases of GIST consisted of 24 cases of gastric GIST, 1 case of hepatic GIST, 1 case of small intestinal GIST, 4 cases of colon GIST, and 1 case of rectal GIST. The age of the patients ranged from 56 year to 84 years with a mean of 71 years. Male to female ratio was 20:11. The size of GIST ranged from 1 cm to 12 cm with a mean of 4.3 cm.
The presenting symptoms were gastrointestinal bleeding in 13 cases, abdominal pain and discomfort in 13 cases, and asymptomatic in 5 cases. Endoscopy and imaging modalities including US, CT and MRI were useful to detect the tumors in all cases, and biopsies confirmed the GIST diagnosis in 21 cases.
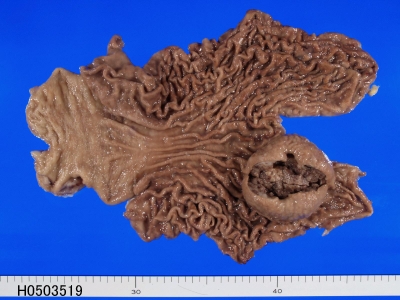
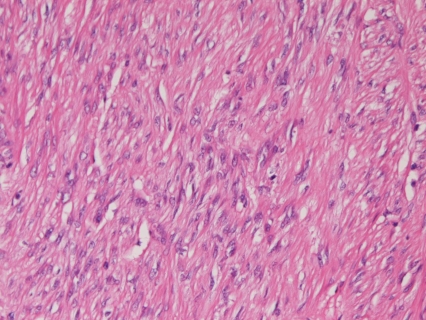
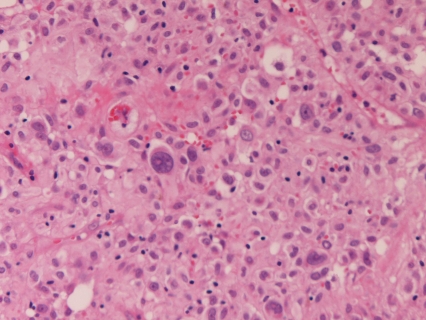
Macroscopically, of the 31 cases of GIST, 23 cases were submucosal tumors (Figure 1), 6 serosa-side tumors, 1 solid tumor in the liver, and 1 rectal polyp. Histologically, of the 31 cases of GIST, 28 were of spindle cell type (Figure 2) and three were of epithelioid type (Figure 3). According to mitotic counts and tumor size, the malignant risk was very low in 4 cases, low in 14 cases, intermediate in 9 cases, and high was 4 cases.
Figure 1.
Gross finding of GIST of the stomach. The GIST shows a submucosal tumor with ulceration
Figure 2.
Spindle cell type of GIST. Spindle cell proliferation is seen. HE, ×200
Figure 3.
Epithelioid type of GIST. HE, ×200
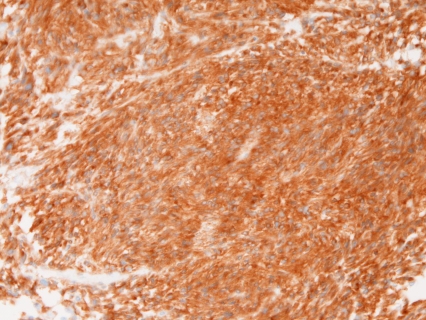
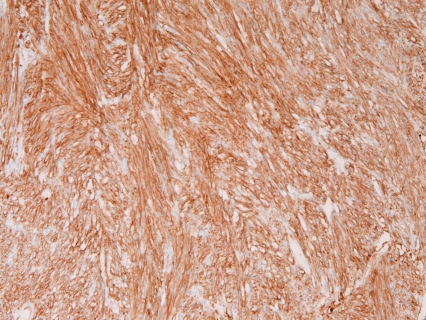
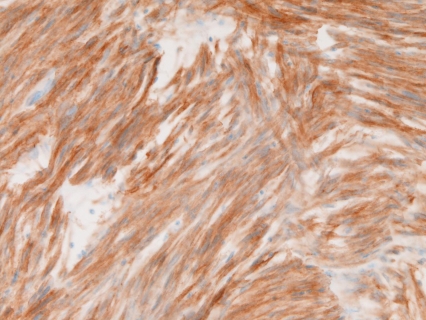
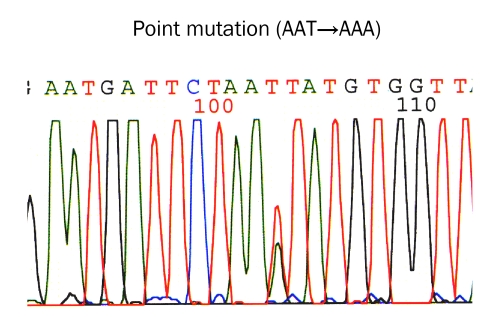
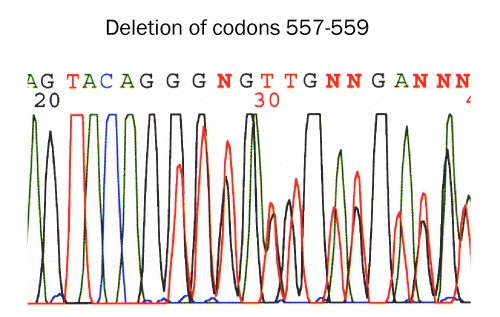
Immunohistochemically, all the 31 cases were positive for KIT (Figure 4) and vimentin, 30 cases for CD34 (Figure 5), and 4 cases for α-smooth muscle actin. None of the 31 GISTS were positive for desmin and S100 protein. Ki67 labeling ranged from 2% to 18%. P53 protein was negative in all cases. PDGFRA was positive in 20 cases among the 24 cases examined (Figure 6). The genetic analysis of the 5 cases of GIST showed point mutations (Figure 7) or deletions (Figure 8) of exon 9 (n=1), exon 11 (n=2) and exon 17 (n=2) of KIT gene. No mutations of PDGFRA gene were recognized.
Figure 4.
KIT is positive in a case of GIST. Immunostaining, ×200
Figure 5.
CD34 is positive in a case of GIST. Immunostaining, ×200
Figure 6.
PDGFRA is positive in a case of GIST. Immunostaining, ×200
Figure 7.
KIT mutation in gastrointestinal stromal tumor. Point mutation at codon 822 (AAT →AAA) in exon 17 (Bar)
Figure 8.
KIT mutation in gastrointestinal stromal tumor. Deletion of codons 557,558, and 559 of exon 17 (Bar)
Four of the 31 cases showed metastases. The chemotherapy was imatinib mesylate in 6 cases, and none in 25 cases. Four cases of high risk group died of GIST, and 27 cases are alive now without tumors.
Discussion
The locations of GIST are highest in the stomach, followed in order by colorectum and small intestine [3, 4]. In the present series, the locations of the 31 cases of GIST was stomach in 24 cases, liver in 1 case, small intestine in 1 case, colon in 4 cases, and rectum in 1 case. GIST occurs in middle or old age [3, 4]. Miettinen et al. [12] who analyzed 1,765 cases of GIST stated that the mean age of GIST was 63 years. In the present study, the age of the patients ranged from 56 year to 84 years with a mean of 71 years. In the present study, male to female ratio was 20:11. The previous reports also indicated male predominance in GIST cases [12]. In the present study, the size of GIST ranged from 1 cm to 12 cm with a mean of 4.3 cm. In the Miettinen's series [12], the size of GIST varied from 0.5 cm to 44cm with a median of 6.0 cm.
In the present series, the presenting symptoms were gastrointestinal bleeding in 13 cases, abdominal pain and discomfort in 13 cases, and asymptomatic in 5 cases. In the Miettinen's series [12], presenting symptoms were mostly gastrointestinal bleedings. In the present study, endoscopy and imaging modalities including US, CT and MRI were useful to detect the tumors in all cases, and biopsies confirmed the GIST diagnosis in 21 cases, suggesting that endoscopy and imaging techniques are useful to detect tumors, and biopsies are useful in GIST diagnosis.
In the present series, of the 31 cases, 23 cases were submucosal tumors, 6 serosa-side tumors, 1 solid tumor in the liver, and 1 rectal polyp. These macroscopic findings are compatible with previous series [3, 4, 12, 13]. In the present study, one case was hepatic GIST. GIST in the liver is extremely rare, and only a few cases have been reported in the English literature [14]. Thus, this hepatic GIST is very interesting for the author.
In the present series, of the 31 cases of GIST, 28 were of spindle cell type and three were of epithelioid type. In general, spindle cell type predominates over epithelioid type [3, 4, 12, 15]. According to the consensus report of GIST by Fletcher et al. [11], the malignant potential of GIST depends on tumor size and mitotic counts. In very low malignant risk group, tumor size is less than 2 cm and mitotic counts are less than 5 per 50 high power field (HPF). In low malignant rick group, tumor size is 2cm <5cm and mitotic counts are < 5/50 HPF. In intermediate rick group, tumor size is 5cm <10cm, and mitotic counts are < 5/50 HPF. In high rick group, tumor size is > 10 cm, and mitotic counts are > 10/50 HPF [11]. In the present study, according to mitotic counts and tumor size [11], the malignant risk was very low in 4 cases, low in 14 cases, intermediate in 9 cases, and high in 4 cases.
Immunohistochemically, more than 90% of GIST expresses KIT and/or CD34 [3, 4]. KIT-negative GIST is present [3, 4]. In practice, positive KIT and/or CD34 are enough to diagnose the lesions as GIST. In KIT-negative cases, a genetic analysis of KIT and PDGFRA is necessary. In the present series, all the 31 cases were positive for KIT and vimentin, 30 cases for CD34, and 4 cases for α-smooth muscle actin. The actin-positive 4 cases are GIST with smooth muscle differentiation. In the present study, none of the 31 GISTS were positive for desmin and S100 protein. Ki-67 labeling ranged from 2% to 18%, suggesting relative low proliferative activities in the present series. P53 protein was negative in all cases, suggesting that p53 mutations are absent in the present 31 cases. In the present study, PDGFRA was positive in 20 cases among 24 cases examined, suggesting that the PDGFRA oncoprotein was present in about 83 % of GIST.
In the present study, the genetic analysis of the 5 cases of GIST showed point mutations or deletions of exons 9, 11, and 17 of KIT gene. No mutations of PDGFRA gene were recognized. KIT and PDGFRA genes are known to be mutually exclusive [3, 4, 5]; so, the present cases showed only KIT gene mutations, sparing PDGFRA gene mutations. The present 5 cases showed hot spot mutations in the KIT and PDGFRA genes. Protein expression of PDGFRA in GIST has not been reported. The present study showed that immunoreactive PDGFRA was expressed in 20/24 cases (83%), suggesting that PDGFRA is present in GIST. In the present series, the 5 cases of GIST which underwent genetic examination were positive for PDGFRA protein, suggesting that PDGFRA protein overexpression is not associated with PDGFRA gene mutations. Much more studies between protein expression and gene mutations of KIT and PDGFRA are required.
Imatinib mesylate (Gleevec) is effective in GIST (3, 4). In the present study, four of the 31 cases showed metastases. The chemotherapy was imatinib mesylate in 6 cases, and none in 25 cases. Four cases of high risk group died of GIST, and 27 cases are alive now without tumors. These findings suggest that high risk patients with GIST may die of GIST and its metastasis.
In summary, the authors reported clinicopathologic features of 31 GIST of our laboratory. Of them, 24 were gastric GIST, 1 hepatic GIST, 1 small intestinal GIST, 4 colon GIST, and 1 rectal GIST. The age of the patients ranged from 56 year to 84 years with a mean of 71 years. Male to female ratio was 21:10. The presenting symptoms were gastrointestinal bleeding in 13 cases, abdominal pain and discomfort in 13 cases, and asymptomatic in 5 cases. The size of GIST ranged from 1 cm to 12 cm with a mean of 4.3 cm. Grossly, 23 cases were submucosal tumors, 6 serosa-side tumors, 1 solid tumor in the liver, and 1 rectal polyp. Histologically, 28 cases were of spindle cell type and 3 of epithelioid type. The malignant risk was very low in 4 cases, low in 14 cases, intermediate in 9 cases, and high in 4 cases. Immunohistochemically, all cases were positive for KIT and vimentin, 30 cases for CD34, and 4 cases were for α-smooth muscle actin. None were positive for desmin and S100 protein. Ki-67 labeling ranged from 2% to 18%. P53 protein was negative in all cases. PDGFRA was positive in 20 cases among 24 cases examined. A genetic analysis showed all the 5 GISTs showed point mutations or deletions in KIT gene, but did not in PDGFRA gene. The 5 cases of GIST were positive for PDGFRA protein, suggesting that PDGFRA overexpression is not associated with PDGFRA gene mutations.
References
- 1.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shimomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumor. Science. 1988;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 2.Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumor. Gastroenterology. 2003;125:660–667. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K. Pathology of gastrointestinal stromal tumor. Pathol Int. 2006;56:1–9. doi: 10.1111/j.1440-1827.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen M, Lasota J. Gastrointestinal strumal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 5.Lasota J, Miettinen M. KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs) Semin Diag Pathol. 2006;23:91–102. doi: 10.1053/j.semdp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. Would J Gastroenterol. 2008;14:7256–7259. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terada T. Gastrointestinal stromal tumor of the uterus: A case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Pathol. 2009;28:29–34. doi: 10.1097/PGP.0b013e3181808000. [DOI] [PubMed] [Google Scholar]
- 8.Terada T. Primary extragastrointestinal stromal tumors of the transverse mesocolon without c-kit mutations but with PDGFRA mutations. Med Oncol. 2009;26:233–237. doi: 10.1007/s12032-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 9.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 10.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;206:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical and molecular study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa T, Matsuno Y, Shimoda T, Hirohashi S. Gastrointestinal stromal tumor: consistent CD117 immunostaining for diagnosis, and prognostic classification based on tumor size and MIB-1 grade. Hum Pathol. 2002;33:669–676. doi: 10.1053/hupa.2002.124116. [DOI] [PubMed] [Google Scholar]
- 14.Hu X, Forster J, Damajanov I. Primary malignant gastrointestinal stromal tumor of the liver. Arch Pathol Lab Med. 2003;127:1606–1608. doi: 10.5858/2003-127-1606-PMGSTO. [DOI] [PubMed] [Google Scholar]
- 15.Singer S, Rubin BP, Lux ML, Chen CJ, Demetri GD, Fletcher JA. Prognostic value of KIT mutation type, mitotic activity and histological subtype in gastrointestinal stromal tumor. J Clin Oncol. 2002;20:3898–3905. doi: 10.1200/JCO.2002.03.095. [DOI] [PubMed] [Google Scholar]