Abstract
There is reliable information about how changes in spleen histology are influenced by the relationship among B and T lymphocytes, macrophages, dendritic cells and myofibroblasts. Moreover, if it can be applied in the day-by-day pathology laboratory. This work intends to elucidate morpho-functional aspects of relationships of these cells in the different spleen compartments, how they are influenced by pathological conditions and how basic immunohistochemical techniques could optimize the histopathological diagnosis. We analyzed the usefulness of the monoclonal antibodies CD45RO, CD20, CD21, CD35, CD68, caldesmon, the smooth muscle α-actin type 1 (SMA-1) in 91 specimens. CD21+ CD35+ follicular dendritic cells were organized into three patterns in agreement with the immune condition of the lymphoid follicle. Smooth muscle α-actin type 1+and caldesmon+myofibroblasts draw two double rings: marginal–perifollicular and germinal–marginal. The latter is closely related to T-cells. CD68+red pulp macrophages had clear and linear configuration. The interruption of this CD68+ linear pattern in splenic marginal zone lymphoma cases could be a criterion to differentiate it from reactive hyperplasia. CD45RO, CD20, CD21, CD68 and SMA-1 provide a basic and quality immunohistochemical battery for a better comprehension of the human spleen and could improve its histopathological diagnosis.
Keywords: Follicular dendritic cells, myofibroblasts, macrophages, spleen, immune system, immunohistochemical, histopathology
Introduction
The spleen is the key organ in the highly-efficient immunologic response. Until a few decades ago the available data regarding the spleen lymphoid follicle organization were just random pieces of information derived from studies on different animal species, mainly rats [1-4]. There was consensus on only a few points. Now recent comparative studies are proving that these previous data were insufficient and inapplicable to the human spleen [5, 6]. These works emphasized the role of dendritic cells (DCs) in the immune dynamic of the spleen. DCs are the most powerful and complex antigen-presenting cells (APC) in any organism, with a high level of specialization, even inside different functional compartments of a given tissue, such as the spleen. Major efforts have been carried out to characterize the biological properties of DCs by means of cell cultures and immunohistochemical and biomolecular techniques. Yet little is known of how the morphologic changes of DCs and their relationship with lymphocytes, macrophages and myofibroblasts and their functional conditions could be useful in the routine pathological laboratory. Thus, we focused our investigation on research defining easy and practical FDC patterns in lymph follicles and how these patterns influence macrophages and myofibroblasts.
To this effect we studied FDCs in different splenic compartments, the population of myofibroblasts and their relation to the macrophage alterations under several pathological circumstances. We selected monoclonal antibodies that would provide accuracy and were easily to reproduced, even on poorly fixed paraffin-embedded section, aiming to obtain a day-by-day immunohistochemical panel. In designing the present work we took into account two definitions
First, the terms used in the scientific literature of perilymphoid [7] or perifollicular [8] zone could lead to conceptual mistakes [5, 8, 9]. New histological findings show this lymphoid band only around follicles. Therefore, it would be better to call it perifollicular zone (PZ). Moreover, this terminology fits adequately with the spleen immune system dynamics: PZ seems to be limited to those zones where recirculating memory B cells are correctly stimulated [5, 10-12].
Second, myofibroblasts could exhibit a variable immunophenotype. Eyden B [13] states that the electronic microscope is the only technique that could reliably identify a certain cell type. At present, knowledge of the definition of myofibroblast has been widely accepted, though inaccurate: a spindle-shaped cell which expresses the α-actin of smooth muscle cells. It is inaccurate because other cell types could be spindle-shaped and can express these molecules. On the contrary, when myofibroblast are located in normal tissues and in certain histological context, it is quite easy to identify them and to exclude other cellular types [13,14].
Material and methods
Human spleens
We performed a retrospective investigation using 91 specimens obtained from the hospital pathology archive, between 10 and 90 years of age (41 females and 50 males). All samples were removed ex vivo in the University Hospital Virgen Macarena between 1996 and 2001. The diagnosis that led to spleen removal is summarized in Table 1.
Table 1.
Human spleens removed ex vivo
| Diagnosis | Number of organs |
|---|---|
| Blunt abdominal trauma | 35 |
| Lymphomas | 12 |
| Gastrointestinal malignancies | 20 |
| Thrombocitopenic purpura and autoimmune anemies | 13 |
| Abdominal surgical procedures | 7 |
| Iatrogenic spleen lesions | 4 |
Blunt abdominal trauma cases were evaluated separately (other studies have considered it from a physiologic perspective).
The lymphoma cases correspond to four splenic marginal zone lymphomas (SMZL), three large B-cell lymphomas, two Hodgkin's lymphomas, a gastric MALT lymphoma, a follicular lymphoma and a T-cell lymphoma. Vascular neoplasms such as angiosarcoma or hemangioma were not included.
Single-staining paraffin antibody sections
Paraffin sections were processed routinely through dewaxing in xylene and rehydrating in graded ethanol. Then, they were washed in double-distilled water and incubated overnight at 37ºC. Immune reactions were visualized using the avidin-biotin complex technique (Novocastra laboratories Ltd, UK) following the manufacturer's instructions and using an automated (Ventana ES) and joint detection (Ventana enhanced DAB) system. For antigen retrieval we used 1600 ml of 0.01 M sodium citrate (except for CD35) boiled in a pressure kettle without closing the lid. For CD35 we used ethylene diamine tetra-acetic acid (EDTA) 1:50 in a microwave for 2 minutes.
All cases were subjected to single staining immunohistochemical techniques with different antibodies. Three to four sections in cases without splenomegaly; twenty to twenty-five in those with lymphoma were obtained. A flexible estimation of the follicles included between fifteen to several dozen per case was made.
A total of 91 cases were stained with CD45RO, CD20 and CD21. CD35 was finally used on 72 cases and CD68 on 70 cases. Table 2 shows monoclonal antibodies used on paraffin-embedded sections, their sources and dilutions.
Table 2.
Monoclonal antibodies
| Monoclonal mouse antibody | Clone | Isotype | Specificity | Dilution | Origin |
|---|---|---|---|---|---|
| CD45RO | Ab | IgG2a | Common leucocitary antigen | Predilution | Ventana Medical System inc. Tucson, USA |
| CD20 | L26 | IgG2a k | Intracytoplasmic epitope linked to CD20 antigen | Predilution | Ventana Medical System inc., Tucson, USA |
| CD21 | 2G9 | IgG2a | Human CD21 antigen | 1:10 | Novocastra Laboratories Ltd. Newcastle, UK |
| CD35 | RLB25 | IgG2b | Human CD 35 antigen | 1:40 | Novocastra Laboratories Ltd. Newcastle, UK |
| CD68 | KP1 | IgG1 k | Human CD68 antigen | 1:200 | Novocastra Laboratories Ltd. Newcastle, UK |
| SMA-1 | 1A4 | IgG2a | Smooth muscle alpha-chain | Predilution | Zymed laboratories inc. San Francisco, CA, USA |
| Caldesmon | TD107 | IgG1 | Human caldesmon | 1:50 | Novocastra Laboratories Ltd. Newcastle, UK |
FDCs were stained with CD21 and CD35 on separated paraffin-embedded sections. Macrophages were stained with CD68, B-lymphocytes with CD20 and active T-lymphocytes with the common leukocyte antigen CD45RO isoform.
Myofibroblasts were stained with caldesmon and monoclonal antibodies against the smooth muscle α-actin type 1 (SMA-1) on 17 cases for each antibody on different paraffin-embedded sections. Only 5 cases were stained with both immunohistochemical markers.
The cases selected to be stained with caldesmon and SMA-1 required having good-quality tissue slides, all previously stained with CD21, CD35, CD45RO and CD20. Ten of the cases corresponded to follicular hyperplasia and the rest were lymphomas (SMZL, large B-cells).
Method
We followed well established criteria [15-17] to identify non-specific reactive changes, incidental splenectomy and after abdominal trauma, spontaneous spleen rupture and the basic features of SMZL [18-19]. Cases were studied by two different observers. All available sections of each given case were reviewed. First, we evaluated all the immunostains of a given specimen, together with their corresponding haematoxylin-eosin section. Then we analyzed the distribution of the lymph follicles, their relation to blood vessels and the presence of germinal centres (GC) and their cell populations and red pulp cell configuration. We compared the undamaged areas of spleen tissue with those affected by a neoplastic population (including non-SMZL cases). Second, we reviewed all slides stained with a single monoclonal antibody, the staining intensity (slight, mild or strong), its cellular localization (cytoplasm or membrane) and distribution. The distinct morphology and distribution of active T lymphocytes helped to differentiate them from monocytes and granulocytes.
Results
Monoclonal antibodies accuracy
Both monoclonal antibodies CD21 and CD35 did characterize follicular dendritic cells (FDCs) network. CD21 was positive in 73.6% of the analyzed cases (n=91) and CD35 in only 36.1% (n=72). Therefore, CD21 was more sensitive, specific and provided a uniform immunohistochemical staining (Figures 1A-1E), with a lower rate of cross-linked reactions than CD35. CD35 had various cross-linked staining reactions, mainly with B-cell lineage. The monoclonal antibody CD68 was positive in 91.4% of the samples (n=70). It showed homogeneous results and lack of significant cross-linked reactions in the red pulp or lymph follicles (Figure 2G). SMA-1 staining (Figures 2I-2K) was more sensitive and specific than caldesmon (Figure 2L) to stain myofibroblasts. SMA-1 were positive in 88.2% of the cases (n=17), whereas caldesmon in only 52.9% (n=17). SMA-1 results were uniform even under different histological tissue conditions.
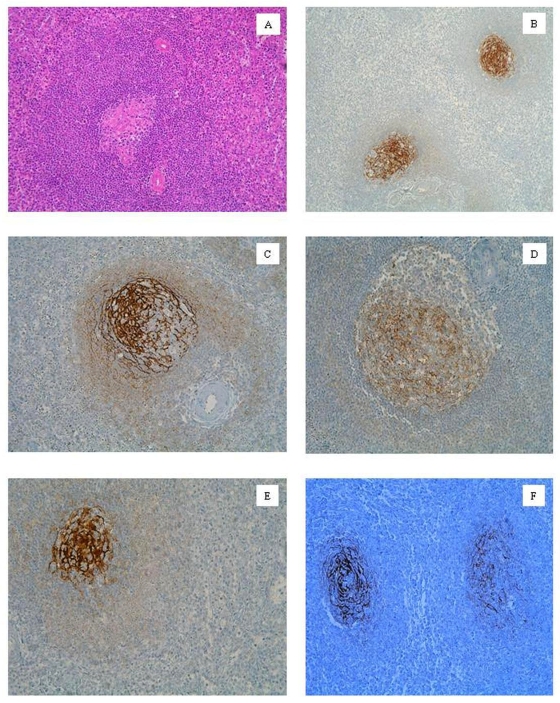
Figure 1.
A. Active secondary lymphoid follicle. SMZL and hyperplasia follicular area. Hematoxilin-eosin. B. FDCs pattern type 1. Primary lymphoid follicle. Follicular hyperplasia. CD21. C. FDCs pattern type 2. Small GC and wide MZ phase. Follicular hyperplasia. CD21. D. Follicle with small GC and wide MZ phase. Follicular hyperplasia. CD21. E. FDCs pattern type 3. SMZL. CD21. F. FDCs meshwork invaded by cells belonging to SMZL. SMZL. CD21.
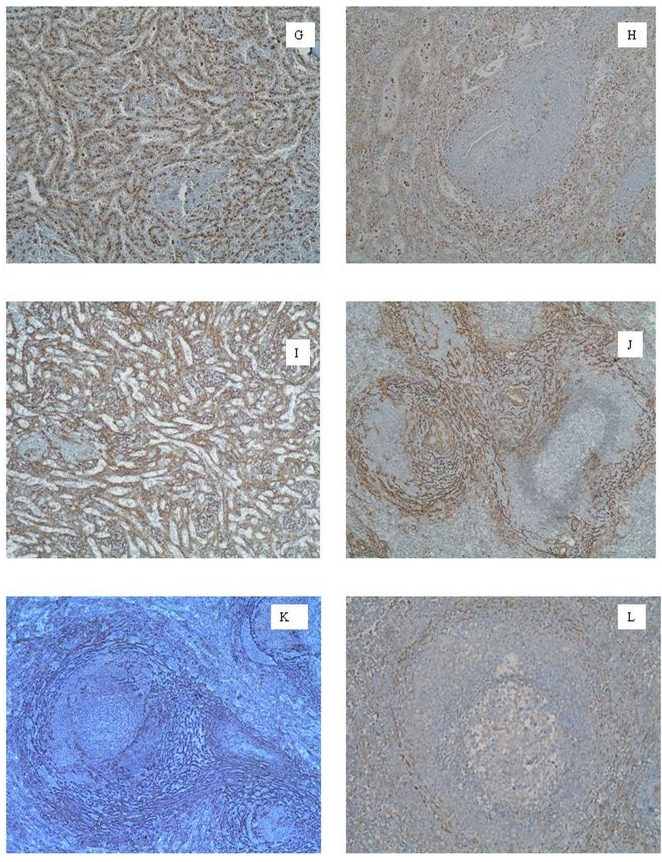
Figure 2.
G. Macrophage lineal drawing the red pulp sinuses. Hodgkin's lymphoma, hyperplasic area. CD68. H. Disturbance in macrophage lineal drawing. Spleen trauma, hyperplasic area. CD68. I. Myofibroblasts associated to red pulp. Follicular hyperplasia, active GC. SMA-1. J. Marginal-perifollicular myofibroblast double ring (MPDR). Follicular hyperplasia, large MZ. SMA-1. K. Germinal-marginal myofibroblast double-ring (GMDR). Large cells LNH B lymphoma, hyperplasia area. SMA-1. L. Germinal-marginal myofibroblast double-ring (GMDR). Follicular hyperplasia, large MZ. Caldesmon.
CD20 and CD45RO were positive in 95.6% and 98.9% of the cases, respectively (n=91).
Macrophages
We observed three different macrophage populations (Figures 2G, 2H). The first one was constituted by isolated cells in the inner part of the sinus lumen, showing a mildly stained polygonal morphology with short cytoplasmic extensions around a small cell body. The second population was parallel to sinus endothelial cells. They had lengthened bodies and slender nuclei and their strong positive staining delineated clearly the whole sinus network. The third population was the macrophages located in the follicles. These cells showed thin and branching bodies, with some medium-sized cytoplasmic extensions. They were isolated in GC, the mantle zone and the inner area of the marginal zone (MZ). Their number increased inside large GC.
Basic patterns of FDCs
We describe three FDCs network patterns based on their immunostaining intensity, the shape of the cell body and the range of the cytoplasmic extensions.
a)Type 1: growing mesh: (Figure 1B). FDCs showed small, oval and slight to mild intensity staining bodies. They were like a scattered, fine and well-developed network of cytoplasmic extensions that could reach the proximity of T-cells ring. FDCs were circumscribed within a small and frayed GC, not clearly limited by the mantle zone.
b) Type 2: full-blown mesh: (Figure 1C). FDCs shaped a wide and spider-shaped network that was limited by an inner T-cell ring. Oval- and small-shaped FDCs bodies with long and thick cytoplasmic extensions in the dark zone (DZ) were observed. These processes spread through the light zone (LZ), showing stronger staining. This mesh could even pass through the mantle zone (Figure 1D) and acquired a frayed appearance in the follicles without a closed inner T-ring. This network could isolate inner heterogeneous and small cellular groups.
c)Type 3: backward mesh: (Figure 1E). The dendritic network is enclosed in a small and round GC. FDCs were scattered, with big, rounded and strong staining bodies. The dendritic processes were difficult to identify. GC showed regressive or atrophic appearances.
Follicular hyperplasia phases
We observed three basic phases in relation to the spleen immune activity at the moment of splenectomy.
Primary follicle: This phase was observed in 21% of cases of benign follicular hyperplasia. The lymphoid follicles had well-structured B and T cell populations. There were numerous extrafollicular foci of B-cells. CD68+ macrophages drew the sinuses network clearly in a mirror configuration (Figure 2G). FDCs mesh could not be seen and was not organized in a type 1 pattern. SMA-1+ and caldesmon+ myofibroblasts showed a slighted and layered distribution in PZ.
Transition between primary and secondary follicles: The transition between primary and secondary follicles included different changes. The extrafollicular foci of B-cells appeared scarce in the red pulp. MZ grew and made itself widen. It began to view CD20+ cells of plasmacytoid configuration. T-cell double ring did not exist in the primary follicles. The organization of T lymphocytes in this ring was one of the main morphological changes to secondary follicles. This ring was not observed in this transition phase. The crossover of two layers of T cells or a unique layer of T-lymphocytes were observed. FDC network began to grow, but it was not limited by the inner ring of T lymphocytes. The cytoplasmic extensions spread themselves to LZ and reached to MZ inner layer. SMA-1+ caldesmon+ myofibroblast were not configured in a parallel double-ring to T-lymphocytes (see ahead). The myofibroblasts were not configured in any organized structure and relatively scarce compared to their presence in the secondary follicles with prominent GC.
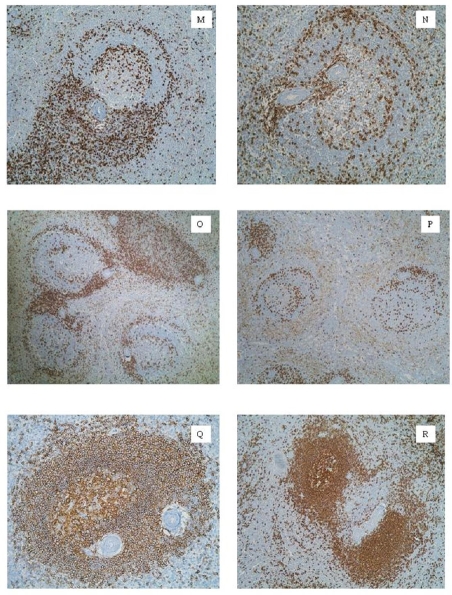
Secondary follicle with prominent GC: We observed this phase in 28% of the specimens. This pattern had uniform and well-developed follicles (Figure 1A): a wide MZ, a defined mantle zone and a prominent GC. There was a large concentration of plasmacytoid or monocytoid CD20+ B cells in the outer layer of MZ. There were also perivascular arteriolar sheaths of B-cells throughout the red pulp (Figure 3R). T-lymphocytes were found delineating a double-ring: an inner circumference limiting GC from mantle zone and an outer one surrounding MZ, separating it from PZ (Figures 3M, 3N). There is a FDCs mesh type 2. Caldesmon+ and SMA-1+ myofibroblast double-crown were also present in a mirror image regarding T cell double-ring, that we have called germinal-marginal double-ring (GMDR) (Figures 2K, 2L).
Figure 3.
M. T-ring emerging from the T-cell meshwork associated to PALS. Follicular hyperplasia, large MZ.CD45RO. N. T-cell double-ring in a follicle with a small GC and wide MZ. Follicular hyperplasia, large MZ. CD45RO. O. Lymphoid follicles with T-cell double-ring in different moments of their organization next to PALS. Follicular hyperplasia, large MZ. CD45RO. P. Follicle with large GC (left). Follicle with small GC and wide MZ (right). Spleen trauma, follicular hyperplasia. CD45RO. Q. Secondary lymphoid follicle. Follicular hyperplasia, developed GC. CD20. R. Secondary lymphoid follicle (left) next to PALS (right). Follicular hyperplasia, large MZ. CD20.
Follicles with small GC and wide MZ: This phase was the most frequently observed in the present study, reaching up to 51% of the total cases – including half the spleen trauma specimens. There was a huge T-cell population in the red pulp and PALS (Figures 3P). The presence of the double T-ring was occasionally viewed. B-lymphocytes configured large MZ. However, B-cell perivascular sheaths were scattered and poorly defined. A backward network of FDCs was observed (pattern 3). Caldesmon+ and SMA-1+myofibroblasts configured a different double-crown. The outer circumference divided PZ from the surrounding red pulp. The inner circumference limited MZ and PZ. We have called this structure the marginal-perifollicular double ring (MPDR) (Figures 2J).
Spleen trauma
Follicular benign hyperplasia was mainly found in a third phase configuration (55%). The presence of primary follicles (11%) or secondary follicles with prominent GC (33%) were observed less frequently. The predominant lineages identified in these large MZ were B cells. The T-cells were scattered and showed no definite distribution in PALS, mantle zone or GC. The mild staining of the CD68+ macrophages revealed a homogeneous population. The red pulp had a spongy appearance surrounding PZ (Figures 2H) and sometimes perifollicular blood congestion was viewed. FDCs networks were configured in a type 3 pattern. Caldesmon+ and SMA-1+myofibroblast population had no double-crown structures.
Splenic marginal zone lymphoma
Non-tumoral and tumoral CD20+ B-lymphocytes were predominant in MZ. The B malignant cells colonized the red pulp and shaped niches between the lymph follicles. CD45RO+ T-lymphocytes were mainly located in the follicles. In general, the macrophage population was handicapped: they drew rather isolated groups in the red pulp. We observed bridges of B malignant lymphocytes that interrupted the continuity and uniformity of the CD68+macrophage meshwork. FDC network had an open-centre that resembled onion layers. Malignant lymphoid cells were located between each of these layers (Figure 1F).
Discussion
CD35 + and CD21+ dendritic cells: about its patterns
Although CD21 was more specific than CD35, neither of them could identify interdigitating dendritic cells, and therefore, changes between inner and outer areas of PALS. Thus, we were not able to report histological findings related to the sequence of DCs maturation in PALS [5, 20, 21-29].
FDCs are more than “passive” APC [27, 30-35]. We found three patterns of FDC mesh configuration. Pattern type 1 belonged to the primary lymph follicle. Pattern 2 was characteristic of secondary lymph follicles with well-developed GC and pattern 3 appeared in secondary lymph follicles with a small LZ, large MZ and plenty of B-cells.
Bofill et al. [36] defined a first AS02+++ CD21+IgM+ FDCs type that is located in primary follicles. They proposed a certain loss of GC structure in the third phase of its development (Table 3).
Table 3.
FDCs patterns and immune status of the lymphoid follicles
| FDCs patterns | Meshwork organization | Bofill et al FDCs types | Follicle's immune status |
|---|---|---|---|
| No staining or cross-linked lymphoid staining | Type 1 | Primary follicles in a latency statement or predesigned secondary follicle statement | |
| Pattern 1 | Mesh in organization | Types 2 and 3 | Activated primary follicle |
| Pattern 2 | FDC blowned network | Types 4 and 5 | Secondary follicles with prominent GC |
| Pattern 3 | Involution or atrophy | Type 6 | Follicle with backwarding GC and wide MZ |
We found no positive staining in primary latent follicles for CD21, CD35, SMA-1 or caldesmon that could identify FDCs or myofibroblasts. Although FDCs mesh became less prominent and the T cell double-ring began to blur, the follicular B-cell population preserved its organization thanks to MPDR. Thus, GC would be not disorganized, but rather it would be a change in the primary roll of cells [36]. On the other hand, we had great difficulties to differentiate B-cell bodies from FDC cytoplasm extensions in the follicles with a GC in regression or inactive primaries follicles. We did not use suitable monoclonal antibodies to obtain this and thus we were unable to view those cells described by Bofill et al.
These findings might indicate that inactive primary follicles have not been colonized by FDCs yet. The FDCs pattern 2 reached and extended itself far from GC: it passed through mantle zone and could reach the inner border of MZ [5, 36] (Figures 1C, 1D) in secondary lymph follicles. The characteristic of this network suggested that cells could play a role in the control of the B-cell maturation sequence [37-43], together with T lymphocytes [37, 38, 44-48].
Myofibroblasts double-crown configurations
The spleen could also be defined as a myofibroblast network with vascular vessels and the immune system throughout [5, 49, 50]. Based on this definition, the weaker staining of the caldesmon antibody (Figure 2L) could be explained by higher cross-linked reactions with erythrocytes and other cell types that densely populate the spleen.
Our work did not provide data on extramedullar haematopoiesis [51]
The splenic myofibroblasts were organized in two double-crowns that limited different morphological compartments (Table 4). A first group was organized in a strong positive staining continuous network throughout the white pulp and limiting it from the red pulp [9, 49, 52, 53] (Figures 2I, 2J). The higher-density staining could be explained by a high concentration of α-actin molecules and the specific activation of these cells [54]. We could not identify a marginal sinus in the human spleen tissue. The barrier role of MPDR [5, 9, 55-59] (Figure 2J) could assume maginal sinus functions in the human spleen tissue, possibly together with macrophages [60-64]. These observations are against the results of classical experiments with microcorrosion cast techniques [51, 65-70]. MPDR configuration suggests that they could correspond to the filtration beds described by Weiss [71, 72] and indeed, they could have cell distribution and filtration functions [73, 74].
Table 4.
Principal morphological characters of myofibroblast
| Myofibroblasts not related to lymphocytes | Myofibroblasts related to lymphocytes |
|---|---|
| SMA-1 and caldesmon high intensity | SMA-1 and caldesmon low intensity |
| Bordering PALS | Inner PALS |
| Marginal-perifollicular myofibroblastic double ring | Germinal-marginal myofibroblastic double ring |
| They would correspond with the filtration beds and barrier cells described by Weiss65,70,71 | In close association with T and B lymphocytes In continuity with FDCs mesh in the seccondary follicles |
The second group of myofibroblasts shaped the GMDR. Its position in lymph follicles was in concordance with the T-cell distribution (Figures 2K, 2L). This configuration might translate immunologic activities [49, 73, 75-77].
Is there a relationship between myofibroblasts and FDCs?
We observed that myofibroblast and FDC networks share common spaces in the inner compartment of the spleen (Figures 1C, 1D, 2K, 2L). The scattered apoptosis images in the backward GC could indicate the need of accurate cell renovation [78-80]. Several reasons could be argued to explain it. Perhaps FDCs are no longer stimulated or have completed a number of cell cycles and then have fallen into apoptosis or alternately might return to a role in myofibroblast mesh [36, 38, 81]. Further studies are needed to clarify it.
Over the last 30 years, two main hypotheses about FDCs origin have been proposed: hematopoietic precursors emigrated from the bone marrow or mesenchymal precursors [27]. Our data could be better explained with findings from different studies that support the mesenchymal hypothesis [5, 39].
The histological proximity of both cells cannot be a coincidence. Muñoz Fernández et al. [82] showed that FDC cell lines could be isolated and supported in fibroblast culture medium. These cell lines could express SMA, so it relates FDC with myofibroblasts. So, under specific conditions, FDC could express a common marker with myofibroblast.
Moreover, other investigators have described the common expression in FDCs and fibroblast cell types of protein antigens [83] such as 3C8 [84] and ASO2 [36] and the presence of FDCs in various non-tumoral pathologies, postulating their development from mesenchymal fibroblasts [38, 48, 85-87].
The macrophage populations: usefulness in pathologic diagnosis
CD68 provided high-quality characterization of the macrophage population in red pulp and lymph follicles. The macrophages appeared as a uniform population in the spleen [78, 79, 89]. The red pulp CD68+ macrophage population was easy to identify by drawing a homogeneous perisinusoidal configuration. We found a loss of integrity of the sinus CD68+delineation [88] locally in splenic traumatic ruptures (Figures 2G, 2H) or tumoral infiltration (i.e. SMZL). It is important to differentiate these cases from “bloody-packed” zones, next to PZ, that are a source of false images of discontinuity. The lack of these lineal CD68+structures could be very useful to the differential diagnosis of SMZL and follicular hyperplasia.
Spleen trauma, follicular hyperplasia and SMZL: key points
The spleens coming from posttraumatic splenectomy were mostly related to those locally or the systemic process provoked an increased production of antibodies (e.g., mononuclear syndromes or lymphomas). Thus it was common to find lymph follicles with large MZ. So B lymphocytes were the predominant observed lineage cell. T lymphocytes seemed to be dispersed in the posttraumatic splenectomy specimens. They were neither a homogenous population in lymph follicles nor in the red pulp. Scattered double T-rings were viewed. In the trauma cases T cells tended to concentrate in PALS, mantle zone and GC. In contrast, in the benign hyperplasia, T cells were clearly in each compartment.
CD68+ macrophages still preserved a medium staining. They acquired a compact aspect in PZ. This phenomenon, due to a rich vasculature of PZ, characterized the spleen trauma cases.
FDC could still be identified in a type 3 pattern. However, SMZL cases are characterized by small B lymphocytes replacing the mantle zone [90]. They even could colonize the reactive follicle center, sweeping away the FDC network.
The importance of FDCs, macrophages and myofibroblasts in diagnosis
The basic immunohistochemical panel for the histopathological study of human spleen that we propose includes the following monoclonal antibodies: CD45RO, CD20, CD21, CD68 and SMA-1. This panel is easy to reproduce in the routine laboratory, it provides enough descriptive data to view the immune compartments of the spleen tissue and, thus, to analyze their alterations. It is essential to consider the extension and patterns of the FDCs network and its relationship to the lymphoid populations in corona and MZ. Infiltrative malignant lymphoid diseases destroy these patterns, setting the dendritic processes apart from each other (Figure 2F). The alteration of CD68+ lineal pattern provides a useful differential diagnosis criterion between SMZL and the follicular hyperplasia.
Acknowledgments
We would like to thank the special and uninterested collaboration in laboratory techniques given by the technical support personal of the department of normal histology and pathology from the University of Seville and the pathology section of the University Hospital Virgen Macarena.
Disclosure of conflict of interest
All authors have not had any financial support in the composition of this article. They have neither had any commercial nor proprietary support in any drug, device, or equipment mentioned in the submitted article.
References
- 1.Fujita T, Kashimura M, Adachi K. Scanning electron microscopy (SEM) studies of the spleen-normal and pathological. Scanning Electron Microsc. 1982;1:435–444. [PubMed] [Google Scholar]
- 2.Kashimura M, Fujita T. A scanning electron microscopy study of human spleen: relationship between microcirculation and functions. Scann Microsc. 1987;1:841–851. [PubMed] [Google Scholar]
- 3.van Krieken JHJM, Te Velde J. Normal Histology of the Human Spleen. Am J Surg Pathol. 1988;12:777–785. doi: 10.1097/00000478-198810000-00007. [DOI] [PubMed] [Google Scholar]
- 4.van Krieken JHJM, Te Velde J, Hermans J, et al. The amount of white pulp in the spleen; a morphometrical study done in methylmethacry-late-embedded splenectomy specimens. Histopathology. 1983;7:167–182. doi: 10.1111/j.1365-2559.1983.tb02289.x. [DOI] [PubMed] [Google Scholar]
- 5.Steiniger B, Barth P. Berlin: Springer-Verlag; 2000. Microanatomy and function of the spleen. [DOI] [PubMed] [Google Scholar]
- 6.Steiniger B, Barth P, Herbst B, Hartnell A, Crocker PR. The species-specific structure of microanatomical compartments in the human spleen: strongly sialoadhesin-positive macrophages occur in the perifollicular zone, but not in the marginal zone. Immunology. 1997;92:307–316. doi: 10.1046/j.1365-2567.1997.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelin-Duclos C, Cattoretti G, Lin K-I, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with blimp-1 expression in vivo. J Immunol. 2000;165:5462–5471. doi: 10.4049/jimmunol.165.10.5462. [DOI] [PubMed] [Google Scholar]
- 8.Steiniger B, Barth P, Hellinger A. The perifollicular and marginal zones of the human splenic white pulp (Do fibroblast guide lymphocyte immigration?) Am J Pathol. 2001;159:501–512. doi: 10.1016/S0002-9440(10)61722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steiniger B, Ruttinger L, Barth PJ. The three-dimensional structure of human splenic white pulp compartments. J Histochem Cytochem. 2003;51:655–664. doi: 10.1177/002215540305100511. [DOI] [PubMed] [Google Scholar]
- 10.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 11.Geberhiwot T, Assefa D, Kortesmaa J, Ingerpuu S, Pedraza C, Wondimu Z, Charo J, Kiessling R, Virtanen I, Tryggvason K, Patarroyo M. Laminin8 (alfa4, beta1, gamma1) is synthesized by lymphoid cells, promotes lymphocyte migration and co-stimulates T cell proliferation. J Cell Science. 2000;114:423–433. doi: 10.1242/jcs.114.2.423. [DOI] [PubMed] [Google Scholar]
- 12.Balázs M, Martin F, Zhou T, Kearney JF. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 13.Eyden B. The myofibroblast: phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J Cell Mol Med. 2008;1:22–37. doi: 10.1111/j.1582-4934.2007.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comin CE, Dini S, Novelli L, Santi R, Asirelli G, Messerini L. h-Caldesmon, a useful positive marker in the diagnosis of pleural malignant mesothelioma, epithelioid type. Am J Surg Pathol. 2006;30:463–469. doi: 10.1097/00000478-200604000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins BS, Wright DH. Cambridge: Cambridge University Press; 2000. Illustrated pathology of the spleen. [Google Scholar]
- 16.Jaffe ES, editor. 2nd. Vol. 16. Philadelphia: W.B. Saunders Company; 1995. Surgical pathology of the lymph nodes and related organs. [Google Scholar]
- 17.Isaacson PG. Conference report: Pathology of the spleen. Paris 1996. Histopatholoy. 1998;32:172–179. doi: 10.1046/j.1365-2559.1998.00311.x. [DOI] [PubMed] [Google Scholar]
- 18.Mollejo M, Menarguez J, Lloret E, Sánchez A, Campo E, Algara P, Cristóbal E, Sánchez E, Piris MA. Splenic marginal zone lymphoma: a distinctive type of low-grade B-cell lymphoma. Am J Pathol. 1995;19:1146–1157. [PubMed] [Google Scholar]
- 19.Mollejo M, Algara P, Mateo MS, Sánchez-Beato M, Lloret E, Medina MT, Piris MA. Splenic small B-Cell lymphoma with predominant red pulp involment; a diffuse variant of splenic marginal zone lymphoma? Histopathology. 2002;40:22–30. doi: 10.1046/j.1365-2559.2002.01314.x. [DOI] [PubMed] [Google Scholar]
- 20.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 21.McIlroy D, Troadec C, Grassi F, Samri A, Barrou B, Autran B, Debré P, Feuillard J, Hosmalin A. Investigation of human spleen dendritic cell phenotype and distribution reveals evidence of in vivo activation in a subset of organ donors. Blood. 2001;97:3470–3477. doi: 10.1182/blood.v97.11.3470. [DOI] [PubMed] [Google Scholar]
- 22.Reis e Sousa C, Henry S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their distribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, de Baetselier P, Urbain J, Leo O, Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Termeer C, Johannsen H, Braun T, Renkl A, Ahrens T, Denfeld RW, Lappin MB, Weiss JM, Simon JC. The role of CD44 during CD40 ligand-induced dendritic cell clustering and maturation. J Leukoc Biol. 2001;70:715–722. [PubMed] [Google Scholar]
- 25.Bjorck P, Flores-Romo L, Liu YJ. Human interdigitating dendritic cells directly stimulate CD40activated naive B cells. Eur J Immunol. 1997;27:1266–1274. doi: 10.1002/eji.1830270531. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Choi K, Ahn H, Song K, Choe J, Lee I. TuJ1 (class III beta-tubulin) expression suggests dynamic redistribution of follicular dendritic cells in lymphoid tissue. Eur J Cell Biol. 2005;84:453–459. doi: 10.1016/j.ejcb.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Banchereau J, Steinman RM. Dendritic cell and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 28.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 29.Hollowood K, Goodlad JR. Germinal centre cell kinetics. J Pathol. 1998;185:229–233. doi: 10.1002/(SICI)1096-9896(199807)185:3<229::AID-PATH86>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y-J, Grouard G, de Boutellier O, Banchereau J. Follicular dendritic cells and germinal centers. Int Rev Cytol. 1996;166:139–179. doi: 10.1016/s0074-7696(08)62508-5. [DOI] [PubMed] [Google Scholar]
- 31.Szakal AK, Kapasi ZF, Masuda A, Tew JG. Follicular dendritic cells in the alternative antigen transport pathway: microenvironment, cellular events, age and retrovirus related alterations. Sem Immunol. 1992;4:257–265. [PubMed] [Google Scholar]
- 32.Qin D, Wu J, Burton GF, Szakal AK, Tew JG. Follicular dendritic cells mediated maintenance of primary lymphocyte cultures for long-term analysis of a functional in vitro immune system. J Immunol Methods. 1999;226:19–27. doi: 10.1016/s0022-1759(99)00038-1. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Zhang X, Kovacic S, Long AJ, Bourque K, Wood CR, Choi YS. Identification of human follicular dendritic cell molecule that stimulates germinal center B cell growth. J Exp Med. 2000;191:1077–1084. doi: 10.1084/jem.191.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young B, Heath JW, editors. Weather's functional histology. Manual and atlas book. 4a. Madrid: Harcourt international iberoamerican division; 2001. Immune system; pp. 216–221. [Google Scholar]
- 35.Hur DY, Kim DJ, Kim S, et al. Role of follicular dendritic cells in the apoptosis of germinal center B cells. Immunol Lett. 2000;72:107–111. doi: 10.1016/s0165-2478(00)00166-8. [DOI] [PubMed] [Google Scholar]
- 36.Bofill M, Akbar AN, Amlot PL. Follicular dendritic cells share a membrane-bound protein with fibroblasts. J Pathol. 2000;191:217–226. doi: 10.1002/(SICI)1096-9896(200006)191:2<217::AID-PATH586>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Carroll M. Role of complement receptors CD21/CD35 in B lymphocyte activation and survival. Curr Top Microbiol Immunol. 1999;246:63–68. doi: 10.1007/978-3-642-60162-0_8. [DOI] [PubMed] [Google Scholar]
- 38.Kosco MH, Pflugfelder E, Gray D. Follicular dendritic cell-dependent adhesion and proliferation of B cells in vitro. J Immunol. 1992;148:2331–2339. [PubMed] [Google Scholar]
- 39.van Nierop K, de Groot C. Human follicular dendritic cells: function, origin and development. Sem Immunol. 2002;14:251–257. doi: 10.1016/s1044-5323(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 40.Tew JG, Wu J, Qin D, Helm S, Burton GF, Szakal AK. Follicular dendritic cells and presentation of antigen and co-stimulatory signals to B cells. Immunol Rev. 1997;156:39–52. doi: 10.1111/j.1600-065x.1997.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 41.Choe J, Li L, Zhang X, Gregory CD, Choi YS. Distinct role of follicular dendritic cells and T cells in the proliferation, differentiation and apoptosis of a centroblast cell line, L3055. J Immunol. 2000;164:56–63. doi: 10.4049/jimmunol.164.1.56. [DOI] [PubMed] [Google Scholar]
- 42.Hollmann C, Gerdes J. Follicular dendritic cells and T cells: nurses and executioners in the germinal centre reaction. J Pathol. 1999;189:147–149. doi: 10.1002/(SICI)1096-9896(199910)189:2<147::AID-PATH433>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Rissoan M-C, Soumelis V, Kadowaki N, Grouard G, Briere F, Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 44.Berney C, Herren S, Power CA, Gordon S, Martinez-Pomares L, Kosco-Vilbois MH. A member of the dendritic cell family that enters B cell follicles and stimulates primary antibody responses identified by a mannose receptor fusion protein. J Exp Med. 1999;190:851–860. doi: 10.1084/jem.190.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butch AW, Kelly K.A, Willbanks MS, Yu X. Human follicular dendritic cells inhibit superan-tigen-induced T-cell proliferation by distinct mechanisms. Blood. 1999;94:216–224. [PubMed] [Google Scholar]
- 46.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 47.Ngo VN, Hyman PL, Luther SA, Förster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organize lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 48.Grouard G, Durand I, Filgueira L, Banchereau J, Liu YJ. Dendritic cells capable of stimulating T cells in germinal centers. Nature. 1996;384:364–367. doi: 10.1038/384364a0. [DOI] [PubMed] [Google Scholar]
- 49.Borrello MA, Phipps RP. Differential Thy-1 expression by splenic fibroblasts defines functionally distinct subsets. Cell Immunol. 1996;179:198–206. doi: 10.1006/cimm.1996.0268. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida K, Tamahashi N, Matsuura N, Takahashi T, Tachibana T. Antigenic heterogeneity of the reticular meshwork in the white pulp of mouse spleen. Cell Tissue Res. 1991;226:223–229. doi: 10.1007/BF00318177. [DOI] [PubMed] [Google Scholar]
- 51.Bowdler AJ, editor. 2nd ed. New Jersey: Humana Press; 2002. The complete spleen: structure, function and clinical disorders; pp. 3–106. [Google Scholar]
- 52.Pinkus GS, Warhol MJ, O'Connor EM, Etheridge CL, Fujiwara K. Immunohistochemical localization of smooth muscle myosin in human spleen, lymph node, and other lymphoid tissues. Unique staining patterns in splenic white pulp and sinuses, lymphoid follicles, and certain vasculature, with ultrastructural correlations. Am J Pathol. 1986;123:440–453. [PMC free article] [PubMed] [Google Scholar]
- 53.Toccainer-Pelte M-F, Skalli O, Kapanci Y, Gabbiani G. Characterization of stromal cells with myoid features in lymph nodes and spleen in normal and pathologic conditions. Am J Pathol. 1987;129:109–118. [PMC free article] [PubMed] [Google Scholar]
- 54.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Camacho FI, García JF, Sánchez-Verde L, Sáez AI, Sánchez-Beato M, Mollejo M, Piris MA. Unique phenotypic profile of monocytoid B cells. Differences in comparison with the phenotypic profile observed in marginal zone B cells and so-called monocytoid B cell lymphoma. Am J Pathol. 2001;158:1363–1369. doi: 10.1016/S0002-9440(10)64087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindhout E, Vissers JLM, Hartgers FC, Huijbens RJF, Scharenborg NM, Figdor CG, Adema GJ. The dendritic cell-specific CC-chemokine DCCK1 is expressed by germinal center dendritic cells and attracts CD 38-negative mantle zone B lymphocytes. J Immunol. 2001;166:3284–3289. doi: 10.4049/jimmunol.166.5.3284. [DOI] [PubMed] [Google Scholar]
- 57.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Perren Cobb J, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 58.Zandvoort D, Lodewijk ME, de Boer NK, Dammers PM, Kroese FGM, Timens W. CD27 expression in the human splenic marginal zone: the infant marginal zone is populated by naive B cells. Tissue Antigens. 2001;58:234–242. doi: 10.1034/j.1399-0039.2001.580403.x. [DOI] [PubMed] [Google Scholar]
- 59.Morente M, Piris MA, Orradre JL, Rivas C, Villuendas R. Human tonsil intraepithelial B cells: a marginal-zone related subpopulation. J Clin Pathol. 1992;45:668–672. doi: 10.1136/jcp.45.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liakka A, Apaja-Sarkkinen M, Karttunen T, Autio-Harmainen H. Distribution of laminin, and types IV and III collagen in fetal, infant and adult human spleens. Cell Tissue Res. 1991;263:245–252. doi: 10.1007/BF00318766. [DOI] [PubMed] [Google Scholar]
- 61.Liakka A, Karjalainen H, Virtanen I, Auto-Harmainen H. Immuno-electron-microscopic localization of types III pN- collagen and IV collagen, laminin and tenascin in developing and adult human spleen. Cell Tissue Res. 1995;282:117–127. doi: 10.1007/BF00319138. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida T, Takaya K. Follicular dendritic reticular cells in the germinal center of the rat lymph node as studied by immuno-electron microscopy. Arch Histol Cytol. 1989;52:327–335. doi: 10.1679/aohc.52.327. [DOI] [PubMed] [Google Scholar]
- 63.Trial J, Rice L, Alfrey CP. Erythropoietin withdrawal alters interactions between young red blood cells, splenic endothelial cells, and macrophages: an in vitro model of neocytolisis. J Invest Med. 2001;49:335–345. doi: 10.2310/6650.2001.33899. [DOI] [PubMed] [Google Scholar]
- 64.Hartnell A, Steel J, Turley H, Jones M, Jackson DG, Crocker PR. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. 2001;97:288–296. doi: 10.1182/blood.v97.1.288. [DOI] [PubMed] [Google Scholar]
- 65.Tablin F, Weiss L. The equine spleen: an electron microscopic analysis. Am J Anat. 1983;166:393–416. doi: 10.1002/aja.1001660403. [DOI] [PubMed] [Google Scholar]
- 66.Uehara K, Miyoshi M. Junctions between the sinus endothelial cells of rat spleen. Cell Tissue Res. 1997;287:187–192. doi: 10.1007/s004410050744. [DOI] [PubMed] [Google Scholar]
- 67.Schmidt EE, MacDonald IC, Groom AC. Comparative aspects of splenic microcirculatory pathways in mammals: the region bordering the white pulp. Scann Microsc. 1993;7:613–628. [PubMed] [Google Scholar]
- 68.Schmidt EE, MacDonald IC, Groom AC. Microcirculatory pathways in normal human spleen, demonstrated by scanning electron microscopy of corrosion cats. Am J Anat. 1988;181:253–266. doi: 10.1002/aja.1001810304. [DOI] [PubMed] [Google Scholar]
- 69.Sandler MP, Kronenberg MW, Forman MB, Wolfe OH, Clanton JA, Partain CL. Dynamic fluctuations in blood and spleen radioactivity: splenic contraction and relation to clinical radionuclide volume circulations. J Am Cardiol. 1984;3:1205–1211. doi: 10.1016/s0735-1097(84)80178-3. [DOI] [PubMed] [Google Scholar]
- 70.Pellas TC, Weiss L. Deep splenic lymphatic vessels in the mouse: a route of splenic exit for recirculating lymphocytes. Am J Anat. 1990;187:347–354. doi: 10.1002/aja.1001870404. [DOI] [PubMed] [Google Scholar]
- 71.Weiss L. The red pulp of the spleen: structural basis of blood flow. Clin Haematol. 1983;12:375–393. [PubMed] [Google Scholar]
- 72.Weiss L. New trends in spleen research: conclusion. Experientia. 1985;41:243–248. doi: 10.1007/BF02002619. [DOI] [PubMed] [Google Scholar]
- 73.Nolte MA, Belien JA, Schadee-Eestermans I, Jansen W, Unger WW, van Rooijen N, Kraal G, Mebius RE. A conduit system distributes chemokines and small blood-borne molecules though the splenic white pulp. J Exp Med. 2003;198:505–512. doi: 10.1084/jem.20021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weiss L. Barrier cells in the spleen. Immunol Today. 1991;12:24–29. doi: 10.1016/0167-5699(91)90108-6. [DOI] [PubMed] [Google Scholar]
- 75.Kesmir C, de Boer RJ. A spacial model of germinal center reactions: cellular adhesion based sorting of B cells results in efficient affinity maturation. J Theor Biol. 2003;222:9–22. doi: 10.1016/s0022-5193(03)00010-9. [DOI] [PubMed] [Google Scholar]
- 76.Saalbach A, Wetzig T, Haustein UF, Anderegg U. Detection of human soluble Thy-1 in serum by ELISA. Fibroblasts and activated endothelial cells are a possible source of soluble Thy-1 in serum. Cell Tissue Res. 1999;298:307–15. doi: 10.1007/s004419900079. [DOI] [PubMed] [Google Scholar]
- 77.MacLennan ICM, Gulbranson-Judge A, Toellner K-M, Casamayor-Palleja M, Chan E, Sze DM-Y, Luther SA, Acha Orbea H. The changing preference of T and B cells for partners as T dependent antibody response develop. Immunol Rev. 1997;156:53–66. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 78.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg R E Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD 4 + lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 79.Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol. 2003;171:909–914. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 80.Charboard P, Rémy-Martin JP, Tamayo E, Bernard G, Keating A, Péault B. Analysis of the microenvironment necessary for engraftment: role of the vascular smooth muscle-like stromal cells. J Haematol Stem Cell. 2000;9:935–943. doi: 10.1089/152581600750062390. [DOI] [PubMed] [Google Scholar]
- 81.Briard D, Brouty-Boyé D, Azzarone B, Jasmin C. Fibroblast from human spleen regulate NK cell differentiation from blood CD34+ progenitors via cell surface IL-15. J Immunol. 2002;168:4326–4332. doi: 10.4049/jimmunol.168.9.4326. [DOI] [PubMed] [Google Scholar]
- 82.Muñoz Fernandez R, Blanco FJ, Frecha C, Martín F, Kimatrai M, Abadia-Molina AC, Garc-ía-Pacheco JM, Olivares EG. Follicular dendritic cells are related to bone narrow stromal cell progenitors and to myofibroblasts. J Immunol. 2006;177:280–289. doi: 10.4049/jimmunol.177.1.280. [DOI] [PubMed] [Google Scholar]
- 83.Sun X, Chang K-C, Abruzzo LV, Lai R, Younes A, Jones D. Epidermal growth factor receptor expression in follicular dendritic cells: a shared feature of follicular dendritic cell sarcoma and Castleman's disease. Hum pathol. 2003;34:835–839. doi: 10.1016/s0046-8177(03)00356-3. [DOI] [PubMed] [Google Scholar]
- 84.Lee Yong I, Choe J. Human follicular dendritic cells and fibroblasts share the 3C8 antigen. Biochem Bioph Res Co. 2003;304:701–707. doi: 10.1016/s0006-291x(03)00649-1. [DOI] [PubMed] [Google Scholar]
- 85.Lindhout E, van Eijk M, van Pel M, Lindeman J, Dinant HJ, de Groot C. Fibroblasts-like synoviocytes from rheumatoid arthritis patients have intrinsic properties of follicular dendritic cells. J Immunol. 1999;162:5945–5956. [PubMed] [Google Scholar]
- 86.Müller-Hamerlink HK, van Gaudecker B, Drenckhahn D, Jaworsky K, Felderman C. Fibroblastic and dendritic reticulum cells of lymphoid tissue. J Cancer Res Clin Oncol. 1981;101:149–164. doi: 10.1007/BF00405075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Imal Y, Yamakawa M. Morphology, function and pathology of follicular dendritic cells. Pathol Int. 1996;46:807–833. doi: 10.1111/j.1440-1827.1996.tb03555.x. [DOI] [PubMed] [Google Scholar]
- 88.Buckley PJ, Dickson SA, Walker WS. Human splenic sinusoidal lining cells express antigens associated with monocytes, macrophages, endothelial cells, and T lymphocytes. J Immunol. 1985;134:2310–2315. [PubMed] [Google Scholar]
- 89.Karlsson MC, Guinamard R, Bolland S, Sankala M, Steinman RM, Ravetch JV. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J Exp Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bahler DW, Ander Pindzola J, Swerdlow SH. Splenic marginal zone lymphoma appear to originate from different B cell types. Am J Pathol. 2002;161:81–88. doi: 10.1016/S0002-9440(10)64159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]