Abstract
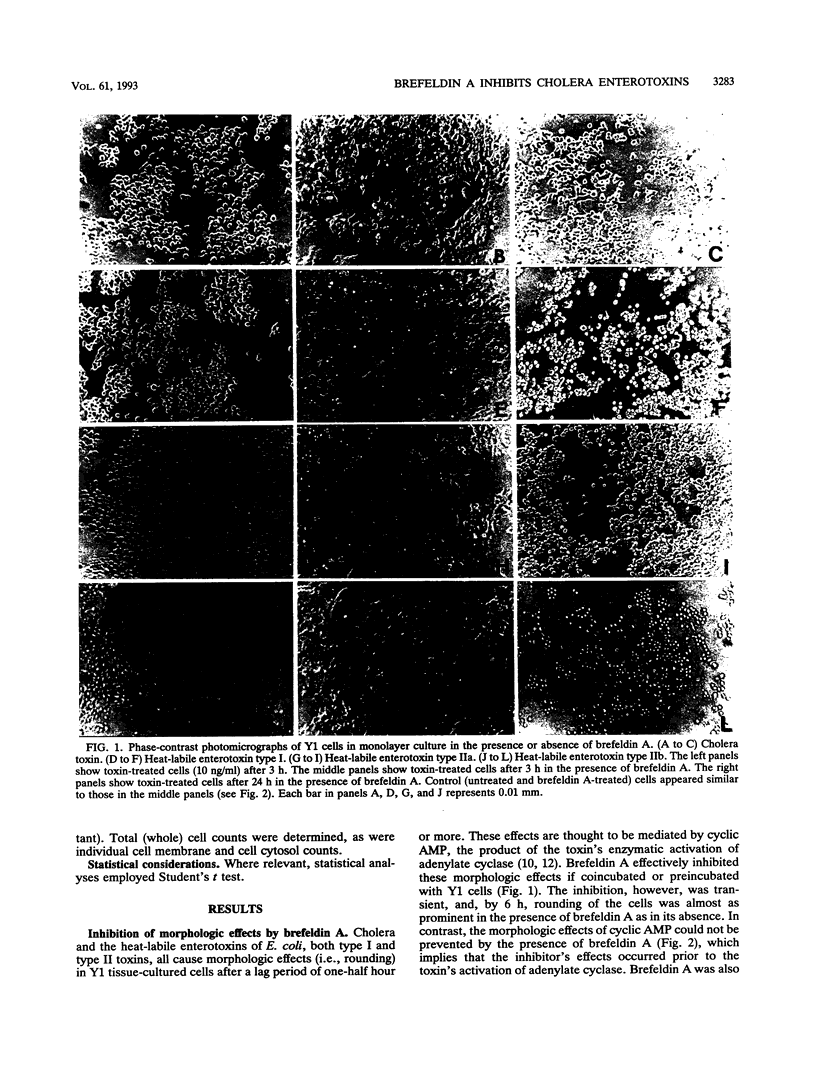
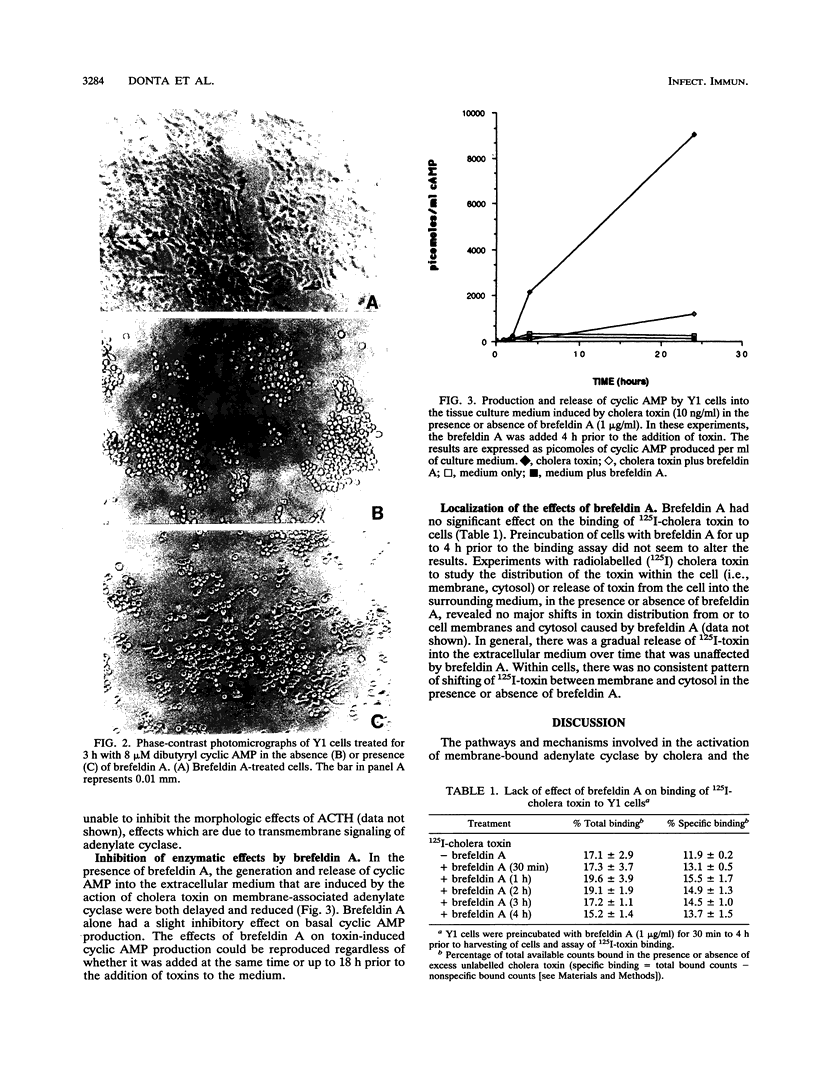
Cholera enterotoxin and the related heat-labile enterotoxins of Escherichia coli enter their target cells through noncoated vesicles, but how the toxins are processed intracellularly and how they get to their targeted enzyme, adenylate cyclase, remain to be defined. Brefeldin A, an inhibitor of the trans-Golgi network, is shown herein to transiently block the morphologic and enzymatic effects of the toxin at a step distal to the initial binding process but prior to activation of adenylate cyclase by the toxin. It is likely, therefore, that these toxins are processed by the Golgi apparatus before trafficking to the membrane adenylate cyclase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres D. A., Rhodes J. D., Meisel R. L., Dixon J. E. Characterization of the carboxyl-terminal sequences responsible for protein retention in the endoplasmic reticulum. J Biol Chem. 1991 Aug 5;266(22):14277–14282. [PubMed] [Google Scholar]
- Cerione R. A., Strulovici B., Benovic J. L., Lefkowitz R. J., Caron M. G. Pure beta-adrenergic receptor: the single polypeptide confers catecholamine responsiveness to adenylate cyclase. Nature. 1983 Dec 8;306(5943):562–566. doi: 10.1038/306562a0. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Damiano-Burbach P., Poindexter N. J. Modulation of enterotoxin binding and function in vitro and in vivo. J Infect Dis. 1988 Mar;157(3):557–564. doi: 10.1093/infdis/157.3.557. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Haddow A. D. Cytotoxic activity of Aeromonas hydrophila. Infect Immun. 1978 Sep;21(3):989–993. doi: 10.1128/iai.21.3.989-993.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta S. T., Poindexter N. J., Ginsberg B. H. Comparison of the binding of cholera and Escherichia coli enterotoxins to Y1 adrenal cells. Biochemistry. 1982 Feb 16;21(4):660–664. doi: 10.1021/bi00533a011. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Sullivan N., Wilkins T. D. Differential effects of Clostridium difficile toxins on tissue-cultured cells. J Clin Microbiol. 1982 Jun;15(6):1157–1158. doi: 10.1128/jcm.15.6.1157-1158.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta S. T., Tomicic T., Holmes R. K. Binding of class II Escherichia coli enterotoxins to mouse Y1 and intestinal cells. Infect Immun. 1992 Jul;60(7):2870–2873. doi: 10.1128/iai.60.7.2870-2873.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Dorner F. Cholera enterotoxin (choleragen). Pharmacol Ther. 1985;27(1):37–47. doi: 10.1016/0163-7258(85)90063-4. [DOI] [PubMed] [Google Scholar]
- Fiorentini C., Malorni W., Paradisi S., Giuliano M., Mastrantonio P., Donelli G. Interaction of Clostridium difficile toxin A with cultured cells: cytoskeletal changes and nuclear polarization. Infect Immun. 1990 Jul;58(7):2329–2336. doi: 10.1128/iai.58.7.2329-2336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res. 1977;8:85–118. [PubMed] [Google Scholar]
- Janicot M., Clot J. P., Desbuquois B. Interactions of cholera toxin with isolated hepatocytes. Effects of low pH, chloroquine and monensin on toxin internalization, processing and action. Biochem J. 1988 Aug 1;253(3):735–743. doi: 10.1042/bj2530735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicot M., Desbuquois B. Fate of injected 125I-labeled cholera toxin taken up by rat liver in vivo. Generation of the active A1 peptide in the endosomal compartment. Eur J Biochem. 1987 Mar 2;163(2):433–442. doi: 10.1111/j.1432-1033.1987.tb10816.x. [DOI] [PubMed] [Google Scholar]
- Janicot M., Fouque F., Desbuquois B. Activation of rat liver adenylate cyclase by cholera toxin requires toxin internalization and processing in endosomes. J Biol Chem. 1991 Jul 15;266(20):12858–12865. [PubMed] [Google Scholar]
- Joseph K. C., Stieber A., Gonatas N. K. Endocytosis of cholera toxin in GERL-like structures of murine neuroblastoma cells pretreated with GM1 ganglioside. Cholera toxin internalization into Neuroblastoma GERL. J Cell Biol. 1979 Jun;81(3):543–554. doi: 10.1083/jcb.81.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of cholera toxin and Escherichia coli heat-labile enterotoxins by ADP-ribosylation factors, a family of 20 kDa guanine nucleotide-binding proteins. Mol Microbiol. 1991 Nov;5(11):2621–2627. doi: 10.1111/j.1365-2958.1991.tb01971.x. [DOI] [PubMed] [Google Scholar]
- Orci L., Tagaya M., Amherdt M., Perrelet A., Donaldson J. G., Lippincott-Schwartz J., Klausner R. D., Rothman J. E. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991 Mar 22;64(6):1183–1195. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Heat shock and the sorting of luminal ER proteins. EMBO J. 1989 Nov;8(11):3171–3176. doi: 10.1002/j.1460-2075.1989.tb08475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmer B. P., Ueda K., Sato G. H. Site of action of adrenocorticotropic hormone (ACTH) in adrenal cell cultures. Biochem Biophys Res Commun. 1968 Sep 6;32(5):806–810. doi: 10.1016/0006-291x(68)90312-4. [DOI] [PubMed] [Google Scholar]
- Tran D., Carpentier J. L., Sawano F., Gorden P., Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnieski B. J., Bramhall J. S. Photolabelling of cholera toxin subunits during membrane penetration. Nature. 1981 Jan 22;289(5795):319–321. doi: 10.1038/289319a0. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Chen C. C., Zhang M. S., Wu H. C. Disruption of the Golgi apparatus by brefeldin A inhibits the cytotoxicity of ricin, modeccin, and Pseudomonas toxin. Exp Cell Res. 1991 Feb;192(2):389–395. doi: 10.1016/0014-4827(91)90056-z. [DOI] [PubMed] [Google Scholar]