Abstract
A 30-year-old male with no previous history of neoplastic disease presented with a 5 cm large testicular tumor. Routine histopathological examination and immunohistochemical investigation showed a classical seminoma with a contiguous 8 mm large nodule. The nodule was separated from the tunica albuginea by tubuli seminiferi showing intratesticular germ cell neoplasi not otherwise specified (NOS). The nodule was composed of spindle cells with low-grade nuclear atypia, nuclear and cytoplasmic S100 protein immunoreactivity in 15% of the cells and a proliferative activity of up to 20%. No other germ cell tumor components were found. To the best of our knowledge, we herein present the first tumor of a pure classical seminoma with an associated low-grade sarcomatous component.
Keywords: Testis, classical seminoma, sarcoma
Introduction
Sarcomatous transformation with different histogenetic differentiation in spermatocytic seminoma are well known to occur [1-4], but there has been no case described where a sarcoma has occurred in a pure classical seminoma. We herein describe, to the best of our knowledge, the first case of a pure classical seminoma in which there was an associated low-grade sarcomatous component.
Clinical summary
A 30-year-old male with no previous history of neoplastic disease presented with a 5 cm large testicular tumor. A radical inguinal orchidectomy was performed. Adjuvant chemotherapy was administered. The patient is alive and well 12 months after surgery.
Materials and methods
A comprehensive immunohistochemical study was performed with routine antibodies according to the instructions of the manufacturers. The following monoclonal antibodies were employed: CD56 (1B6, 1:100, Novocastra, Newcastle, UK), CD30 (Ber-H2, 1:100, DakoCytomation, Glostrup, Denmark), CD31 (JC/70A, 1:50, Dako, Glostrup, Denmark), CD34 (QBEnd/10, 1:800, NeoMarkers, Westinghouse, CA, USA), inhibin (R1, 1:30, Bio-Innovation, Oxford, UK), desmin (D33, 1:2000, DakoCytomation, Glostrup, Denmark), smooth muscle actin (1A4, DAKO, Glostrup, Denmark), p63 (4A4, 1:500, NeoMarkers, Westinghouse, CA, USA), calretinin (5A5, Novocastra, Newcastle, UK), cytokeratins: (CAM 5.2, 1:200, Becton Dickinson, Erembogeden, Belgium), cytokeratin 5/6, D5/16B4, 1:100, DakoCytomation, Glostrup, Denmark), S100 protein (S1/61/69, 1:50, Novocastra, Newcastle, UK), EMA (E29, 1:500, Dako, Glostrup, Denmark), Ki-67 (MIB1, 1:1000, DakoCytomation, Glostrup, Denmark), p53 (DO-7, 1:50, DakoCytomation, Glostrup, Denmark), Melan A (A 103, 1:100, DakoCytomation, Glostrup, Denmark), MTF (D5, 1:25, NeoMarkers, Westinghouse, CA, USA), MyoD1 (5.8A, 1:50, DakoCytomation, Carpinteria, CA, USA), HMB-45 (HMB45, 1:200, DakoCytomation, Carpinteria, CA, USA), vimentin (V9, 1:1000, NeoMarkers, Westinghouse, CA, USA), ALK-1 (p80, 1:400, Novocastra, Newcastle, UK), bcl-2 (clone 124, 1:300, DakoCytomation, Glostrup, Denmark). Polyclonal antibodies listed below were used for our study: myoglobin (DakoCytomation, 1:1000, Glostrup, Denmark), CD117 (DakoCytomation, 1:150, Glostrup, Denmark), alpha-fetoprotein (DakoCytomation, 1: 500, Glostrup, Denmark), hCG (DakoCytomation, 1: 2000, Glostrup, Denmark) and OCT ¾ (Santa Cruz, 1:3200, Vienna, Austria). A molecular genetic study was performed using FISH analysis with premixed probes 12p / CEP 12 (Vysis/Abbott, IL, USA) for isochromosome 12p and probe 9p21 (Vy-sis/Abbott, IL, USA) to detect deletion of 9p21.
Results
The tumor was confined to the testis, i.e. did not grow through the tunica albuginea or into the epididymis. The size of the tumor was 5 cm and was homogenously grey on gross section. No necrotic areas were seen.
The whole tumor was embedded in paraffin and 4μm thin sections were cut and stained with Hematoxylin and Eosin. Routine histopathological examination showed a classical seminoma with no other germ cell tumor components (Figure 1). In addition, there was an 8 mm large nodule contiguous to the seminoma (Figure 2). The nodule was separated from the tunica albuginea by tubuli seminiferi showing intratesticular germ cell neoplasia, not otherwise specified (NOS) (Figure 3). The lesional tissue in the nodule displayed a completely different morphology. It was composed of a well delineated, non-encapsulated, moderately cellular, spindle cell lesion with cells having slender cytoplasmic processes arranged in fascicles and with scattered intra-lesional lymphocytes (Figure 4). On high-power examination, lesional cells showed low-grade nuclear atypia. Mitotic figures were easily found (Figure 5). The immunohistochemical study showed that lesional spindle cells were positive for vimentin (100% of cells) and S100 protein (including nuclear positivity) in approximately 15% of the cells (Figure 6). The neoplastic cells were negative for all other antibodies. The proliferation index as measured with immunohistochemistry (Mib-1/Ki-67) was approximately 20% (Figure 7). In the molecular genetic study amplification of isochromosome 12p (Figure 8), but no deletion of 9p21 was found in the seminomatous component. The FISH-study in the sarcomatous component was not successful.
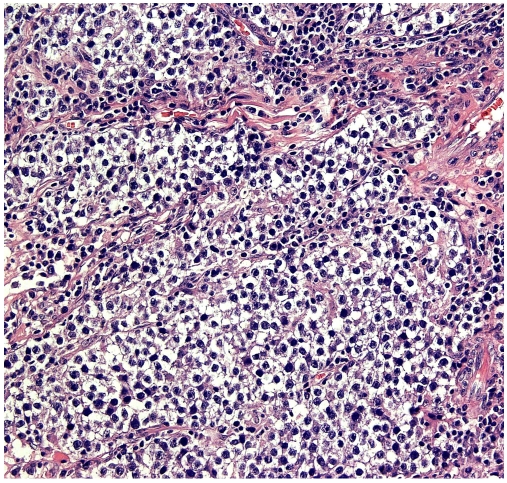
Figure 1.
Photomicrograph of a Hematoxylin and Eosin stained section from the main tumor component showing histomorphological features of a classical seminoma.
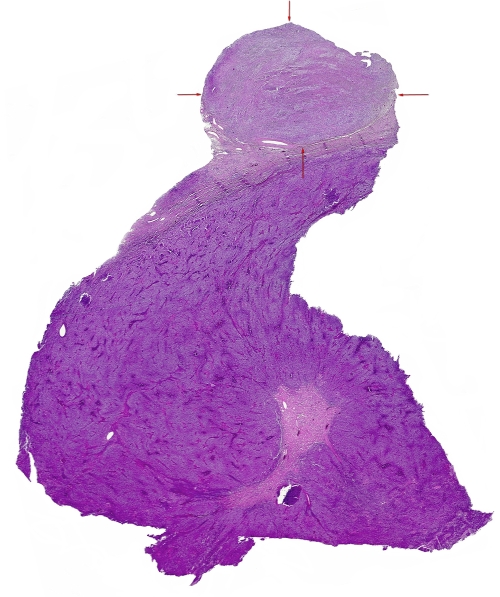
Figure 2.
Photomicrograph of a whole mounted section stained with Hematoxylin and Eosin of a classical seminoma with a 8 mm nodule in the periphery (depicted by the arrows) with a spindle cell lesion with a low-grade sarcomatous morphology.
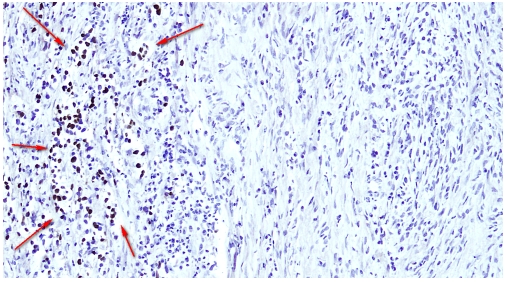
Figure 3.
Photomicrograph from the immunohistochemical study; OCT 3/4 showing tubuli seminiferi with intratesticular germ cell neoplasia NOS located contiguous to the low-grade sarcoma. The nuclear positivity is depicted by arrows.
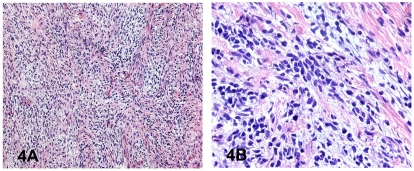
Figure 4A,B.
Photomicrographs of Hematoxylin and Eosin stained sections from the low-grade sarcomatous area in a seminoma showing moderate cellularity. Neoplastic cells have a predominantly spindle cell morphology. There are intralesional lymphocytes.
Figure 5.
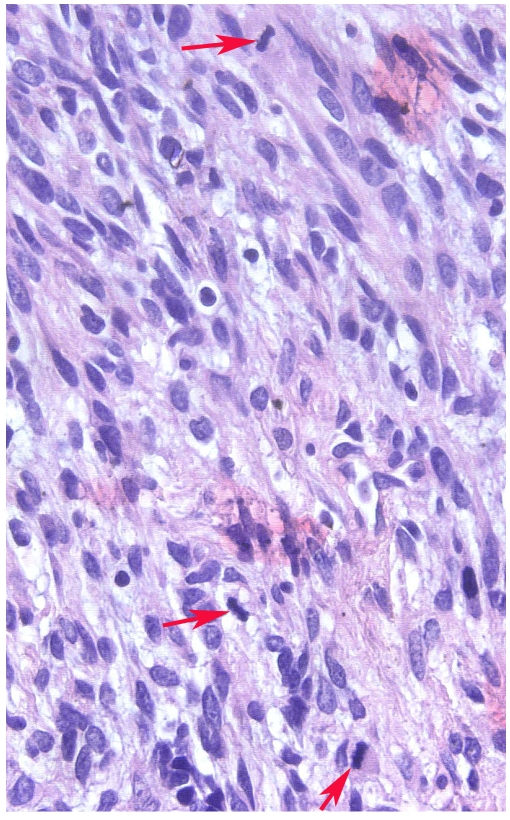
Photomicrograph (High-power) of Hematoxylin and Eosin stained section from the sarcomatous area in a classical seminoma showing nuclear abnormalities, i.e. hyperchromasia and irregular shape and three mitotic figures (arrows).
Figure 6.
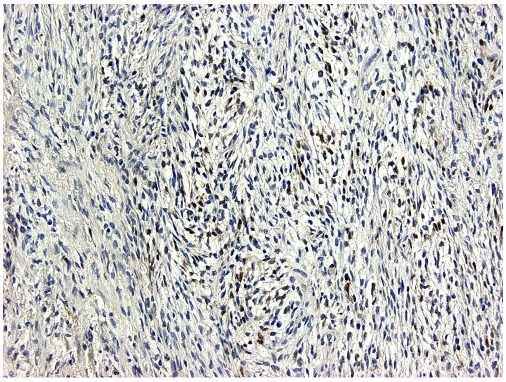
Photomicrograph of section from the low-grade sarcomatous area in a classical seminoma that have been immunohistochemically stained for S100 protein. About 15% of neoplastic cells show both nuclear and cytoplasmic reactivity for S100 protein.
Figure 7.
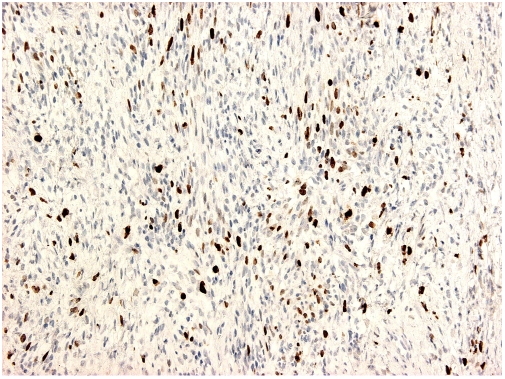
Photomicrograph from the low-grade sarcomatous area in a classical seminoma that have been immunohistochemically stained for Ki-67 protein. About 20% of neoplastic cells show nuclear positive reaction.
Figure 8.
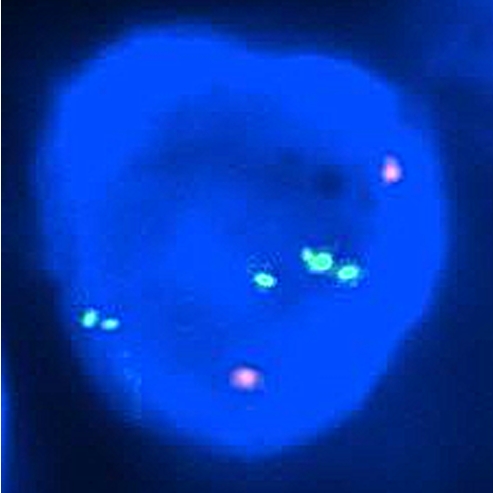
Photomicrographs from the molecular genetic study showing a nucleus from the classical seminomatous component. FISH analysis using 12p (Spectrum Green) and CEP 12 (Spectrum Orange) probe. Visualization under Dual Band Pass filter. Amplified five green (12p) and two red (CEP 12) signals are presented in the nucleus.
Discussion
Spermatocytic seminoma occurring together with a sarcomatous component is a well recognized phenomenon [1-4]. Such tumors are usually diagnosed as high stage neoplasms with metastatic disease at time of surgery. The sarcomatous component in these tumors is variable (rhabdomyosarcoma, chondrosarcoma or undifferentiated sarcoma). Our case differs from spermatocytic seminoma by the absence of the characteristic three cell population of spermatogonia-like cells, small lymphocyte-like cells and intermediate cells. Moreover, the tumor lacked the oedematous stroma typically seen in spermatocytic seminoma. That the germ cell tumor presented herein is a classical and not a spermatocytic seminoma is supported by the molecular genetic study showing amplification of isochromosome 12p and lack of deletion of 9p21.
No tumor similar to the one presented here has been described in the testis, but there are two similar cases on record. One case with a rhabdomyosarcoma occurring in a dysgerminoma of the ovary where no other germ cell tumor (GCT) component was found [5] and one case with the development of rhabdomyosarcoma in a retroperitoneal GCT composed of a pure seminoma [6]. Generally, primary testicular sarcomas are extremely rare neoplasms with only a limited number of cases reported in the literature. These sarcomas have been variously classified [7-14 15, 16 1, 17-22]. Testicular teratomas with somatic malignant transformation including sarcoma are rare but well documented and characterized [20, 22-24]. Furthermore, there are primary testicular tumors on record that are classified as “malignant mixed gonadal stromal tumor with mesenchymal heterologous elements of the testis” [21], “testicular mixed gonadal stromal tumor with osteosarcoma component” [25], “sarcomatoid Leydig cell tumor” [26] and “Leydig cell tumors with adipose differentiation, calcification, ossification, and spindle-shaped tumor cells” [27]. In a recent, and to date the largest, study on testicular GCTs with sarcomatous components by Guo et al [28], 33 cases were presented. Of these 33 cases, 19 had a sarcomatous component in the testicular tumor only, in 11 cases the sarcoma was found in the metastasis only and in 3 cases both in the testicular tumor and the metastasis. All testicular GCTs contained a teratomatous component (pure in 3 cases and mixed GCTs in the rest). In none of the cases was there a pure classical seminoma associated with a sarcoma. As inclusion criterion, Guo et al employed that suggested by Ulbright et al [29] (“Expansile sarcomatous tumor growth of at least one low-power field (4 x objective) replacing the GCT component”), a quantitative criterion that was met by the sarcomatous component in the case presented by us. In only one of the cases presented by Guo et al, was there a low-grade sarcoma (“low-grade unclassified myxoid sarcoma”), the rest were high-grade sarcomatous neoplasms. Based on the verbal description and the presented picture (Figure 4) in the paper by Guo et al, this low-grade unclassified myxoid sarcoma show morphological similarities to the low-grade sarcomatous component presented herein. However, the authors found no immunohistochemical evidence suggesting any specific line of differentiation in this case (also true for additional four cases in their study). This is in contrast to the distinct immunoreactivity for S100 protein that was detected in our case. Based on (1) the presence of low-grade but distinct nuclear atypia and (2) a high proliferative activity and (3) the lack of histological features as well as immunohistochemical findings in line with any previously described benign entity in this anatomical location, we conclude that the spindle cell component in our case represents a low-grade sarcoma. The well circumscribed, non-encapsulated border of the spindle cell lesion does not preclude a diagnosis of sarcoma. Small sarcomas of various types are well known for having a relatively good circumscription. This is true for example in minute synovial sarcomas of the hands and feet [30] and small superficial epithelioid sarcomas as well as small alveolar soft part sarcomas in the tongue and endocervix. We found no light microscopical or immunohistochemical evidence of melanocytic, endothelial, smooth muscle, rhabdomyoblastic, myofibroblastic or mesothelial differentiation. The positivity for S100 protein may indicate a peripheral nerve sheath differentiation and it is well known that malignant peripheral nerve sheath tumors (MPNST) can be very well differentiated and that positivity for the S100 protein in MPNST is usually focal, if positive at all. However, we cannot substantiate a Schwann-cell differentiation with ultrastructural findings because material for an ultrastructural study was lacking. The negative immunostaining for calretinin and inhibin argues against a sarcomatous or spindle cell variant of Leydig-Sertoli cell origin A granulomatous and/or fibrotic reaction should also be considered. These types of tissue reactions can be seen in classical seminoma and can, at times, be extensive. However, we do not believe that the spindle cell lesion described and characterized in this paper is a reactive process. Firstly, there was no granulomatous change in either the spindle cell - low grade sarcomatous - lesion or the seminomatous component. Secondly, there was no substantial amount of collagenous matrix - fibrosis in neither the low grade sarcomatous nor the seminomatous component. The lack of immunohistochemical reactivity for smooth muscle actin and to some extent the ALK-1 protein as well as the absence of plasma cells are findings that are not consistent with an inflammatory myofibroblastic tumor. A pseudosarcomatous myofibroblastic process (fibrous periorchitis or a proliferative funiculitis-type lesion) typically displays tissue-culture type lesional cells with prominent nucleoli which is in contrast to the slender spindle cells with nuclear hyperchromasia lacking prominent nucleoli in our case Moreover, the immunohistochemical study failed to reveal any evidence of myofibroblastic differentiation. The possibility of a testicular fibroma of gonadal stromal origin or of testicular tunics has to be seriously considered. However, as described by Jones et al 31 in three testicular fibromas of gonadal stromal origin, these rare neoplasms show morphological features identical to those of ovarian fibromas and immunohistochemically all tumors in their study were negative for S100 protein and two of the three presented tumors were focally positive for actin and desmin. Moreover, their tumors lacked an inflammatory cell component and the acellular plaques of hyalinized collagen that the authors found were completely lacking in our case. Hence, both the histological features and immunohistochemical findings in the series of fibromas of gonadal stromal origin by Jones et al are in sharp contrast to the findings in our case. Similarly, all the five cases of fibromas of testicular tunics presented by the same authors were negative for S100 protein in their immunohistochemical study and three cases were positive for CD34. The lesional cells displayed bland cytomorphological features without nuclear atypia and they found no mitotic activity. The solitary fibrous tumor-like areas reported by the authors were completely lacking in our case. Again, both the immunohistochemical findings and histological features of the fibromas of testicular tunics that Jones et al describe are significantly different from the low-grade sarcomatous component presented here. Regarding the histogenesis of the sarcomatous component in the case presented herein, several hypotheses can be put forward: (1) There could have been a teratomatous component (as in the cases presented by Guo et al [28]) within the tumor that had been overgrown by the seminomatous component or was so small that it was not detected, although the whole tumor was embedded. (2) The sarcoma could be a coincidentally developed tumor or (3) The sarcomatous component could be derived from the classical seminoma. We tried to investigate (3) with a molecular genetic study but as stated above, this was not successful. Thus, the histogenetic origin of the sarcoma component remains unclear.
The case was recently diagnosed precluding any conclusion regarding the potential impact of such a sarcomatous component on prognosis. However, based on the fact that the sarcomatous area was small and did not show any high-grade malignant features, we feel that a negative impact on prognosis is not to be expected. Moreover, the results in the study by Guo et al [28] suggest that patients with a sarcomatous component confined to the testicular GCT may not have a higher risk of mortality than those at a comparable stage without a sarcomatous component. In summary, we describe for the first time a low-grade sarcoma occurring together with a pure classical seminoma and immunohistochemically characterize this and discuss its histogenesis. We suggest that, based on morphology and the focal positive immunoreactivity for S100 protein, this low-grade sarcoma may be of Schwann-cell origin.
References
- 1.Floyd C, Ayala AG, Logothetis CJ, Silva EG. Spermatocytic seminoma with associated sarcoma of the testis. Cancer. 1988;61:409–14. doi: 10.1002/1097-0142(19880115)61:2<409::aid-cncr2820610234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.True LD, Otis CN, Delprado W, Scully RE, Rosai J. Spermatocytic seminoma of testis with sar-comatous transformation. A report of five cases. Am J Surg Pathol. 1988;12:75–82. doi: 10.1097/00000478-198802000-00001. [DOI] [PubMed] [Google Scholar]
- 3.True LD, Otis CN, Rosai J, Scully RE, Delprado W. Spermatocytic seminoma of testis with sarcomatous transformation. Am J Surg Pathol. 1988;12:806. doi: 10.1097/00000478-198810000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Matoska J, Talerman A. Spermatocytic seminoma associated with rhabdomyo-sarcoma. Am J Clin Pathol. 1990;94:89–95. doi: 10.1093/ajcp/94.1.89. [DOI] [PubMed] [Google Scholar]
- 5.Akhtar M, Bakri Y, Rank F. Dysgerminoma of the ovary with rhabdomyosarcoma. Report of a case. Cancer. 1989;64:2309–12. doi: 10.1002/1097-0142(19891201)64:11<2309::aid-cncr2820641121>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Malagon HD, Valdez AM, Moran CA, Suster S. Germ cell tumors with sarcomatous components: a clinicopathologic and immunohistochemical study of 46 cases. Am J Surg Pathol. 2007;31:1356–62. doi: 10.1097/PAS.0b013e318033c7c4. [DOI] [PubMed] [Google Scholar]
- 7.Fuzesi L, Rixen H, Kirschner-Hermanns R. Cytogenetic findings in a metastasizing primary testicular chondrosarcoma. Am J Surg Pathol. 1993;17:738–42. doi: 10.1097/00000478-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Hachi H, Bougtab A, Amhajji R, Otmany F, al Bouzidi A, Laalou L, et al. [A case report of testicular leiomyosarcoma] Med Trop (Mars) 2002;62:531–3. [PubMed] [Google Scholar]
- 9.Pellice C, Sabate M, Combalia, Ribas E, Cosme M. [Leiomyosarcoma of the testis] J Urol (Paris) 1994;100:46–8. [PubMed] [Google Scholar]
- 10.Singh R, Chandra A, O'Brien TS. Primary intra-testicular leiomyosarcoma in a mixed race man: a case report. J Clin Pathol. 2004;57:1319–20. doi: 10.1136/jcp.2004.018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takizawa A, Miura T, Fujinami K, Kawakami S, Osada Y, Kameda Y. Primary testicular leiomyosarcoma. Int J Urol. 2005;12:596–8. doi: 10.1111/j.1442-2042.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 12.Wakhlu A, Chaudhary A. Massive leiomyosarcoma of the testis in an infant. J Pediatr Surg. 2004;39:e16–7. doi: 10.1016/j.jpedsurg.2004.03.081. [DOI] [PubMed] [Google Scholar]
- 13.Washecka RM, Mariani AJ, Zuna RE, Honda SA, Chong CD. Primary intratesticular sarcoma. Immunohistochemical ultrastructural and DNA flow cytometric study of three cases with a review of the literature. Cancer. 1996;77:1524–8. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1524::AID-CNCR15>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Yachia D, Auslaender L. Primary leiomyosarcoma of the testis. J Urol. 1989;141:955–6. doi: 10.1016/s0022-5347(17)41068-8. [DOI] [PubMed] [Google Scholar]
- 15.Kostakopoulos A, Delakas D, Deliveliotis C, Dimopoulos MA, Sofras F. Rhabdomyosarcoma of the testis. Acta Urol Belg. 1989;57:863–5. [PubMed] [Google Scholar]
- 16.Kumar PV, Khezri AA. Pure testicular rhabdomyosarcoma. Br J Urol. 1987;59:282. doi: 10.1111/j.1464-410x.1987.tb04626.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee JS, Choi YD, Choi C. Primary testicular osteosarcoma with hydrocele. Virchows Arch. 2004;445:210–3. doi: 10.1007/s00428-004-1073-6. [DOI] [PubMed] [Google Scholar]
- 18.Mathew T, Prabhakaran K. Osteosarcoma of the testis. Arch Pathol Lab Med. 1981;105:38–9. [PubMed] [Google Scholar]
- 19.Tazi H, Karmouni T, Ouali M, Koutani A, Hachimi M, Lakrissa A. Osteosarcoma of the testis. Int J Urol. 2006;13:323–4. doi: 10.1111/j.1442-2042.2006.01285.x. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Amsterdam A, Prieto V, Sheinfeld J, Murty VV, Mazumdar M, et al. Teratoma with malignant transformation: diverse malignant histologies arising in men with germ cell tumors. J Urol. 1998;159:133–8. doi: 10.1016/s0022-5347(01)64035-7. [DOI] [PubMed] [Google Scholar]
- 21.Oosterhuis JW, Castedo SM, de Jong B, Seruca R, Dam A, Vos A, et al. A malignant mixed gonadal stromal tumor of the testis with heterologous components and i(12p) in one of its metastases. Cancer Genet Cytogenet. 1989;41:105–14. doi: 10.1016/0165-4608(89)90114-3. [DOI] [PubMed] [Google Scholar]
- 22.Terrier-Lacombe MJ, Martinez-Madrigal F, Porta W, Rahal J, Droz JP. Embryonal rhabdomyosarcoma arising in a mature teratoma of the testis: a case report. J Urol. 1990;143:1232–4. doi: 10.1016/s0022-5347(17)40235-7. [DOI] [PubMed] [Google Scholar]
- 23.Ulbright TM, Loehrer PJ, Roth LM, Einhorn LH, Williams SD, Clark SA. The development of non-germ cell malignancies within germ cell tumors. A clinicopathologic study of 11 cases. Cancer. 1984;54:1824–33. doi: 10.1002/1097-0142(19841101)54:9<1824::aid-cncr2820540910>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Banfield GK, Brookstein R. Rhabdomyosarcoma arising in teratoma of the testis. J R Army Med Corps. 1995;141:167–8. doi: 10.1136/jramc-141-03-07. [DOI] [PubMed] [Google Scholar]
- 25.Reuvekamp PT, Stulp RP, Schraffordt Koops H, Oosterhuis JW, Scheffer H, Buys CH. Analysis of a metastasizing testicular mixed gonadal stromal tumor with osteosarcoma components suggests that a malignant tumor with the histology of osteosarcoma may develop without primary involvement of RB1 and TP53. Cancer Res. 1992;52:6705–7. [PubMed] [Google Scholar]
- 26.Richmond I, Banerjee SS, Eyden BP, Sissons MC. Sarcomatoid Leydig cell tumour of testis. Histopathology. 1995;27:578–80. doi: 10.1111/j.1365-2559.1995.tb00332.x. [DOI] [PubMed] [Google Scholar]
- 27.Ulbright TM, Srigley JR, Hatzianastassiou DK, Young RH. Leydig cell tumors of the testis with unusual features: adipose differentiation, calcification with ossification, and spindle-shaped tumor cells. Am J Surg Pathol. 2002;26:1424–33. doi: 10.1097/00000478-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Guo CC, Punar M, Contreras AL, Tu SM, Pisters L, Tamboli P, et al. Testicular Germ Cell Tumors With Sarcomatous Components: An Analysis of 33 Cases. Am J Surg Pathol. 2009 doi: 10.1097/PAS.0b013e3181adb9d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulbright TM AM, Young RB. Atlas of Tumor Pathology, third series. Washington, DC: Armed Forces Institute of Pathology; 1999. Tumors of the Testis, Adnexa, Spermatic Cord and Scrotum; pp. 147–64. [Google Scholar]
- 30.Michal M, Fanburg-Smith JC, Lasota J, Fetsch JF, Lichy J, Miettinen M. Minute synovial sarcomas of the hands and feet: a clinicopathologic study of 21 tumors less than 1 cm. Am J Surg Pathol. 2006;30:721–6. doi: 10.1097/00000478-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Jones MA, Young RH, Scully RE. Benign fibromatous tumors of the testis and paratesticular region: a report of 9 cases with a proposed classification of fibromatous tumors and tumor-like lesions. Am J Surg Pathol. 1997;21:296–305. doi: 10.1097/00000478-199703000-00005. [DOI] [PubMed] [Google Scholar]