Abstract
A 41-year-old Burmese man presented with nephrotic syndrome, a creatinine level of 150 µmol/L and limited clinical history. His renal biopsy demonstrated glomerulopathy with large eosinophilic deposits in the mesangium and capillary loops that were negative for Congo red, slightly positive for periodic acid-Schiff and blue with Masson trichrome stain. Immunofluorescence microscopy with a routine antibody panel was unhelpful. Electron microscopy demonstrated extensive, moderately electron-dense deposits in the subendothelial space, subepithelial space and mesangium that could be differentiated from adjacent basement membrane by their increased electron density. The deposits contained finely granular material and occasional filaments with variable diameter ranging from 9-16 nm. Fibronectin glomerulopathy was suspected from anti-fibronectin immunohistochemistry that showed positive staining of thickened capillary loops. This staining was subsequently confirmed by immunoelectron microscopy demonstrating the presence of cellular fibronectin (cFN) in deposits. Significantly, deposition of fibronectin appeared to have occurred in the absence of thickening or folding of the adjacent basement membrane. The prominent mesangial location of deposits containing a cFN isotype may indicate that retention of local fibronectin produced in the mesangium has contributed to this pathology.
Keywords: Kidney, fibronectin, histology, electron microscopy, immunocytochemistry
Introduction
Fibronectin glomerulopathy (FGP) is an uncommon relatively recently described disease [1] thought to be hereditary with an autosomal dominant pattern of inheritance [2]. It may follow an indolent course [3] or lead to endstage renal disease in the second to fourth decade of life [4].
FGP must be distinquished from other chronic non-amyloid glomerulopathies with organised deposits that do not display positive Congo red staining including cryoglobulinemia, fibrillary glomerulonephritis, immunotactoid glomerulopathy, collagenofibrotic glomerulopathy and other non-specific collagen deposition diseases [2]. Historically, these various entities have been diagnosed primarily on the basis of the ultrastructural features of filaments and microtubular structures contained in the deposits. Immunofluorescence staining with standard renal antibody panels is generally of lesser value. Diagnosis by immunohistochemical staining with anti-fibronectin is possible but may not be requested if family history is not indicated, or available, or if this antibody is not included in the routine antibody panel. Diagnosis by electron microscopy may be routine requiring the identification of randomly arranged 12 – 16 nm filaments in deposits of the mesangium and capillary loops. However, this too may be frustrated either by lack of filamentous structure in deposits [5, 2], or if observed, the requirement for careful morphometric analysis to establish the filament dimensions.
We report a case of FGP which was diagnosed by the conventional methods in combination with immunoelectron microscopy. Routine electron microscopy was not conclusive due to the paucity of filamentous structure in the deposits and ultimately it was immunoelectron microscopy that verified the diagnosis and gave some insight into the pathogenesis.
Clinical history
A 41-year-old male Burmese refugee presented with nephrotic syndrome, a creatinine level of 150 μmol/L and hypertension. He had a negative vasculitic screen and no diabetes mellitis. His blood showed no immune abnormalities to explain proteinuria or renal impairment but his C3c level was slightly low.
He reported that his uncle had died of renal disease but that his other two siblings had no illnesses and both parents were still alive with no kidney disease. His first presentation had been with oedema aged 18 years in Burma. Three years later he had a renal biopsy performed in Burma but the results of this were unknown, as was the medication he was subsequently prescribed. At the age of 35 years he had been treated with Prednisolone with little effect. He arrived in Australia aged 41 years.
A renal biopsy from the left kidney was performed and the specimen was submitted for routine investigation by histochemistry, immunohistochemistry and electron microscopy. Based on the results of this biopsy, described in this report, a diagnosis of FGP was made. When reviewed recently 10 months after the renal biopsy his creatinine was 105 μmol/L and he had heavy proteinuria. His blood pressure was 114/70 and he was currently stable.
Materials and methods
The renal biopsy tissue was received by the laboratory fresh in phosphate buffered saline at 4 degrees Celsius and comprised three cores of tissue measuring 8, 8 and 7 mm in length. Tissue was distributed according to a standard laboratory protocol with two cores being embedded in paraffin wax for histology and one core divided for immunofluorescence and electron microscopy. Processing for histochemical investigation was routine with sections prepared for staining with haematoxylin and eosin, Masson trichrome, periodic acid- Schiff, Congo red and methanamine silver stains.
Initial immunofluorescence microscopy was carried out using cryostat sections cut from an unfixed frozen specimen that had been attached to a cryostat chuck with OCT medium and quenched in liquid nitrogen. A standard panel of antibodies comprising IgA, IgG, IgM, KLC, LLC, C1q, C3c and fibrinogen was applied using a Sequenza immunostaining system to serial 3 um cryostat sections that had been air dried to glass slides coated with 3- aminopropyltriethoxy-silane.
Subsequent immunohistochemical studies were performed using 3 μm tissue sections from a paraffin embedded block. Test slides together with positive and negative controls were deparaffinised in xylol and hydrated through graded alcohols. The tissue underwent heat-induced epitope retrieval using EDTA buffer (pH 8.0) before immunostaining using the streptavidin-biotin method done on an immunoautostainer (Dako Denmark A/S). Endogenous peroxidase was quenched in all sections with 3% hydrogen peroxide for 5 minutes. The sections were sequentially incubated with primary monoclonal antibody Fibronectin clone 568, (Novocastra Laboratories, U.K.) for 10 minutes then biotinylated secondary antibody (Dako Denmark A/S) and peroxidaselabelled streptavidin reagent (Dako Denmark A/S) each incubated for 10 minutes at room temperature. Diaminobenzedine was used as the colour chromogen with incubation for 5 minutes and slides were counterstained with Harris haematoxylin. In addition to positive controls, negative controls were treated identically with the exception that the slides were incubated with negative control reagent (Dako Denmark A/S) replacing primary antibody.
Tissue for transmission electron microscopy (EM) was sampled from the frozen immunofluorescence microscopy block as the original EM specimen taken from the fresh tissue did not contain a glomerulus. Tissue processing was routine as described previously [6]. The grids were viewed in a Morgagni 268D transmission electron microscope (FEI, The Netherlands) using an accelerating voltage of 80 kV and digital images were acquired using the integrated 1360×1024 pixel CCD digital camera (Olympus SIS, Germany). Image composites were prepared using Adobe Photoshop CS2 (Adobe Systems Inc., USA).
Immunoelectron microscopy was carried out using an immunolabeling protocol devised for routinely processed plastic embedded tissue [7]. Briefly, ultrathin sections 100 nm thick were placed on 300 mesh nickel grids then incubated for 1 hour in monoclonal anti-fibronectin antibody (NCL-FIB, Novacastra Laboratories, UK) followed by incubation for 1 hour in a 1:40 dilution of protein A colloidal gold 10 nm (Aurion, The Netherlands). Mean density of gold probes was determined by computerbased image analysis. Images of deposits taken at 28,000x were segmented into two areas that included either deposit and GBM (test region) or podocyte cytoplasm, endothelial cell cytoplasm and capillary lumen (nonspecific staining region). Statistical significance was determined using mean probe density per unit area scores from test and control regions in five digital images. Probe density was compared in deposit regions and areas of non-specific staining using Student's t-test (SPSS v15).
This work was carried out in due regard for the provisions of the Declaration of Helsinki. The patient has been informed of the preparation of this case report and has gladly given consent for its publication.
Results
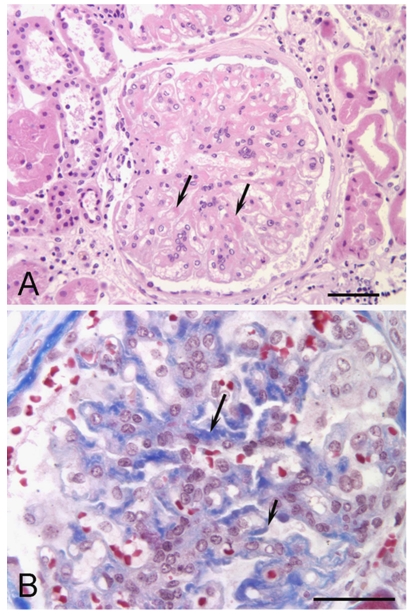
The renal biopsy included cortex that showed severe chronic disease with loss or damage of glomeruli and tubules. Of the 11 glomeruli present, only 4 were viable, the others globally sclerosed. The viable glomeruli showed mesangial and capillary wall thickening with large amounts of eosinophilic deposits (Figure 1) that were weakly positive with periodic acid-Schiff and variably blue to orange with the Masson trichrome stain. There was no significant increase in mesangial cells. The deposits were negative for amyloid by Congo red stain and negative with methenamine silver stain. There was widespread and severe tubular loss and atrophy, severe interstitial fibrosis and chronic inflammation. There were many tubular casts and blood vessels were mildly thickened.
Figure 1.
Fibronectin glomerulopathy histochemistry. A. Haematoxylin and eosin stained section showing eosinophilic material in mesangial regions (arrows) in a slightly lobulated glomerulus (bar equals 100 μm). B. Masson trichrome stained section showing blue staining of deposits associated with the mesangium (long arrow) and capillary loops (short arrow) (bar equals 50 μm)
Investigation by immunofluorescence microscopy with our routine antibody panel showed no staining for IgA, IgG, kappa light chain, lambda light chain or C1q. There were mesangial deposits of IgM, C3c and fibrinogen.
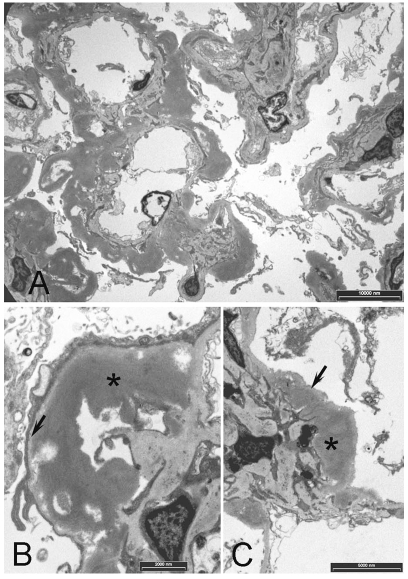
Electron microscopy showed heavy and diffuse globular deposits occupying the subepithelial and subendothelial spaces of the GBM and adjacent the mesangial matrix (Figure 2). The deposits showed moderate electron-density that allowed them to be differentiated from the less electron-dense basement membrane. Accumulated material in deposits was mainly amorphous in structure with occasional filaments showing variable diameter in the range of 9-16 nm. Deposition appeared to be heaviest in the mesangium spreading into the capillary loops. Heavily deposited capillary loops showed degeneration and atrophy of enclosed capillaries (Figure 2).
Figure 2.
Transmission electron microscopy. A. Prominent deposits associated with the mesangium and GBM. B. Higher power view showing a deposit (*) in the subendothelial space adjacent the internal aspect of the GBM (arrow). C. Deposit (*) adjacent the mesangium with basement membrane (arrow). Note that the deposits are more electron dense than basement membrane
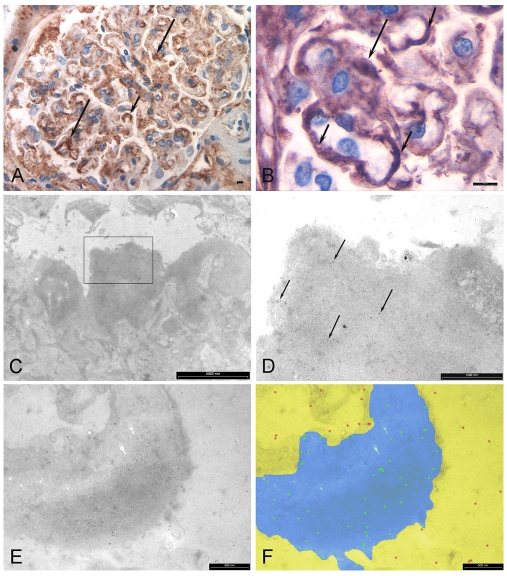
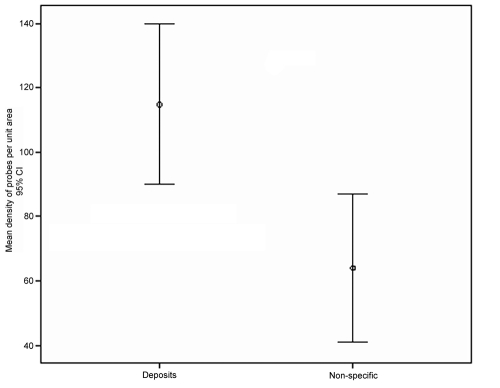
Immunohistochemical staining with anti-fibronectin antibody showed positive staining in the mesangial matrix and occasional capillary loops (Figure 3). Deposits were variably positive with foci of strong staining embedded in thickened regions of negatively stained material. Immunoelectron microscopy using the same antibody confirmed positive labelling for fibronectin in the deposits (Figure 3). The labelling mean density was significantly higher in deposits (P<0.05) than in adjacent regions considered to represent sites of non-specific labelling, including endothelial cell cytoplasm, podocyte cytoplasm and capillary lumen (Figure 4). The confirmation of positive immunohistochemical staining by immunoelectron microscopy allowed a diagnosis of FGP to be made.
Figure 3.
Immunohistochemistry and immunoelectron microscopy with anti-fibronectin. A. Immunohistochemistry showing positive staining associated with the mesangium (long arrows) and capillary loops (short arrows). Bar equals 5 μm. B. Higher power view showing positive staining of deposits adjacent the mesangium (long arrow) and around capillary loops (short arrows). Bar equals 5 μm. C. Immunoelectron microscopy section showing a mesangial deposit (inside box). D. Higher power view of box area in (C) showing immunogold probes (arrows) attached to the deposit. E. Immunogold labelling of a deposit adjacent the GBM (bar equals 500 nm). F. Same image as (E) enhanced with Photoshop showing image analysis segmentation into deposit/ GBM test region (blue) and non-specific region (yellow). Probes attached to deposit are highlighted in green and non-specific labelling is shown in red.
Figure 4.
Mean density of colloidal gold probes attached to deposits compared with adjacent areas of nonspecific staining. Counts were significantly higher in deposits than for areas of non-specific staining (P<0.05)
Discussion
Diagnosis of FGP can present several challenges particularly if there is no known family history or clinical suspicion and no serum analysis provided, as was the case with our patient. Our initial routine histochemical investigations showed the presence of mesangial deposits extending into the capillary loops that were negative with Congo red staining, suggestive of FGP. Periodic acid-Schiff (PAS) which is generally strong for fibronectin [5] in this case was weakly positive and it was unclear whether the staining was associated with the deposits. Immunofluorescence microscopy with our routine antibody panel showed positive staining for IgM, C3c and fibrinogen only which also may be seen in fibronectin glomerulopathy [2].
Electron microscopy showed moderately electron dense deposits that were globular in shape containing mainly amorphous or granular material with occasional filaments. The moderate electron density of the deposits compared to adjacent basement membrane was consistent with fibronectin deposits described in a previous ultrastructural study [8]. However, attempts to measure the diameter of the filaments gave variable dimensions ranging between 9-16 nm. Fibronectin filament dimensions have been reported in the range of 10 to 16 nm [3, 4, 9, 2] and this dimension must then be distinguished from 10 nm amyloid filaments and the larger amyloid fibril [10] and 18-22 nm fibrils of fibrillary glomerulopathy [11]. Ultimately, because of the paucity of filamentous structure in this case we felt ultrastructural morphometry was inadequate to make an unequivocal diagnosis of FGP, prompting us to investigate immunoelectron microscopy as an alternative approach.
Initial immunohistochemical staining with antifibronectin was variably positive in capillary loops and the mesangium but it was not clear if the positive staining was due to deposit formation or thickening of the basement membrane. Immunoelectron microscopy was carried out using the same antibody. This showed positive labelling of distinct globular deposits adjacent to the GBM and mesangial matrix providing confirmation of the diagnosis of FGP. The resolution of immunoelectron microscopy allowed specific localisation of fibronectin to the actual deposits rather than just the region of the capillary loop including both GBM and deposit. However, we were unable to quantify the amount of fibronectin present in the deposits or determine other constituents.
The source of the fibronectin in the deposits of FGP remains unknown. Fibronectin is a normal constituent of the GBM predominantly along the endothelial aspect as well as the mesangium [14, 12]. It has been suggested that abnormalities in the metabolism of circulating fibronectin may play a pathogenetic role in the disease [1] and studies of family members diagnosed with hereditary fibronectin glomerulopathy show massive glomerular deposits in the subendothelial space with strong positive staining with antibody to common fibronectin [9] or plasma fibronectin (pFN) [2]. However, locally produced cellular fibronectin (cFN) is widely distributed in the extracellular matrix [12] and is known to be secreted by mesangial cells [13]. The deposits in our case showed positive staining with a monoclonal antibody raised against an isotype of fibronectin secreted by cultivated human fibroblasts, a cFN isotype. We could not find data to indicate whether our antibody to the cell-attachment domain of fibronectin was a universal marker that would also label soluble pFN but specificity testing of an antibody to the 120K collagenbinding fragment of fibronectin found it to recognise human fibronectin and to show cross species reactivity [15] suggesting that these epitopes are conserved.
The process of deposit formation in fibronectin glomerulopathy is also poorly understood. Experimental studies in animals have identified some conditions in which fibronectin staining in the glomerulus is increased [16, 17, 12] and generally this was found to be associated with thickening of the basement membrane. So, it is important in any assessment of fibronectin deposition firstly to confirm that the increased staining is localised to a deposit and not thickened GBM or mesangial matrix. In our case the deposits were clearly adjacent the lamina densa of the GBM and mesangial matrix. We do not consider that thickening of the GBM or mesangial matrix initiated the deposition of fibronectin as deposit formation occurred in the absence of obvious thickening or folding of basement membrane. We speculate, as have others [12], that changes either in the structure of the basement membrane or in circulating pFN may lead to restriction of pFN but cannot rule out the possibility that locally produced mesangial cell cFN may also contribute to these deposits, as may occur in IgA nephropathy [18].
In the present case, we were able to determine that at least one close relative had previously died from renal disease suggesting that we were looking at a hereditary case. We have localised fibronectin to globular deposits adjacent the GBM and mesangial matrix though identification of the source of fibronectin will require markers that can discriminate the various isotypes of pFN and cFN. There is currently no definitive treatment for fibronectin glomerulopathy and prognosis is uncertain, in some cases leading to chronic renal failure requiring renal dialysis or renal transplantation. This study adds another case to the small series already reported and is one of the first to confirm the diagnosis by immunoelectron microscopy.
References
- 1.Strøm EH, Banfi G, Krapf R, Abt AB, Mazzucco G, Monga G, Gloor F, Neuweiler J, Reiss R, Stosiek P, et al. Glomerulopathy associated with predominant fibronectin deposits: a newly recognised hereditary disease. Kidney Int. 1995;48:163–170. doi: 10.1038/ki.1995.280. [DOI] [PubMed] [Google Scholar]
- 2.Iskandar SS, Herrera GA. Glomerulopathies with organized deposits. Semin Diagn Pathol. 2002;19:116–32. [PubMed] [Google Scholar]
- 3.Assmann KJ, Koene RA, Wetzels JF. Familial glomerulonephritis characterized by massive deposits of fibronectin. Am J Kidney Dis. 1995;25:781–791. doi: 10.1016/0272-6386(95)90555-3. [DOI] [PubMed] [Google Scholar]
- 4.Hildebrandt F, Strahm B, Prochoroff A, Cybulla M, Gemperle O, Krapf R, Brandis M. Glomerulopathy associated with predominant fibronectin deposits: exclusion of the genes for fibronectin, villin and desmin as causative genes. Am J Med Genet. 1996;63:323–327. doi: 10.1002/(SICI)1096-8628(19960503)63:1<323::AID-AJMG54>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Sato H, Matsubara M, Marumo R, Soma J, Kurosawa K, Taguma Y, Saito T. Familial Lobular glomerulopathy: first case report in Asia. Am J Kidney Dis. 1998;31:E3. doi: 10.1053/ajkd.1998.v31.pm10074583. [DOI] [PubMed] [Google Scholar]
- 6.Killingsworth MC. Angiogenesis in early choroidal neovascularisation secondary to age-related macular degeneration. Graefe's Arch Clin Exp Ophthalmol. 1995;233:313–323. doi: 10.1007/BF00200479. [DOI] [PubMed] [Google Scholar]
- 7.Stirling JW, Graff PS. Antigen unmasking for immunoelectron microscopy: labeling is improved by treating with sodium ethoxide or sodium metaperiodate, then heating on retrieval medium. J Histochem Cytochem. 1995;43:115–23. doi: 10.1177/43.2.7529784. [DOI] [PubMed] [Google Scholar]
- 8.Eyden B. The myofibroblast: an assessment of controversial issues and a definition useful in diagnosis and research. Ultrastruct Pathol. 2001;25:39–50. doi: 10.1080/019131201300004672. [DOI] [PubMed] [Google Scholar]
- 9.Fujigaki Y, Kimura M, Yamashita F, Yonemura K, Ikegaya N, Taniguchi Y, Hishida A, Kaneko E. An isolated case with predominant glomerular fibronectin deposition associated with fibril formation. Nephrol Dial Transplant. 1997;12:2717–22. doi: 10.1093/ndt/12.12.2717. [DOI] [PubMed] [Google Scholar]
- 10.Ghadially FN. 4th edn. Boston: Butterworth-Heinemann; 1997. Ultrastructural pathology of the cell and matrix. [Google Scholar]
- 11.Yang GC, Nieto R, Stachura I, Gallo GR. Ultrastructural immunohistochemical localization of polyclonal IgG, C3, and amyloid P component on the Congo red-negative amyloid-like fibrils of fibrillary glomerulopathy. Am J Pathol. 1992;141:409–419. [PMC free article] [PubMed] [Google Scholar]
- 12.Van Vliet AI, van Alderwegen IE, Baelde HJ, De Heer E, Bruijn JA. Fibronectin accumulation in glomerulosclerotic lesions: Self-assembly sites and heparin II binding domain. Kidney Inter. 2002;61:481–489. doi: 10.1046/j.1523-1755.2002.00159.x. [DOI] [PubMed] [Google Scholar]
- 13.Ishimura E, Sterzel RB, Budde K, Kashgarian M. Formation of extracellular matrix by cultured rat mesangial cells. Am J Pathol. 1989;134:843–55. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YZ, Lee HS. Quantitative changes in the glomerular basement membrane components in human membranous nephropathy. J Pathol. 1997;183:8–15. doi: 10.1002/(SICI)1096-9896(199709)183:1<8::AID-PATH1079>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 15.Tarone G, Amedeo MR, Di Renzo MF, Comoglio P. Monoclonal antibodies to the collagen binding domain of human plasma fibronectin. Exp Cell Biol. 1984;52:225–236. doi: 10.1159/000163266. [DOI] [PubMed] [Google Scholar]
- 16.Asar M, Kayisli Ü, Izgüt-Uysal VN, Akkoyunlu G. Immunohistochemical and ultrastructural changes in the renal cortex of cadmium-treated rats. Biol Trace Elem Res. 2004;97:249–63. doi: 10.1385/BTER:97:3:249. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura T, Muso E, Kamata T, Suyama K, Oyama A, Ono T, Yoshida H, Miyawaki S, Sasayama S. Ultrastructural Localization of dominantly increased fibronectin in the markedly thickened glomerular basement membrane in a selectively mated murine high IgA strain (HIGA Mice) Nephron. 1999;83:146–53. doi: 10.1159/000045492. [DOI] [PubMed] [Google Scholar]
- 18.Mattii L, Segnani C, Cupisti A, D'Alessandro D, Moscato S, Meola M, Barsotti G, Marino M, Bianchi F, Dolfi A, Bernardini N. Kidney expression of RhoA, TGF-beta1, and fibronectin in human IgA nephropathy. Nephron Exp Nephrol. 2005;101:e16–23. doi: 10.1159/000086035. [DOI] [PubMed] [Google Scholar]