Abstract
Poorly differentiated synovial sarcomas are diagnostically challenging soft tissue tumors. They can be indistinguishable from other “small blue cell tumors” based on morphology and even immunohistochemical studies. Here we report a rare case of poorly differentiated metastatic synovial sarcoma to lung without known primary, diagnosed with molecular genetic analysis. The tumor was negative for EMA and cytokeratin, previously reported as the most sensitive immunostaining markers for synovial sarcomas. SYT-SSX gene fusion, characteristic for synovial sarcoma, was identified in this case by FISH and RT-PCR assays.
Keywords: Synovial sarcoma, lung, metastatic, molecular genetic
Introduction
Synovial sarcoma was initially described by Simon in 1865 [1]. It was named “synovial sarcoma” in 1934 by Sabrazes due to its resemblance to the developing synovial tissue under light microscopy [2]. The term “synovial sarcoma”, however, is a misnomer. The tumor cells do not share the same immunohistochemical and ultrastructural features of the normal synovium [3]. Recent cDNA microarray based studies found that the gene expression profile of synovial sarcoma is closely related to neural crest-derived malignant peripheral nerve sheath tumor [4]. In addition, treatment of synovial sarcoma cell lines with all-trans-retinoic acid, recombinant human fibroblast growth factor 2 and bone morphogenetic protein 4 causes growth inhibition and enhanced expression of neural tissue-related genes [5]. These findings suggest that the cellular origin of synovial sarcoma is probably neural crest-derived cells.
Synovial sarcoma has an annual incidence of 2.5 per 100,000. It accounts for approximately 8% of all human soft tissue sarcomas [6], and commonly occurs in children and young adults with a male to female ratio of 1.2: 1 [7]. The tumor typically arises in the peri-articular soft tissue of the lower extremeties and often invades locally. It can also metastasize distantly, especially to the lung and lymph nodes [8]. Classical synovial sarcoma has a biphasic appearance and is composed of sheets of spindle cells and sharply segregated epithelial cells forming gland-like areas. A second form of synovial sarcoma is monophasic consisting of only a sarcomatous component. The large majority of synovial sarcomas are of these two forms [8]. A relatively rare form of synovial sarcoma is the poorly differentiated form, which shows hypercellular small spindle or round cells with high nuclear to cytoplasmic ratio and active mitosis, resembling “small blue round cell tumors”, i.e. Ewing sarco-ma/PNET, rhabdomyosarcoma, neuroblastoma or lymphoma [9]. Synovial sarcoma, especially monophasic and poorly differentiated types are often diagnostically challenging.
In a 2006 study, cluster analysis of immunohistochemical profiles in selected groups of sarcomas showed that EMA was the most sensitive (91%) and specific (82%) marker for synovial sarcoma [10]. Cytokeratin stains in general are not as sensitive and specific as EMA, with a sensitivity of 70% and specificity of 68%. Among the different cytokeratin subsets, CK7 was found to be the most specific, and AE1/AE3 the most sensitive. Positive staining for both EMA and CK7 was less sensitive (52%), but more specific (100%). CD56 and bcl-2 were positive in 26% and 48% of synovial sarcomas, are thus considered as second tier stains to EMA and cytokeratins. These two markers were more prevalent in cytokeratin negative synovial sarcomas, and were useful in such cases. Although positive CD99 (usually membrane stain) was seen in 70% of synovial sarcomas, this staining was non-specific, and was also found in 93% of Ewing sarcomas and 43% of malignant peripheral nerve sheath tumors [10], which may enter the differential diagnosis and are often morphologically similar to poorly differentiated synovial sarcoma. Up to now, there has been no ideal immunohistochemical marker for diagnosis of synovial sarcoma, especially for its poorly differentiated variant.
A specific chromosomal translocation of t(X;18)(p11.2;q11.2) can be detected in over 95% of synovial sarcomas. This translocation results in the fusion between SYT gene at 18q11 and one of the SSX family genes, SSX1 or SSX2, at Xp11 [8]. Nearly all classic bio-phasic synovial sarcomas carry SYT-SSX1 fusion, while most monophasic tumors have SYTSSX2 fusion [8]. For the poorly differentiated form of synovial sarcoma, the gene fusion type has not been well characterized. Here we report a diagnostically challenging case of metastatic poorly differentiated synovial sarcoma to lung with unknown primary, proven by both FISH- and PCR-based molecular assays.
Clinicopathological approach and molecular genetic analysis
Clinical findings
A 28-year-old male with a history of juvenile rheumatoid arthritis presented with progressive shortness of breath, cough, hemoptysis and weight loss. He denied any fever, chills or night sweats. Chest X-ray and CT scan showed bilateral lung masses and mediastinal lmyphadenopathy. Physical examination, CT scan, scrotal ultrasound and radionucleotide bone scan revealed no additional mass lesions.
Pathologic findings
The core biopsy showed an undifferentiated malignant neoplasm composed of small cells with scant vacuolated cytoplasm and irregular nuclei with clumped chromatin and inconspicuous nucleoli (Figure 1). The differential diagnosis included lymphoma, undifferentiated small cell sarcoma and, less likely, carcinoma. Immunohistochemical stains reveals weak CD99 and cyclin D-1 positivity (Figure 2). EMA, pancytokeratin, keratin AE1/AE3, cytokeratin 7, CD20, CD3, CD5, CD10, CD30, pax-5, CD79a, TdT, ALK, myeloperoxidase, TTF-1, myogenin, myoD1, S-100, synaptophysin, chromogranin, PLAP, CD31, CD34 and Oct3/4 were all negative. A CD99 positive small “blue round cell” tumor with negative lymphoid markers raised the possibility of Ewing sarco-ma/Peripheral neuroectodermal tumor (PNET), although CD99 staining was weak and cytoplasmic (Figure 2). We resorted to FISH and PCR assays for EWSR1 gene rearrangement to rule out Ewing sarcoma/PNET. However, both PCR and FISH tests were negative, which essentially precludes the diagnosis of Ewing sar-coma/PNET.
Figure 1.
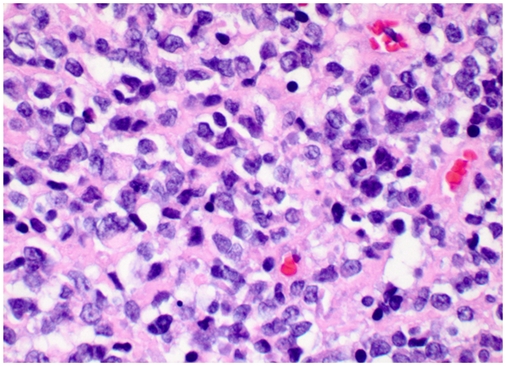
Representative histologic sections of the tumor (hematoxylin and eosin staining, X 400 magnification). Tumor cells are small with scant vacuolated cytoplasm, irregular clu mped nuclei and inconspicuous nucleoli.
Figure 2.
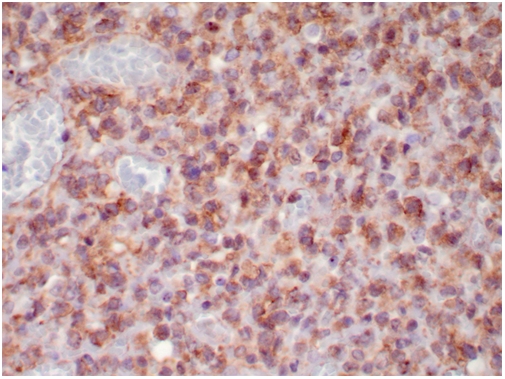
Immunohistochemical staining of CD99 in the tumor (X 400 magnifiction). The CD99 staining is weak and cytoplasmic.
Although CD99 is not specific marker, the weak positive stain raised the possibility of synovial sarcoma as well. We therefore performed molecular analysis for SYT associated genetic alteration. Interestingly, the tumor was found to be positive for SYT gene break-apart rearrangement by FISH (Figure 3), indicating a diagnosis of synovial sarcoma. In addition, we performed RT-PCR to detect the SYT associated gene fusion plus DNA sequencing, and found that the tumor was positive for SYTSSX1 gene fusion (Figure 4), which confirmed the result of FISH and revealed the gene fusion type. The presence of multiple nodules in the lung favors a metastatic tumor over a lung primary, even though a primary tumor could not be identified after exhaustive metastatic workup.
Figure 3.
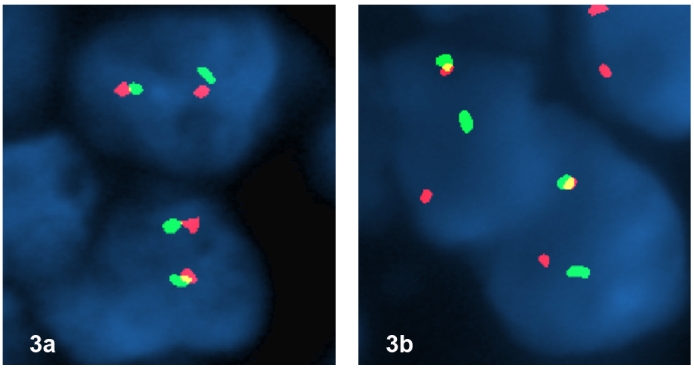
(a) FISH with break-apart probe for EWS gene shows no separation of the red and green signals, indicating no EWS associated gene rearrangement. (b) FISH with break-apart probe for SYT gene shows one intact yellow signal, one separated red and green signal per nucleus in tumor cells indicating the presence of a t(X;18) translocation.
Figure 4.
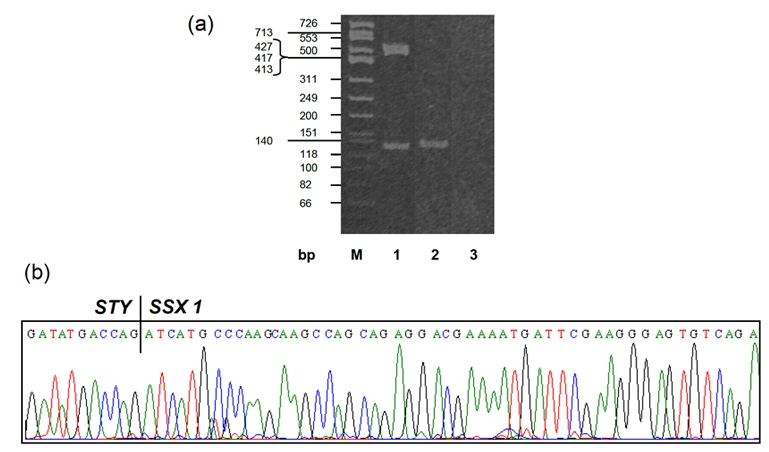
(a) RT-PCR analysis for SYT/SSX gene fusion. M: DNA molecular marker; Lane 1: positive control; Lane2: patient RNA, positive for SYT/SSX gene fusion; Lane 3: negative control. (b) DNA sequence with SYT/SSX1 gene fusion.
Clinical follow-up
With a definitive diagnosis of synovial sarcoma, the patient underwent an appropriate chemotherapy using a MAI regimen (Mesna, Adriamycin and Ifosfamide). After two cycles of treatment, the patient showed a positive response. Follow-up chest CT showed that the tumor nodules had decreased in both size and number with some small lesions completely resolved. The patient is now still alive with close clinical follow-up.
Discussion
Multiple pulmonary metastases of synovial sarcoma as the only clinical manifestation are exceedingly rare. The only reported case is of a 48-year-old Japanese female patient with the classic biphasic form of metastatic synovial sarcoma [11]. Interestingly, she developed a palpable nodule in the tendon of lower extremity more than three years after the pneumonectomy, which proved to be synovial sarcoma [11]. In our case, the patient had poorly differentiated synovial sarcoma with more diagnostically challenging. Based on the morphology alone, the differential diagnosis was broad and included Ewing/PNET, lymphoma, leukemia, poorly differentiated carcinoma, primitive synovial sarcoma, neuroblastoma and rhabdomyosarcoma. CD99, a non-specific marker for synovial sarcoma, showed only weak cytoplasmic positivity. The molecular analysis of SYT gene alteration played an important role in the diagnosis of synovial sarcoma. The gene fusion type of SYT-SSX1, as identified in this case, has been reported with worse prognosis than that with SYT-SSX2 [12]. The patient of this case may have an occult primary, like the Japanese case, and should be followed up closely.
Patients with metastatic synovial sarcoma can not be managed with surgical excision, the treatment of choice for primary tumors [13]. A definitive diagnosis of sarcoma with specific subtypes, however, is important in the selection of effective agents for adjuvant chemotherapy. For example, typical regimens for small-cell sarcoma, especially Ewing's sarco-ma/PNET and rhabdomosarcoma, include the combination of vincristine, doxorubicin and cyclophosphamide [14]. While for synovial sarcoma, ifosfamide-based chemotherapy appears more effective [15]. The patient underwent two cycles of treatment with combined adriamycin + ifosfamide regimen and showed significant tumor regression. The long-term outcome of this patient, however, remains unkown. With known genetic alteration in synovial sarcoma, new therapies targeting altered DNA, RNA or protein are under investigation and seem promising. In a recent report, vaccination of synovial sarcoma patients with SYTSSX junction peptide was tested in a phase I pilot trial. The peptide was found to be safe and immunogenic. However, its therapeutic efficacy is still under evaluation [16]. Retinoic acid and its derivatives, well-studied redifferentiation agents, have been used successfully in the treatment of promyelocytic leukemia. Recent studies have shown that all-trans-retinoic-acids could induce the differentiation of synovial sarcoma cell lines and inhibit cell growth both in vitro and in vivo, suggesting that they are potential drugs for the treatment of synovial sarcoma as well [5]. In conclusion, molecular analysis of SYT associated genetic alteration for synovial sarcoma demonstrated great value in diagnosis, prognosis and potentials in prediction of response to therapy.
Acknowledgments
The authors would like to thank Dr. Shahandeh Haghir from Samaritan Medical Center in Watertown, NY, for allowing us to review this case in consultation.
References
- 1.Simon G. Exstirpation einer sehr grossen, mit dicken Stiele angewachsenen Kneigelenkmaus mit gluklichem Erfolge. Arch Klin Chir. 1865;6:573–576. [Google Scholar]
- 2.Sabrazes J, Loubat E, de Grailly R, Magendie J. Synovial sarcomes. Gaz Hebd Sc Med Bordeaux. 1934;55:754–762. [Google Scholar]
- 3.Miettinen M, Virtanen I. Synovial sarcoma-a misnomer. Am J Pathol. 1984;117:18–25. [PMC free article] [PubMed] [Google Scholar]
- 4.Nagayama S, Katagiri T, Tsunoda T, Hosaka T, Nakashima Y, Araki N, Kusuzaki K, Nakayama T, Tsuboyama T, Nakamura T, Imamura M, Nakamura Y, Toguchida J. Genome-wide analysis of gene expression in synovial sarcomas using a cDNA microarray. Cancer Res. 2002;62:5859–5866. [PubMed] [Google Scholar]
- 5.Ishibe T, Nakayama T, Aoyama T, Nakamura T, Toguchida J. Neuronal differentiation of synovial sarcoma and its therapeutic application. Clin Orthop Relat Res. 2008;466:2147–2155. doi: 10.1007/s11999-008-0343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scully SP, Temple HT, Harrelson JM. Synovial sarcoma of the foot and ankle. Clin Orthop Re-lat Res. 1999;364:220–226. doi: 10.1097/00003086-199907000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Haldar M, Randall RL, Capecchi MR. Synovial sarcoma: from genetics to genetic-based animal modeling. Clin Orthop Relat Res. 2008;466:2156–2167. doi: 10.1007/s11999-008-0340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazar A, Abruzzo LV, Pollock RE, Lee S, Czerniak B. Molecular diagnosis of sarcomas: chromosomal translocations in sarcomas. Arch Pathol Lab Med. 2006;130:1199–1207. doi: 10.5858/2006-130-1199-MDOS. [DOI] [PubMed] [Google Scholar]
- 9.Folpe AL, Schmidt RA, Chapman D, Gown AM. Poorly differentiated synovial sarcoma: immunohistochemical distinction from primitive neuroectodermal tumors and high-grade malignant peripheral nerve sheath tumors. Am J Surg Pathol. 1998;22:673–682. doi: 10.1097/00000478-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Olsen SH, Thomas DG, Lucas DR. Cluster analysis of immunohistochemical profiles in synovial sarcoma, malignant peripheral nerve sheath tumor, and Ewing sarcoma. Mod Pathol. 2006;19:659–668. doi: 10.1038/modpathol.3800569. [DOI] [PubMed] [Google Scholar]
- 11.Itoh A, Tsuneta Y, Kikuchi Y, Kawai T, Okuno T, Nishiura Y, Kanda M. A case report of multiple pulmonary tumors as a sole manifestation of synovial Sarcoma. Nihon Kyobu Shikkan Gakkai Zasshi. 1992;30:118–122. [PubMed] [Google Scholar]
- 12.Inagaki H, Nagasaka T, Otsuka T, Sugiura E, Nakashima N, Eimoto T. Association of SYTSSX fusion types with proliferative activity and prognosis in synovial sarcoma. Mod Pathol. 2000;13:482–488. doi: 10.1038/modpathol.3880083. [DOI] [PubMed] [Google Scholar]
- 13.Murray PM. Soft tissue sarcoma of the upper extremity. Hand Clin. 2004;20:325–333. doi: 10.1016/j.hcl.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Bertuzzi A, Castagna L, Nozza A, Quagliuolo V, Siracusano L, Balzarotti M, Compasso S, Alloisio M, Soto Parra H, Santoro A. High-dose chemotherapy in poor-prognosis adult small round-cell tumors: clinical and molecular results from a prospective study. J Clin Oncol. 2002;20:2181–2188. doi: 10.1200/JCO.2002.08.033. [DOI] [PubMed] [Google Scholar]
- 15.Randall RL, Schabel KL, Hitchcock Y, Joyner DE, Albritton KH. Diagnosis and management of synovial sarcoma. Curr Treat Options Oncol. 2005;6:449–459. doi: 10.1007/s11864-005-0024-z. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi S, Wada T, Ida K, Sato Y, Nagoya S, Tsukahara T, Kimura S, Sahara H, Ikeda H, Shimozawa K, Asanuma H, Torigoe T, Hiraga H, Ishii T, Tatezaki SI, Sato N, Yamashita T. Phase I vaccination trial of SYT-SSX junction peptide in patients with disseminated synovial sarcoma. J Transl Med. 2005;3:1–9. doi: 10.1186/1479-5876-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


