Abstract
Childhood dermal tumors with melanocytic features is an unusual tumor that can create diagnostic confusion. Among them, paraganglioma-like melanocytic tumors- previous included in melanocytic tumors of uncertain malignant potential- has some particular histopathologic and immunohistochemical features. We describe a case of 13 years old girl with a paraganglioma-like dermal melanocytic tumor of the left leg.
Keywords: Paraganglioma like dermal melanocytic lesion
Introduction
Intradermal tumors with melanocytic features combined which lack of jonctional or intraepithelial components represent a special group of lesions with particular microscopic features and clinical behaviour. Primary dermal melanoma, solitary metastatic melanoma [1], solitary dermal melanoma [2], clear cell sarcoma [3] are several lesions included in differential diagnosis of paraganglioma like dermal melanoytic tumor (PDMT) which is very rare lesion with a benign behaviour compared with previous ones. Clinical and therapeutic management of PDMT are totally different from other melanocytic lesion. No PDMT was previous described in children. Many authors observed particular pattern of tumor blood vessels and low proliferative index of tumor cells in PDMT but no data are available concerning proliferative index of endothelial cells lining tumor vasculature neither characterization of other factors involved in PDMT angiogenesis.
Case presentation
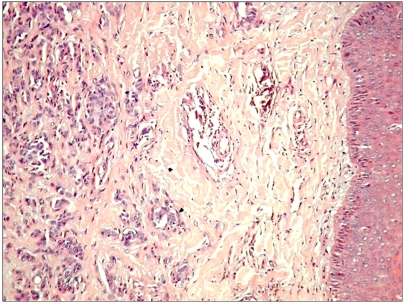
A 13 years old girl was admitted one year ago with unique nodular non pigmented and painless lesion 2-2, 5 cm in size on the left leg. Medical history revealed no other family members with similar lesions, slow tumor growth rate and no other associated clinical symptoms. Excisional biopsy was performed. Microscopically, we found a pseudo-nodular lesion restricted in the deep dermis without any contact with the overlying normal epidermis (Figure 1). Lymphocyte rich inflamatory infiltrate was found deep in the dermis, as a border between the proliferation and normal tissue. Tumor cells were large acidophilic oval or round in shape with distinct large nuclei and proeminent nucleoli mixed with spindle cells arranged in small nests, cords and sheets separated by fibrous septae. No mitosis or necrosis was seen. There was no cytoplasmic melanin pigment in tumor cells by conventional microscopy.
Figure 1.
Tumor view at low magnification .Note that tumor is restricted to the deep dermis without any contact with normal overlying epidermis. (H↦E staining, ×100)
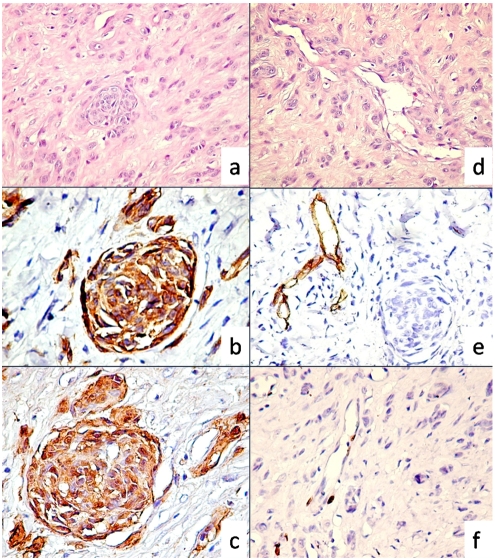
Immunostaining for vimentin, S-100 protein and Melan A revealed a strong positive reaction indicating the melanocytic lineage for tumor cells (Figure 2b, c). Low proliferative index (<1%) for Ki67 was observed in neoplastic cells. By contrast with tumor cells, endothelial cells had an unusual high proliferation rate, most of tumor blood vessels exhibited between 2-4 Ki67 positive endothelial nuclei (Figure 2f). No positive reaction was found for D2-40, smooth muscle actin, AE1/AE3 cytokeratin, CD68 and HMB45, neither for neuro filament associated protein and neuron specific enolase in tumor cells. Also, tumor cells were negative for desmin and glial fibrilar acidic protein.Vascular markers (CD31, CD34, FVIII related antigen) positive reaction was restricted to tumor blood vessels endothelium and highlighted a rich intratumoral vascular network with highly branched pattern between neoplastic structure suggesting an intense angiogenic process (Figure 2d, e). Lymphovascular or perineural invasion were not found on studied specimens.
Figure 2.
Nested and cord like arrangements of tumor cells (a, H&E, ×200). Tumor immunophenotype revealed intense positive reaction for Melan A(b) and S100 protein(c). Highly branched intratumor blood vessels (d) were CD34 positive (e, note that tumor cells were negative) and had numerous proliferative Ki67 positive endothelial cells. In contrast tumor cells had a low proliferative index for Ki67 (f).
No local recurrences, other similar lesions or distant metastases were registered after one year follow up of the case.
Discussion
To our knowledge this is the first case of paraganglioma like dermal melanocytic tumor (PDMT) reported in a 13 years old child. PDMT was described first by Deyrup et al in 2004 [4], as a benign dermal lesion with melanocytic features found in extremities, predominantly on female patients aged 18-35. There were reported only 9 cases of PDMT in the medical literature, 8 of them retrospectively diagnosed after reviewing the initial diagnosis. Usually, PDMT was described as unique or multiple lesion with nodular well circumscribed, encapsulated pattern and occasionally with infiltration of the surrounding dermis, aspect also found in our case. Two important microscopic features were present in all cases described until now: (i) no evidences of melanocytic atypia or epidermal hyperplasia in the overlying epithelium; (ii) presence of a distinctive partitioning of the tumor into small and large packets, nests, or short cords by delicate fibrous septa. Descriptive term of “paraganglioma” derived from nested appearance of the tumor toghether with a particular architecture of highly branched tumor blood vessels network and not from a common filliation with paraganglia cells. But this observation does not exclude differential diagnosis with primary cutanous paraganglioma, a very rare tumor in childhood (only one case of 10 years old boy reported in the literature).
A particular aspect of this case report was found on endothelial cells of tumor blood vessels. The proliferative rate of endothelial cells-a queiscent cells in normal condition- was higher than those of tumor cells. We observed clusters of proliferating endothelial cells in almost all tumor blood vessels. Previous studies [4, 5] described proeminent blood vessels but no other features of endothelial cells lining these capillaries.
An accurate differential diagnosis from other benign dermal tumor as cellular blue naevus or malignant dermal tumor such as melanoma or clear cell sarcoma represents an important step for patient's evolution and prognosis [6]. Lack of peripheral infiltration of the dermis by pigmented spindled and dendritic cells found in neurocristic hamartoma and cellular blue nevus could exclude them from diagnosis. Large size at presentation, low mitotic rate and low nuclear grade characterise PDMT and represent useful criteria to differentiate this lesion from dermal melanoma. Primary dermal melanoma [7, 8] nodular melanoma or metastatic melanoma should be included in differential diagnosis of any dermal tumor with melanocytic features by molecular and genetic analysis [9, 10]. In conclusion dermal melanocytic lesions, including PDMT, actually classified by AFIP as “melanocytic tumor of uncertain malignant potential” [11] should be carefully diagnosed and managed as distinctive lesions by establishing specific morphologic, immunohistochemical, molecular and prognostic markers.
Concluding remarks
Paraganglioma-like is a descriptive term for such a tumor composed of cells disposed in nests surrounding by o rich network of branching proliferative capillaries. It represents a rare dermal melanocytic lesion, unusual in children that should be carefully diagnosed by histopathologic observation, immunohistochemistry (mandatory) and molecular diagnostic if it is needed. No local reccurences, nodal metastasis and excellent prognosis reported in already described cases.
References
- 1.Bowen GM, Chang AE, Lowe L, Hamilton T, Patel R, Johnson TM. Solitary melanoma confined to the dermal and/or subcutaneous tissue: evidence for revisiting the staging classification. Arch Dermatol. 2000;136(11):1397–1399. doi: 10.1001/archderm.136.11.1397. [DOI] [PubMed] [Google Scholar]
- 2.Lee CC, Faries MB, Ye X, Morton DL. Solitary dermal melanoma: beginning or end of the metastatic process? Ann Surg Oncol. 2009;16(3):578–584. doi: 10.1245/s10434-008-0272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim DH, Choi KH, Cho YD. Clear cell sarcoma of the upper thoracic back muscle. J Korean Neurosurg Soc. 2009;45(2):112–4. doi: 10.3340/jkns.2009.45.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deyrup AT, Althof P, et al. Paraganglioma-like dermal melanocytic tumor: a unique entity distinct from cellular blue nevus, clear cell sarcoma, and cutaneous melanoma. Am J Surg Pathol. 2004;28:1579–1586. doi: 10.1097/00000478-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Saadat P, Cesnorek S, et al. Primary cutaneous paraganglioma of the scalp. J Am Acad Dermatol. 2006;54:220–223. doi: 10.1016/j.jaad.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Sarma DP, Teruya B, Wang B. Paragangliomalike dermal melanocytic tumor: a case report. Cases J. 2008:1–48. doi: 10.1186/1757-1626-1-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swetter SM, Ecker PM, Johnson DL, et al. Primary dermal melanoma: a distinct subtype of melanoma. Arch Dermatol. 2004;140:99–103. doi: 10.1001/archderm.140.1.99. [DOI] [PubMed] [Google Scholar]
- 8.Cassarino DS, Cabral ES, et al. Primary dermal melanoma: distinct immunohistochemical findings and clinical outcome compared with nodular and metastatic melanoma. Arch Dermatol. 2008;144:49–56. doi: 10.1001/archdermatol.2007.16. [DOI] [PubMed] [Google Scholar]
- 9.Hida Y, Kubo Y, et al. Primary dermal melanoma: A case report and molecular characterization. J Dermatol. 2009;36:346–352. doi: 10.1111/j.1346-8138.2009.00650.x. [DOI] [PubMed] [Google Scholar]
- 10.Erickson LA, Letts GA, Shah SM, et al. TRPM1 (Melastatin-1/MLSN1) mRNA expression in Spitz nevi and nodular melanomas. Mod Pathol. 2009:56. doi: 10.1038/modpathol.2009.56. doi: 10.1038/modpathol.2009. [DOI] [PubMed] [Google Scholar]
- 11.Elder DE, Murphy GF. Washington, DC: Armed Forces Institute of Pathology; Atlas of Tumor Pathology: Melanocytic Tumors of the Skin; pp. 183–185. [Google Scholar]