Abstract
Melanomas are the most prevalent cancers in 25–29 year old females and compose roughly 12% of cancers in 20–40 year old women; under the age of 40, women have a higher incidence of melanomas than do men. Within the past few decades, the alarming trend to use commercial sunlamps for cosmetic pigmentation is of particular concern, especially since 71% of those patrons are women with 50% of patrons under the age of 29. A major problem may be the use of UVA-rich sunlamps which produce a visible tan but afford little to no protection from subsequent UV exposure. We hypothesize that the additional exposure of adolescents to unnaturally large amounts of UVA from artificial UV sources is implicated in the increasing incidence of malignant melanomas disproportionately in young women.
Keywords: melanoma, young women, UVA, sunlamps, tanning
INTRODUCTION
Appearance may only be skin deep but society has always judged us by that whether we like it or not. Social pressure to ‘enhance’ skin pigmentation has driven some fair-skinned individuals to engage in risky and unhealthy behaviors, such as with the alarming trend of increased UVA-rich sunlamp use by young women over the last 20–30 years. Not surprisingly, younger generations have ignored public health warnings in favor of extending their potentially carcinogenic exposure to UV. Of concern is the lack of a strong public health policy to protect vulnerable adolescents who receive careless intermittent sunburns from artificial sources of UV. Emerging data on the incidence rates of skin cancers in young women under the age of 40 compared to men of that age group suggest that their etiology is due, at least in part, to excessive repeated intermittent exposures to unnaturally large amounts of UVA from UVA-rich sunlamps. Regrettably, much of this excessive UV exposure is preventable, but public health officials, physicians and parents continually underestimate the use of sunlamps by adolescents in the same way that other risky behaviors in our society were once dismissed, e.g. smoking.
Those who argue against the correlation between sunlamps and the increased risk of melanoma and other types of skin cancers typically state that one cannot readily separate sunlamp exposure from normal solar exposure. Although this argument has some validity, the contribution of sunlamps should not be minimized. There is overwhelming evidence that UV is the cause of nonmelanoma skin cancers so the argument to have additional sources of UV exposure (not administered by medical personnel) other than the sun is a disservice to public health. Obviously, UV is a ubiquitous carcinogen, having both harmful and beneficial effects. UV is impossible to avoid completely, but exercising intelligent levels of exposure is critical to minimizing erythema, immune suppression, photoaging and skin cancer. The deleterious effects of UV have been known for a long time, but it is only in recent decades that artificial sources of UV have become available that contribute to the etiology of skin cancer. Our skin has protected us for millennia from the environment, but it is particularly vulnerable to increased exposure to UVA that can penetrate much deeper into the skin.
The unnaturally high UVA doses provided by UVA-rich sunlamps (which are now predominantly used in the tanning industry) are particularly deceiving to young adolescents because they are being promoted as ‘damage-free’ tans yet recent studies show that UVA offers no protective effects from further UV exposure and is not risk-free. Furthermore, the justification for sunlamp use to increase vitamin D levels in a young population that lacks rickets and has access to dietary vitamin D supplementation further underscores the fact that unnecessary additional UV exposure poses a preventable risk.
We hypothesize that the increasing incidence of melanomas, particularly in young women, reflects their increased intermittent use of UVA-rich sunlamps with increased annual UV doses and stronger dose rates compared to solar exposure. In the following sections, we provide evidence in the literature that strengthens this hypothesis.
1. Melanoma in young adults
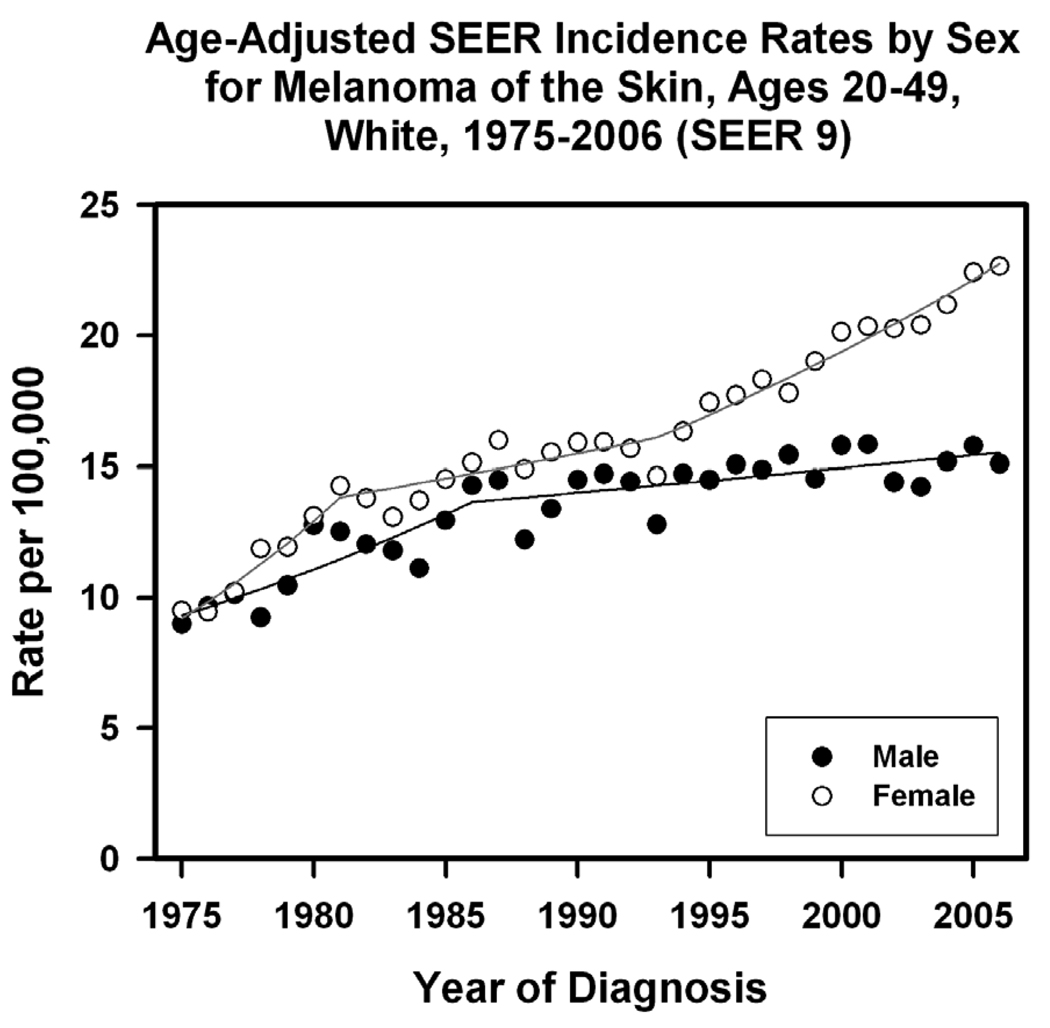
Cutaneous melanomas in young adults from 1975–2000 in the United States were the most prevalent cancers in 25–29 year old females and represented roughly 12% of cancers in 20–40 year old women (Bleyer et al., 2006).Under the age of 49, fair-skinned women have a higher incidence of melanomas than do fair-skinned men and within the last 10 years there has been an increasing divergence in incidence rates between genders (Figure 1) (Fast Stats: 2009).According to NCI SEER 9 data among white individuals between the ages of 20–49, this rapid change in incidence rates in women seems to diverge after 1995 and has steadily increased thereafter (Fast Stats: 2009). Previously, Purdue MP et al. had noted this increase among young women and provided further compelling evidence on gender-specific estimated annual percent change in melanoma incidence and incidence according to the year an individual was born (Purdue et al., 2008).In addition, according to skin cancer registry data from England, more women than men under the age of 40 were identified having melanoma although more deaths occurred in men(Morris et al., 2009).
Figure 1.
Age-Adjusted SEER Incidence Rates by Sex for Melanoma. Cancer sites include invasive cases only unless otherwise noted. Incidence source: SEER 9 areas (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, and Atlanta). Rates are per 100,000 and are age-adjusted to the 2000 US Std Population (19 age groups - Census P25–1130). Regression lines are calculated using the Joinpoint Regression Program Version 3.3.2, June 2008, National Cancer Institute.
2. The role of pigment-related genes in the induction of melanoma
The variation in skin pigmentation arises not only from melanosome size, shape and distribution throughout the epidermis, but also on ratios of eumelanin or pheomelanin produced in melanocytes. Three critical genes are responsible for the balance between eumelanin vs pheomelanin production: melanocortin 1 receptor (MC1R), and its ligands α-melanocyte stimulating hormone (αMSH) and agouti signaling protein (ASIP). The MC1R is activated by αMSH but is antagonized by ASIP and those interactions determine the types and amounts of melanins produced, and thus the resulting skin pigment phenotype. When activated, MC1R signals through the cAMP pathway, stimulating the expression and function of microphthalmia-associated transcription factor (MITF). In turn, MITF regulates the function of a cascade of melanocyte-specific genes that encode (among other things) the critical melanogenic enzyme tyrosinase, the rate-limiting factor in melanin biosynthesis (Tadokoro et al., 2002).Variants of the gene encoding MC1R are associated with the light skin/red hair phenotype that is extremely sensitive to UV and at an increased risk for melanoma (Flanagan et al., 2000; Healy et al., 2000).In studies of two separate populations, a haplotype near the ASIP locus associated with red hair variants showed increased risk for melanoma (OR = 1.45, 1.68) (Gudbjartsson et al., 2008; Nan et al., 2009).The relevance of MC1R function with respect to risk of skin cancer derives from the fact that in addition to regulating skin pigmentation, the MC1R also has dramatic effects on DNA repair, which is of course a critical process in UV-related photocarcinogenesis (Abdel-Malek et al., 2009). In addition, a European case-control study found an increased risk of melanoma in other genetic variants of pigment-related genes such as TYRP1 (OR = 1.15) and TYR (OR = 1.21) (Gudbjartsson et al., 2008).
3. Photoprotection (perceived vs real) of constitutive and facultative pigmentation
Differences in the rates of photocarcinogenesis of light versus dark skin types underscores the advantage of having a darker naturally defined constitutive pigmentation level. Darker skin types have a protection factor of about 13 (Johnson et al., 1998).In the United States, black individuals actually have a 18-fold (women) and 26-fold (men) lower incidence of melanoma than do white individuals (Horner et al., 2009).Constitutive pigmentation of the skin with higher melanin content protects the epidermis (specifically the lower epidermis) from DNA damage in the form of cyclobutane pyrimidine dimers and 6,4-photoproducts (Tadokoro et al., 2003; 2005; Yamaguchi et al., 2006).In fair-skinned individuals, physiologically relevant levels of repetitive UV doses do increase visible pigmentation, but do not increase apoptosis levels (as found in darker skin types) and provide only modest protective gains compared to constitutive pigmentation (Miller et al., 2008; Yamaguchi et al., 2008). Taken together, these data suggest that any UV-induced tan in fair-skinned individuals is of modest protective value.
It has become clear that there are distinct effects of facultative pigmentation induced by different types of UV, i.e. UVA or UVB. Chemical assays showed that UVB increases melanin production (Wolber et al., 2008) and affords only a slight UV protection factor of ~2 from subsequent UV exposure (Sheehan et al., 2002).UVA has the ability to induce visible skin pigmentation in the absence of new melanin synthesis, potentially through the more efficient distribution of existing melanin and/or the oxidation of melanogenic precursors (DHICA and 6H5MI2C) in the basal and suprabasal layers of the epidermis (Miyamura et al., 2009).However UVA-induced tans do not increase photoprotection or melanin production (Wolber et al., 2008).These new data regarding UVA are of particular concern because UVA-rich sunlamps are being used for tanning by fair skinned individuals.
4. Role of sex steroid hormones and vitamin D in gender differences
The current available data in the literature do not support a role of sex steroid hormones in the increased incidence of melanomas in young women. A review of the literature indicated there was no significant evidence to suggest an association between melanoma and oral contraceptive use or endogenous female hormones (International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer, 2007; La and Bosetti, 2009; Lens and Bataille, 2008).Melanocytes in normal skin and nevi as well as melanoma cells stain positively for estrogen receptor B, however its role in melanoma is unclear (Ohata et al., 2008).However, considering that estrogen receptor B expression is lost with increasing melanoma thickness and is increased in thinner melanomas, it is possible that estrogen receptor B plays a role in malignant transformation and/or progression (de Giorgi et al., 2009)
Less clear is the potential role in melanoma, if any, of the lack of vitamin D in young women. Even as early as 1940, the Council on Physical Therapy steered clear of indicating that vitamin D production from sunlamps is as beneficial as that produced by solar exposure (Council on Physical Therapy, 1940).Indications of lower mortality from melanoma was found in individuals with a lifetime of UV exposure (Berwick et al., 2005) and UV-induced vitamin D is tied to that outcome (Egan et al., 2005; Holick, 2001).The active form of vitamin D (1,25-dihydroxyvitamin D3) binds to the vitamin D receptor (VDR), which has many polymorphisms, among them being a variant (Fok1) associated with increased melanoma risk (Gandini et al., 2009; Kostner et al., 2009).In addition, limited data on vitamin D intake have indicated a potential protective effect from melanoma, based on a very small meta-analysis sample (Gandini et al., 2009). However a larger cohort study indicated no association between vitamin D intake and melanoma risk in either gender (Asgari et al., 2009). The potential for vitamin D production from sunlamp exposure remains controversial and whether it plays some role in the increased incidence rate of young women with skin cancers merits further investigation.
5. UV burden
Typical solar exposure taken at noon on a clear day in July near Washington, DC is composed of ~95% UVA and ~5% UVB (Miller et al., 1998).Compared to UVB, UVA makes up a greater percentage of UV wavelengths that actually reach the basal layer of the epidermis in human skin. However, the question remains, does UVA play a role in melanomagenesis? There is some controversy as to whether UVA contributes to cutaneous melanoma and several animal models strengthen that argument. The fact that HGF/SF-transgenic mice exposed to a single UV dose produced melanomas was a sobering discovery (Noonan et al., 2001), and subsequent research with those HGF/SF-transgenic mice suggested that UVA does not play a role in melanoma induction (DeFabo et al., 2004). However, Setlow et al. showed that repetitive UV exposures at wavelengths >304 nm induce melanomas in Xiphophorus fish, and subsequently demonstrated that the induction of reactive melanin radicals using UVA (at 365 nm) was the cause of melanoma induction in the presence of melanin (Setlow et al., 1989; 1993; Wood et al., 2006).In addition, they found a correlation between gender and the number of UVA-induced melanomas, with male fish having twice as many melanomas as female fish (Setlow et al., 1993).A melanoma model developed in Monodelphis domestica opposums reported that thrice weekly irradiations with UVA for 81 weeks was just as successful as UVB in producing melanoma precursor lesions (Ley, 1997).
Consequently, the role of UVA in melanomagenesis merits further investigation. It is known that frequent users of UVA-rich sunlamps double their amount of annual UVA exposure(Miller et al., 1998) and in countries such as Norway the UVA irradiances of tanning beds are 3–3.5 times that of summer solar exposure (Nilsen et al., 2008).Of particular concern are the high pressure UVA tanning beds with dose rates up to 13 times that of the summer sun, which would quadruple the annual UVA amounts received by frequent tanners (Miller et al., 1998).
6. Why Sunlamps matter?
In the first half of the twentieth century, the medical community was already wrestling with the implications of sunlamp use towards the induction of skin cancer (even before the emergence of fluorescent lamps). At that time, scientists believed that the sunbathing fad would die down (Coblentz, 1948).With the advent of fluorescent lamps, sunlamp products such as tanning beds, booths, and portable home units exploded onto the market in the 1980’s and became widely available to the public. Over the years, the Federal Trade Commission has identified major manufacturers of tanning devices that have misrepresented the safety of sunlamps by identifying them as “safe” and alleging no contributing risk towards skin damage, skin aging and/or skin cancer (U.S.Federal Trade Commission, 1988).However, this type of behavior is not new and as early as the 1930’s, the medical community was warning manufacturers to stop advertising the health benefits of sunlamps that was not backed by scientific evidence (Council on Physical Therapy, 1934).Adding to public perceptions, regulatory agencies do not consider sunlamps to be medical devices because the therapeutic benefit cannot be isolated from the byproduct of increased skin pigmentation. This quandary feeds into the argument of perceived benefits and allows sunlamp salons to remain ungoverned at the federal level (although sunlamp manufacturers are regulated), leaving it to individual state and local jurisdictions to set guidelines for sunlamp use in their communities.
The National Electronic Injury Surveillance System of the U.S. Consumer Product Safety Commission collects data from a sample of US hospitals to identify the number of individuals treated in emergency rooms due to injuries from consumer products. That database indicates that 75% of sunlamp-related injuries from 1980–2006 (excluding eye injuries) occurred in young women compared to only 25% in men (Table 1)(US Consumer Product Safety Commission, 2009) with a large portion of those being individuals under the age of 20. Although this is a relatively small sample of US hospitals, and does not allow for proper extrapolation, one has to be startled by how severe these sunburns must have been to warrant a visit to an emergency room.
Table 1.
Sunburn Injuries from Sunlamps Treated in Hospital Emergency Rooms Reported to the U.S.Consumer Product Safety Commission’s National Electronic Injury Surveillance System
| Sample # of Hospitals |
Cases | Female | Female under 20 |
% | Age | Male | Male under 20 |
Age | % | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1980 | 74 | 36 | 25 | 16 | 69 | 19.8 | 11 | 4 | 27.6 | 31 |
| 1981 | 74 | 41 | 28 | 15 | 68 | 21.3 | 13 | 6 | 24.1 | 32 |
| 1982 | 73 | 25 | 22 | 17 | 88 | 19.1 | 3 | 2 | 19.3 | 12 |
| 1983 | 73 | 25 | 18 | 9 | 72 | 22.4 | 7 | 3 | 26.1 | 28 |
| 1984 | 72 | 22 | 16 | 12 | 73 | 18.5 | 6 | 2 | 29.3 | 27 |
| 1985 | 64 | 22 | 15 | 14 | 68 | 17.3 | 7 | 2 | 23.6 | 32 |
| 1986 | 64 | 14 | 13 | 8 | 93 | 21.0 | 1 | 0 | 24.0 | 7 |
| 1987 | 62 | 14 | 10 | 6 | 71 | 20.2 | 4 | 3 | 27.5 | 29 |
| 1988 | 62 | 18 | 13 | 11 | 72 | 18.8 | 5 | 3 | 25.4 | 28 |
| 1989 | 62 | 20 | 16 | 9 | 80 | 21.3 | 4 | 2 | 22.3 | 20 |
| 1990 | 65 | 6 | 5 | 4 | 83 | 17.6 | 1 | 0 | 32.0 | 17 |
| 1991 | 91 | 13 | 10 | 8 | 77 | 20.6 | 3 | 1 | 25.7 | 23 |
| 1992 | 91 | 19 | 14 | 8 | 74 | 20.7 | 5 | 3 | 22.0 | 26 |
| 1993 | 91 | 8 | 6 | 2 | 75 | 26.0 | 2 | 0 | 61.5 | 25 |
| 1996 | 90 | 5 | 4 | 0 | 80 | 33.5 | 1 | 0 | 24.0 | 20 |
| 1997 | 101 | 5 | 3 | 1 | 60 | 26.3 | 2 | 2 | 17.0 | 40 |
| 1998 | 101 | 3 | 3 | 1 | 100 | 23.3 | 0 | 0 | N/A | N/A |
| 1999 | 101 | 3 | 3 | 2 | 100 | 23.0 | 0 | 0 | N/A | N/A |
| 2000–06 | 100 | 15 | 11 | 7 | 73 | 22.6 | 4 | 3 | 17.8 | 27 |
| Total | 314 | 235 | 150 | 75 | 21.8 | 79 | 36 | 26.4 | 25 | |
The U.S. Consumer Product Safety Commission collected and kindly provided the sunlamp injury data (product code: 1609) from 1980–2006 used to compile this table from its National Electronic Injury Surveillance System (NEISS). The NEISS database collects data on injuries from consumer products leading to emergency room visits from each of its sample hospitals. Eye only injuries and other injuries from sunlamps not related to sunburns were excluded from the data set. Due to the small number of reported injuries from 2000–2006, those data were combined for simplicity. The percentage (%) of females or males was calculated from the total number from each gender against the total number of cases for each individual year and for the entire data set in the row labeled “Total”.
That said, this sample may significantly underestimate the number of sunburns caused by sunlamps (and the larger number of visits to the emergency room by women is in line with data indicating that they are the predominate consumer). Consequently, these severe sunburn events occurred during the emergence of UVA-rich sunlamps (which require longer exposure times) which ironically should avoid the risk of sunburn injuries.
7. Use of sunlamps and sunburn prevalence by young adults
What if UVA-rich sunlamp use potentially shortens the latency period between induction of melanoma and/or nonmelanoma skin cancers? Among the risky behaviors by young adults, excessive sunlamp use may not be a major parental concern. Compared to other high risk behaviors which have potentially serious acute consequences, UV exposure risks are viewed as having long-term consequences whose effects will not materialize in the immediate future. This allows the commercial sunlamp industry to continue targeting young adults, specifically young women who make up 71% of their patrons (Swerdlow and Weinstock, 1998).Interestingly, of the ~25,000 professional tanning salons in the US, females own 50% of those compared to 25% in other women-owned businesses (Bizzozero, 2005).With roughly 1.75 billion dollars worth of tanning equipment in US tanning salons and more than 75% of those salons reporting plans for expansion (Bizzozero, 2005), there is no doubt that this is a highly profitable enterprise. Moreover, the customer base targeted in many instances is the young adolescent, such as in the Denver metropolitan area where tanning salon advertisements appeared in 11 of 23 high school newspapers, with 38% of those announcing unlimited tanning (Freeman et al., 2006).
An estimated 18–55% of commercial sunlamp users in Europe and in North America have reported sunburns. A 1996 US National Longitudinal Study of Adolescent Health indicated that young white adolescents, aged 13–19, with a tanning bed lifetime use of more than 20 visits were predominately made up of young women (Demko et al., 2003).In a 1999 US Nurses Health Study of young white adolescents aged 12–18, it was noted that more young women (14.4%) than young men (2.4%) had used a tanning bed within the previous year (Geller et al., 2002).The number of sunburns reported in the previous summer was also higher (40%) in young women than in young men (30%) (Geller et al., 2002).More recently, data from two national American Cancer Society telephone surveys indicated that 61.1% of females and 44.6% of males who used sunlamps reported receiving sunburns (Cokkinides et al., 2009).
Commercial sunlamp use and sunburn prevalence is not only an issue in the US. In a German study, 4% of young men compared to 35% of young women in the same age group (14–18 years) reported using sunbeds, and 31% of the young women using sunbeds indicated that they were high frequency users (>10 times a year) (Borner et al., 2009).A Swedish study of young adolescents aged 13–19 found a significant tendency for young women to engage in tanning bed use (Boldeman et al., 2001).They reported overwhelming use of tanning beds by minors with 20% of young women vs 6% of young men aged 13–16 and 52% of young women vs 25% of young men aged 17–19 (Boldeman et al., 2001).In addition, 4% and 17% of young women in the age groups 13–16 and 17–19, respectively, reported a sunburn using a tanning bed within the last year compared to their male counterparts who reported 1% and 5%, respectively.
Summary and future directions
The etiology of melanoma has been suggested to involve large intermittent doses of UV and suggests that age-specific risks are not determined by total lifetime UV doses in contrast to nonmelanoma skin cancers (Fears et al., 1977). Social controversies about UV-induced pigmentation from sunlamps have been explored and the risks and benefits will continue to be debated (Schulman and Fisher, 2009; Tran et al., 2008).The International Agency for Research on Cancer (IARC) recently raised the classification of UV-emitting tanning devices to “carcinogenic to humans”. Importantly, the IARC reviewed case-control studies where the first exposure to a sunbed was before the age of 35 and found a 75% increase in melanoma risk (International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer, 2007).This recent policy change reinforces our hypothesis that the increasing rates of melanoma in young women may reflect intermittent sunlamp exposures. This is of particular concern for sunlamps that are UVA-rich and purport to induce ‘safe’ tans, despite the fact that significant cellular damage occurs from UVA and that the resulting skin ‘tan’ actually has no photoprotective benefit. The latter aspect is particularly detrimental since it gives a false sense of security that might induce the tanner to feel they are protected from damage due to subsequent UV exposure.
Current data have dramatic implications for healthy young fair-skinned women who predominantly use UVA-rich sunlamps to produce tans. The mounting evidence of increased melanoma risk for young adults suggests that setting a minimum age requirement for sunlamp use may be appropriate as the current guidelines do not seem to be effective in preventing the increasing incidence rate of skin cancers. If indeed minors (under 18) are not the age group being economically targeted by sunlamp salon owners, then it would seem prudent that the industry embrace legislation proposed in some U.S. counties that would prohibit minors under 18 from using sunlamps (County Council of Baltimore County, 2009;Howard County Health Department, 2009).However, the Indoor Tanning Association actively rallied support from its member tanning salons to fight that legislation [http://www.theita.com/Home/HowardBaltimoreCountyNew.htm]. Despite their opposition and lobby, Howard County in Maryland became the first county in the U.S. to prohibit minors under 18 from using sunlamps (Kromm, 2009).In addition, requirements for parental consent or permission do not seem to be a deterrent, but rather is a strong indicator for sunlamp use by young adolescents (Cokkinides et al., 2009).Based on a nationwide study, in states with youth restriction laws, parents may be complicit in allowing minors under the age of 18 to engage in tanning (Pichon et al., 2009).Of particular concern is the fact that 89% of indoor tanning facilities examined were not in compliance with government guidelines for 3 or fewer sunlamp exposures in the first week (Pichon et al., 2009).Young adults, regardless of gender, should be deterred from using tanning beds. Perhaps modern education programs designed for a peer-to-peer networking generation might be effective in deterring excessive UV exposure by motivating individuals to make healthy, informed decisions about UV exposure. Additionally, smart and effective compromises between lawmakers, physicians, and parents in protecting the young adolescent population from increasing skin cancer risks and economic exploitation is necessary.
Our hypothesis that the increasing use of UVA-rich sunlamps underlies, at least in part, the dramatic rise in melanomas disproportionately in young females, will no doubt be tested as statistics on skin cancers in the current young generation become available in future years.
ACKNOWLEDGEMENTS
This research was supported in part by the Intramural Research Program of the National Cancer Institute at NIH.
REFERENCES
- Abdel-Malek ZA, Ruwe A, Kavanagh-Starner R, Kadekara AL, Haskell-Luevano C, Kokov L, Kittel JJ. alpha-MSH tripeptid analogs activate the melanocortin 1 receptor and reduce UV-induced DNA damage in human melanocytes. Pigment Cell Melanoma Res. 2009;22:635–644. doi: 10.1111/j.1755-148X.2009.00598.x. [DOI] [PubMed] [Google Scholar]
- Asgari MM, Maruti SS, Kushi LH, White E. A cohort study of vitamin D intake and melanoma risk. J. Invest Dermatol. 2009;129:1675–1680. doi: 10.1038/jid.2008.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick M, Armstrong BK, Ben Porat L, Fine J, Kricker A, Eberle C, Barnhill R. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97:195–199. doi: 10.1093/jnci/dji019. [DOI] [PubMed] [Google Scholar]
- Bizzozero J. Up Close The State of the Industry Report '05. Looking Fit. 2005 Sep 5; 9-5-2005. URL: http://www.lookingfit.com/articles/591cover.html. [Google Scholar]
- Bleyer A, O'Leary M, Barr R, Ries LA. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975–2000. Bethesda, MD: National Cancer Institute; NIH Pub. No. 06-5767. 2006.
- Boldeman C, Branstrom R, Dal H, Kristjansson S, Rodvall Y, Jansson B, Ullen H. Tanning habits and sunburn in a Swedish population age 13–50 years. Eur. J. Cancer. 2001;37:2441–2448. doi: 10.1016/s0959-8049(01)00310-0. [DOI] [PubMed] [Google Scholar]
- Borner FU, Schutz H, Wiedemann P. A population-based survey on tanning bed use in Germany. BMC. Dermatol. 2009;9:6. doi: 10.1186/1471-5945-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coblentz WW. Experimental production of cancer of the skin by ultraviolet radiation; its implications in the use of sunlamps. J. Am. Med. Assoc. 1948;136:1040–1043. doi: 10.1001/jama.1948.72890330002009. [DOI] [PubMed] [Google Scholar]
- Cokkinides V, Weinstock M, Lazovich D, Ward E, Thun M. Indoor tanning use among adolescents in the US, 1998 to 2004. Cancer. 2009;115:190–198. doi: 10.1002/cncr.24010. [DOI] [PubMed] [Google Scholar]
- Council on Physical Therapy. Acceptance of Sunlamps. JAMA. 1934;102:42–44. [Google Scholar]
- Council on Physical Therapy. Acceptance of Sunlamps. JAMA. 1940;114:325–326. [Google Scholar]
- County Council of Baltimore County, M. 82-09 Tanning Beds - Minors. [82-09. 10-19-2009]; http://resources.baltimorecountymd.gov/Documents/CountyCouncil/bills/b08209.pdf.
- de Giorgi V, Mavilia C, Massi D, Gozzini A, Aragona P, Tanini A, Sestini S, Paglierani M, Boddi V, Brandi ML, Lotti T. Estrogen receptor expression in cutaneous melanoma: a real-time reverse transcriptase-polymerase chain reaction and immunohistochemical study. Arch. Dermatol. 2009;145:30–36. doi: 10.1001/archdermatol.2008.537. [DOI] [PubMed] [Google Scholar]
- DeFabo EC, Noonan FP, Fears TR, Merlino G. Ultraviolet B but not ultraviolet A radiation initiates melanoma. Cancer Res. 2004;64:6372–6376. doi: 10.1158/0008-5472.CAN-04-1454. [DOI] [PubMed] [Google Scholar]
- Demko CA, Borawski EA, Debanne SM, Cooper KD, Stange KC. Use of indoor tanning facilities by white adolescents in the United States. Arch. Pediatr. Adolesc. Med. 2003;157:854–860. doi: 10.1001/archpedi.157.9.854. [DOI] [PubMed] [Google Scholar]
- Egan KM, Sosman JA, Blot WJ. Sunlight and reduced risk of cancer: is the real story vitamin D? J Natl Cancer Inst. 2005;97:161–163. doi: 10.1093/jnci/dji047. [DOI] [PubMed] [Google Scholar]
- Fast Stats. An interactive tool for access to SEER cancer statistics. [10-26-2009];Surveillance Research Program, National Cancer Institute; http://seer.cancer.gov/faststats.
- Fears TR, Scotto J, Schneiderman MA. Mathematical models of age and ultraviolet effects on the incidence of skin cancer among whites in the United States. Am. J. Epidemiol. 1977;105:420–427. doi: 10.1093/oxfordjournals.aje.a112400. [DOI] [PubMed] [Google Scholar]
- Flanagan N, Healy E, Ray A, Philips S, Todd C, Jackson IJ, Birch-Machin MA, Rees JL. Pleiotropic effects of the melanocortin 1 receptor (MC1R) gene on human pigmentation. Hum. Mol. Gen. 2000;9:2531–2537. doi: 10.1093/hmg/9.17.2531. [DOI] [PubMed] [Google Scholar]
- Freeman S, Francis S, Lundahl K, Bowland T, Dellavalle RP. UV tanning advertisements in high school newspapers. Arch. Dermatol. 2006;142:460–462. doi: 10.1001/archderm.142.4.460. [DOI] [PubMed] [Google Scholar]
- Gandini S, Raimondi S, Gnagnarella P, Dore JF, Maisonneuve P, Testori A. Vitamin D and skin cancer: a meta-analysis. Eur. J. Cancer. 2009;45:634–641. doi: 10.1016/j.ejca.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Geller AC, Colditz G, Oliveria S, Emmons K, Jorgensen C, Aweh GN, Frazier AL. Use of sunscreen, sunburning rates, and tanning bed use among more than 10 000 US children and adolescents. Pediatrics. 2002;109:1009–1014. doi: 10.1542/peds.109.6.1009. [DOI] [PubMed] [Google Scholar]
- Gudbjartsson DF, Sulem P, Stacey SN, Goldstein AM, Rafnar T, Sigurgeirsson B, Benediktsdottir KR, Thorisdottir K, Ragnarsson R, Sveinsdottir SG, Magnusson V, Lindblom A, Kostulas K, Botella-Estrada R, Soriano V, Juberias P, Grasa M, Saez B, Andres R, Scherer D, Rudnai P, Gurzau E, Koppova K, Kiemeney LA, Jakobsdottir M, Steinberg S, Helgason A, Gretarsdottir S, Tucker MA, Mayordomo JI, Nagore E, Kumar R, Hansson J, Olafsson JH, Gulcher J, Kong A, Thorsteinsdottir U, Stefansson K. ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat. Genet. 2008;40:886–891. doi: 10.1038/ng.161. [DOI] [PubMed] [Google Scholar]
- Healy E, Flannagan N, Ray A, Todd C, Jackson IJ, Matthews JNS, Birch-Machin MA, Rees JL. Melanocortin-1 receptor gene and sun sensitivity in individuals without red hair. Lancet. 2000;355:1072–1073. doi: 10.1016/S0140-6736(00)02042-0. [DOI] [PubMed] [Google Scholar]
- Holick MF. Sunlight "D"ilemma: risk of skin cancer or bone disease and muscle weakness. Lancet. 2001;357:4–6. doi: 10.1016/S0140-6736(00)03560-1. [DOI] [PubMed] [Google Scholar]
- Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altedruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975–2006. 2009 http://seer.cancer.gov/csr/1975_2006/
- Howard County Health Department. Regulations pertaining to Tanning Facilities in Howard County, Maryland. [11-10-2009]; http://www.co.ho.md.us/health/docs/tanningregulations.pdf.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int. J. Cancer. 2007;120:1116–1122. doi: 10.1002/ijc.22453. [DOI] [PubMed] [Google Scholar]
- Johnson BL, Moy R, White GM. Ethnic skin: medical and surgical. Toronto: Mosby Publ; 1998. [Google Scholar]
- Kostner K, Denzer N, Muller CS, Klein R, Tilgen W, Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009;29:3511–3536. [PubMed] [Google Scholar]
- Kromm EE. Howard County becomes first in nation to ban minors from tanning beds – Board of Health votes to approve regulations. [11-10-2009]; http://www.co.ho.md.us/health/docs/MR-tanning111009.pdf.
- La VC, Bosetti C. Oral contraceptives and neoplasms other than breast and female genital tract. Eur. J. Cancer Prev. 2009;18:407–411. doi: 10.1097/CEJ.0b013e32832caaca. [DOI] [PubMed] [Google Scholar]
- Lens M, Bataille V. Melanoma in relation to reproductive and hormonal factors in women: current review on controversial issues. Cancer Causes Control. 2008;19:437–442. doi: 10.1007/s10552-008-9110-4. [DOI] [PubMed] [Google Scholar]
- Ley RD. Ultraviolet radiation a-induced precursors of cutaneous melanoma in Monodelphis domestica. Cancer Res. 1997;57:3682–3684. [PubMed] [Google Scholar]
- Miller SA, Coelho SG, Zmudzka BZ, Bushar HF, Yamaguchi Y, Hearing VJ, Beer JZ. Dynamics of pigmentation induction by repeated ultraviolet exposures: dose, dose interval and ultraviolet spectrum dependence. Br. J. Dermatol. 2008;159:921–930. doi: 10.1111/j.1365-2133.2008.08708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochemistry. and Photobiology. 1998;68:63–70. [PubMed] [Google Scholar]
- Miyamura Y, Coelho SG, Schlenz K, Batzer J, Smuda C, Choi W, Brenner M, Passeron T, Wolber R, Hearing VJ. The deceptive nature of UVA-tanning on human skin versus the modest protective effects of skin pigmentation elicited by UVB. 2009 doi: 10.1111/j.1755-148X.2010.00764.x. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S, Cox B, Bosanquet N. Cost of skin cancer in England. Eur J Health Econ. 2009;10:267–273. doi: 10.1007/s10198-008-0127-0. [DOI] [PubMed] [Google Scholar]
- Nan H, Kraft P, Hunter DJ, Han J. Genetic variants in pigmentation genes, pigmentary phenotypes, and risk of skin cancer in Caucasians. Int. J. Cancer. 2009;125:909–917. doi: 10.1002/ijc.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen LT, Hannevik M, Aalerud TN, Johnsen B, Friberg EG, Veierod MB. Trends in UV irradiance of tanning devices in Norway: 1983–2005. Photochem. Photobiol. 2008;84:1100–1108. doi: 10.1111/j.1751-1097.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- Noonan FP, Recio JA, Takayama H, Duray P, Anver MR, Rush WL, DeFabo EC, Merlino G. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–272. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- Ohata C, Tadokoro T, Itami S. Expression of estrogen receptor beta in normal skin, melanocytic nevi and malignant melanomas. J. Dermatol. 2008;35:215–221. doi: 10.1111/j.1346-8138.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- Pichon LC, Mayer JA, Hoerster KD, Woodruff SI, Slymen DJ, Belch GE, Clapp EJ, Hurd AL, Forster JL, Weinstock MA. Youth access to artificial UV radiation exposure: practices of 3647 US indoor tanning facilities. Arch. Dermatol. 2009;145:997–1002. doi: 10.1001/archdermatol.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdue MP, Freeman LE, Anderson WF, Tucker MA. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J. Invest Dermatol. 2008;128:2905–2908. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman JM, Fisher DE. Indoor ultraviolet tanning and skin cancer: health risks and opportunities. Curr. Opin. Oncol. 2009;21:144–149. doi: 10.1097/CCO.0b013e3283252fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc. Natl. Acad. Sci. USA. 1993;90:6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow RB, Woodhead AD, Grist E. Animal model for ultraviolet radiation-induced melanoma: platyfish-swordtail hybrid. Proc. Natl. Acad. Sci. U. S. A. 1989;86:8922–8926. doi: 10.1073/pnas.86.22.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JM, Cragg N, Chadwick CA, Potten CS, Young AR. Repeated ultraviolet exposure affords the same protection against DNA photodamage and erythema in human skin types II and IV but is associated with faster DNA repair in skin type IV. J. Invest. Dermatol. 2002;118:825–829. doi: 10.1046/j.1523-1747.2002.01681.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. Journal of the American Academy of Dermatology. 1998;38:89–98. doi: 10.1016/s0190-9622(98)70544-4. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Kobayashi N, Beer JZ, Zmudzka BZ, Wakamatsu K, Miller SA, Lamoreux ML, Ito S, Hearing VJ. "The biochemistry of melanogenesis and its regulation by ultraviolet radiation,". In: Ortonne JP, Ballotti R, editors. Mechanisms of Suntanning. London: Martin Dunitz Publishing; 2002. pp. 67–78. [Google Scholar]
- Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, Korossy KS, Miller SA, Beer JZ, Hearing VJ. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB. J. 2003;17:1177–1179. doi: 10.1096/fj.02-0865fje. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, Miller SA, Wolber R, Beer JZ, Hearing VJ. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J. Invest Dermatol. 2005;124:1326–1332. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- Tran TT, Schulman J, Fisher DE. UV and pigmentation: molecular mechanisms and social controversies. Pigment Cell Melanoma Res. 2008;21:509–516. doi: 10.1111/j.1755-148X.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S.Federal Trade Commission. 1988 Annual Report. 1988 http://www.ftc.gov/os/annualreports/ar1988.pdf.
- US Consumer Product Safety Commission. National Electronic Injury Surveillance System (NEISS) 2009 https://www.cpsc.gov/cgibin/NEISSQuery/home.aspx.
- Wolber R, Schlenz K, Wakamatsu K, Smuda C, Nakanishi Y, Hearing VJ, Ito S. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res. 2008;21:487–491. doi: 10.1111/j.1755-148X.2008.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SR, Berwick M, Ley RD, Walter RB, Setlow RB, Timmins GS. UV causation of melanoma in Xiphophorus is dominated by melanin photosensitized oxidant production. Proc. Natl. Acad. Sci. USA. 2006;103:4111–4115. doi: 10.1073/pnas.0511248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Coelho SG, Zmudzka BZ, Takahashi K, Beer JZ, Hearing VJ, Miller SA. Cyclobutane pyrimidine dimer formation and p53 production in human skin after repeated UV irradiation. Exp. Dermatol. 2008;17:916–924. doi: 10.1111/j.1600-0625.2008.00722.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, Berens W, Beer JZ, Hearing VJ. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006;20:1486–1488. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]