Abstract
Microglia of the central nervous system have a dual role in the ability to influence the survival of neighboring cells. During inflammatory cell activation, microglia can lead to the disposal of toxic cellular products and permit tissue regeneration, but microglia also may lead to cellular destruction with phagocytic removal. For these reasons, it is essential to elucidate not only the underlying pathways that control microglial activation and proliferation, but also the factors that determine microglial survival. In this regard, we investigated in the EOC 2 microglial cell line with an oxygen-glucose deprivation (OGD) injury model of oxidative stress the role of the “O” class forkhead transcription factor FoxO3a that in some scenarios is closely linked to immune system function. We demonstrate that FoxO3a is a necessary element in the control of early and late apoptotic injury programs that involve membrane phosphatidylserine externalization and nuclear DNA degradation, since transient knockdown of FoxO3a in microglia preserves cellular survival 24 hours following OGD exposure. However, prior to the onset of apoptotic injury, FoxO3a facilitates the activation and proliferation of microglia as early as 3 hours following OGD exposure that occurs in conjunction with the trafficking of the unphosphorylated and active post-translational form of FoxO3a from the cytoplasm to the cell nucleus. FoxO3a also can modulate apoptotic mitochondrial signal transduction pathways in microglia, since transient knockdown of FoxO3a prevents mitochondrial membrane depolarization as well as the release of cytochrome c during OGD. Control of this apoptotic cascade also extends to progressive caspase activation as early as 1 hour following OGD exposure. The presence of FoxO3a is necessary for the expression of cleaved (active) caspase 3, 8, and 9, since loss of FoxO3a abrogates the induction of caspase activity. Interestingly, elimination of FoxO3a reduced caspase 9 activity to a lesser extent than that noted with caspase 3 and 8 activities, suggesting that FoxO3a in relation to caspase 9 may be more reliant upon other signal transduction pathways potentially independent from caspase 3 and 8.
Keywords: Apoptosis, bromodeoxyuridine, caspases, cytochrome c, forkhead transcription factors, FoxO3a, immune system, inflammation, microglia, mitochondria, oxidative stress, proliferating cell nuclear antigen
INTRODUCTION
Oxidative stress has a vital role in the pathology of multiple disease processes that can involve metabolic disorders (Barbosa et al., 2008; Duarte et al., 2008; Gossai and Lau-Cam, 2009; Guarnieri et al., 2009; Hao et al., 2009; Maiese, 2008a; Maiese, 2009b; Ruf et al., 2009; Szabo, 2009; Wu et al., 2009), cognitive dysfunction (Erol, 2009; Newman et al., 2007; Power et al., 2008; Toledano et al., 2008), ischemic injury (Park et al., 2009; Thomas et al., 2008; Zhou et al., 2009), cardiac, lung, and liver disease [109, 110, 111], psychiatric disorders [112, 113, 114], seizures (Lehtinen et al., 2009; Sales Santos et al., 2009), drug toxicity (Lu et al., 2009; North et al., 2003; Rosa et al., 2007), and infertility [115, 116, 117]. Closely tied to oxidant cell injury is the process of apoptosis. Apoptosis consists of the destruction of genomic DNA (Maiese et al., 1999; Maiese and Vincent, 2000a, b) as a later event during apoptotic injury (Dombroski et al., 2000; Jessel et al., 2002; Kang et al., 2003b; Maiese and Vincent, 2000b) and the early externalization of membrane phosphatidylserine (PS) residues (Chong et al., 2005b; Maiese et al., 2008f). The loss of membrane phospholipid asymmetry leads to the exposure of membrane PS residues on the cell surface and the activation and proliferation of inflammatory microglial cells to target cells for phagocytosis (Chong et al., 2003c; Kang et al., 2003a, b; Maiese and Chong, 2003; Mallat et al., 2005).
Interestingly, in many instances, the ultimate outcome of cells and their survival may be controlled by neighboring inflammatory cells such as microglia (Gilfillan and Rivera, 2009; Maiese et al., 2009c). For example, during neurodegenerative disorders that can involve the loss of cognition and activation of microglial cells, phagocytic removal of both neurons and vascular cells can ensue (Chong et al., 2007a; Maiese et al., 2008d; Maiese et al., 2005b). Within these periods of inflammatory cell activation, microglia rely upon their own intrinsic cytoprotective pathways (Chong et al., 2007b; Li et al., 2006b) to proliferate and remove cells that are no longer functional (Li et al., 2005; Mallat et al., 2005). Although microglia can be beneficial during periods of activation and proliferation to remove toxic cell products (Geijtenbeek and Gringhuis, 2009; Salminen and Kaarniranta, 2009) and allow for tissue regeneration (Chong et al., 2007b; Dringen, 2005), microglia also can generate reactive oxygen species to lead to additional cell and tissue injury (Bakshi et al., 2008; Denes et al., 2008; Maiese, 2008b, 2009a; Maiese et al., 2008a; Maiese et al., 2008b; Williams et al., 2009; Zhao et al., 2009). As a result, it becomes critical to understand the pathways that can control not only the activation and proliferation of inflammatory microglia, but also the mechanisms that can limit survival of microglia.
One novel pathway that may be a viable candidate to modulate microglial function and survival involves the family of forkhead transcription factors. In particular, forkhead transcription factors of the “O” class (FoxOs) have intricate relationships with several vital cellular functions, such as metabolism (Maiese et al., 2007a, 2008c; Maiese et al., 2008e, f; Maiese et al., 2007c) and immune surveillance (Maiese et al., 2007b, 2008c). FoxOs are expressed in the reproductive system, cardiac and skeletal muscle, lung, liver, pancreas, spleen, thymus, and the nervous system (Lappas et al., 2009; Maiese et al., 2008e, 2009c, d). Furthermore, FoxOs, such as the family member FoxO3a, can determine cellular survival in systems that involve metabotropic glutamate receptors (Chong et al., 2006b), neurotrophins (Zheng et al., 2002), cancer (Fei et al., 2009; Maiese et al., 2008e, 2009c), and cytokine (Chong and Maiese, 2007). For modulation of the immune system, FoxO3a activation may be required during disorders such as systemic lupus erythematosus (Sela et al., 2006), T cell hyperactivity (Lin et al., 2004), and immune complex-mediated inflammatory arthritis (Jonsson et al., 2005; Ludikhuize et al., 2007).
Given the ability of FoxO3a to oversee immune system function and cellular survival in a number of scenarios, we investigated the role of FoxO3a to modulate cerebral microglial activation and proliferation as well as intrinsic cellular pathways that are tied to apoptotic injury during oxidative stress. Here we show in the EOC 2 microglial cell line with an oxygen-glucose deprivation (OGD) injury model and transient gene knockdown of FoxO3a that early microglial activation and proliferation during oxidant stress induction depends upon FoxO3a. Furthermore, subsequent apoptotic injury programs with PS externalization and nuclear DNA degradation of microglia proceed through mitochondrial membrane depolarization, cytochrome c release, and activation of caspase 3, 8, and 9 that require the presence and intracellular trafficking of unphosphorylated (active) FoxO3a.
MATERIALS AND METHODS
Microglia Cell Cultures
The microglial cell line EOC 2 was obtained from American Type Culture Collection (ATTC, Manassas, VA.). Cells were maintained in Dulbecco’s modified Eagle medium (ATTC, Manassas, VA), supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO), 50 µg/ml penicillin and streptomycin and 20% media from the LADMAC cell line (ATCC, Manassas, VA) which contains colony stimulating factor-1 (CSF-1) secreted by LADMAC cells. Cells were seeded onto 24-well plates or 35 mm culture dishes at a density of 1.5 × 106 cells per well or 4 × 106 cells per dish.
Experimental Treatments
Oxygen-glucose deprivation (OGD) in microglia was performed by replacing the media with glucose-free HBSS containing 116 mmol/L NaCl, 5.4 mmol/L KCl, 0.8 mmol/L MgSO4, 1 mmol/L NaH2PO4, 0.9 mmol/L CaCl2, and 10 mg/L phenol red (pH 7.4) and cultures were maintained in an anoxic environment (95% N2 and 5% CO2) at 37 °C per the experimental paradigm.
Assessment of Cell Survival
Microglial injury was determined by bright field microscopy using a 0.4% trypan blue dye exclusion method 24 hours following treatment with OGD per our previous protocols (Chong et al., 2007b; Chong and Maiese, 2007; Kang et al., 2003a, b). The mean survival was determined by counting eight randomly selected non-overlapping fields with each containing approximately 10–20 cells (viable + non-viable). Each experiment was replicated 6 times independently with different cultures.
Assessment of DNA Fragmentation
Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay (Chong et al., 2002a; Kang et al., 2003b). Briefly, microglial cells were fixed in 4% paraformaldehyde/0.2% picric acid/0.05% glutaraldehyde and the 3’-hydroxy ends of cut DNA were labeled with biotinylated dUTP using the enzyme terminal deoxytransferase (Pro-mega, Madison, WI) followed by streptavidin-peroxidase and visualized with 3,3’-diaminobenzidine (Vector Laboratories, Burlingame, CA).
Assessment of Membrane Phosphatidylserine (PS) Residue Externalization
Phosphatidylserine (PS) exposure was assessed through the established use of annexin V. Per our prior protocols (Chong et al., 2004a; Kang et al., 2003b), a 30 µg/ml stock solution of annexin V conjugated to phycoerythrin (PE) (R&D Systems, Minneapolis, MN) was diluted to 3 µg/ml in warmed calcium containing binding buffer (10 mmol/L Hepes, pH 7.5, 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 1.8 mmol/L CaCl2). Plates were incubated with 500 µl of diluted annexin V for 10 minutes. Images were acquired with "blinded" assessment with a Leitz DMIRB microscope (Leica, McHenry, IL) and a Fuji/Nikon Super CCD (6.1 megapixels) using transmitted light and fluorescent single excitation light at 490 nm and detected emission at 585 nm.
Assessment of Microglial Activation and Proliferation
Proliferating cell nuclear antigen (PCNA) expression for microglial activation (Williams et al., 2002) and bromideoxyuridine (BrdU) uptake for microglial proliferation (Martinez-Contreras et al., 2002) was performed with anti-mouse monoclonal antibody PCNA (1:1000) or BrdU (1:6000) (Sigma, St Louis, MO) conjugated with biotinylated anti-mouse IgG (1:100) and visualized through fluorescein avidin (1:100) for PCNA and Texas Red streptavidin (Vector laboratories, Burlingame, CA) for BrdU. BrdU (10 µM) and fluorodexyuridine (1µM) (Sigma, St. Louis, MO) were applied 1 hour prior to the time of fixation.
Expression of Phosphorylated FoxO3a, total FoxO3a, and Active Caspase 3, 8, and 9
Cells were homogenized and each sample (50 µg lane−1) was subjected to SDS-polyacrylamide gel electrophoresis (7.5% FoxO3a; 12.5% caspase 3, 8, and 9). After transfer, the membranes were incubated with a rabbit polyclonal antibody against a rabbit antibody against phospho-FoxO3a (1:1000) (p-FoxO3a, Ser253, Cell Signaling, Beverly, MA), a rabbit antibody against total FoxO3a, and a rabbit antibody against cleaved (active) caspase 9 (37 kDa) (1:1000), a rabbit antibody against cleaved (active) caspase 8 (18 kDa) (1:1000), or a rabbit antibody against cleaved (active) caspase 3 (17 kDa) (1:1000) (Cell signaling Technology, Beverly, MA). Following washing, the membranes were incubated with a horseradish peroxidase (HRP) conjugated secondary antibody goat anti-rabbit IgG (1:2000, Zymed Laboratories, Carlsbad, CA). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) and band density was performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Transient Gene Knockdown of FoxO3a with Small Interfering RNA (siRNA)
To silence FoxO3a gene expression, the following sequences were synthesized (Ambion, Austin, Texas): the FoxO3a target sequence 5’- AAATCTAACTCATCTGCAA GT -3’, the siRNA sense strand 5’-AUCUAACUCAUCUG CAAGUUU -3’, and the antisense strand 5’-ACUUGCAG AUG AGUUAGAUUU -3’. Transfection of siRNA duplexes was performed with Lipofectamine 2000 reagent according to manufacturer guidelines (Invitrogen, Carlsbad, CA). Experimental assays were performed 72 hours post-transfection. For each siRNA assay, positive controls contain multiple siRNAs including the target siRNA and negative controls are absent of the target siRNA.
Assessment of Mitochondrial Membrane Potential
The fluorescent probe JC-1 (Molecular Probes, Eugene, OR), a cationic membrane potential indicator, was used to assess the mitochondrial membrane potential. Microglia in 35 mm dishes were incubated with 2 µg/ml JC-1 in growth medium at 37 °C for 30 min. The cultures were washed three times using fresh growth medium. Mitochondria were then analyzed immediately under a Leitz DMIRB microscope (Leica, McHenry, IL, USA) with a dual emission fluorescence filter with 515–545 nm for green fluorescence and emission at 585–615 nm for red fluorescence.
Preparation of Mitochondria for the Analysis of Cytochrome c Release
Per our prior protocols (Chong et al., 2005a; Chong et al., 2002a, 2003a, b, 2004a; Chong et al., 2005f; Chong et al., 2002b; Kang et al., 2003a, b), after washing once with ice-cold PBS, cells were harvested at 10,000g for 15 min at 4°C and the resulting pellet was re-suspended in buffer A (20 mM HEPES, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 phenyl-methylsulfonylfluoride) containing 250 mM sucrose and used as the mitochondrial fraction. The supernatant was subjected to ultracentrifugation at 50,000 g for 1 hour at 4 °C with the resultant supernatant used as the cytosolic fraction.
Immunocytochemistry for FoxO3a and Caspase 3
For immunocytochemical staining of FoxO3a and cleaved caspase 3 (active form), microglial cells were fixed with 4% paraformaldehyde and permeabilized using 0.2% Triton X-100. Cells were then incubated with rabbit anti-FoxO3a (1:100, Cell Signaling Technology, Beverly, MA) or rabbit anti-cleaved caspase 3 (1:200, Cell Signaling Technology, Beverly, MA) over night at 4 °C and then with biotinylated anti-rabbit IgG (1:50, Vector laboratories) for 2 hours followed by Texas Red streptavidin (1:50, Vector laboratories) for 1 hour. Cells are washed in PBS, then stained with DAPI (Sigma, St. Louis, MO) for nuclear identification. FoxO3a and caspase 3 proteins was imaged with fluorescence at the wavelengths of 565 nm (red) and 400 nm (DAPI nuclear staining).
Statistical Analysis
For each experiment, the mean and standard error were determined. Statistical differences between groups were assessed by means of analysis of variance (ANOVA) from 6 replicate experiments with the post-hoc Dunnett's test. Statistical significance was considered at p<0.05.
RESULTS
Oxygen-Glucose Deprivation (OGD) Results in Cell Injury to Microglia
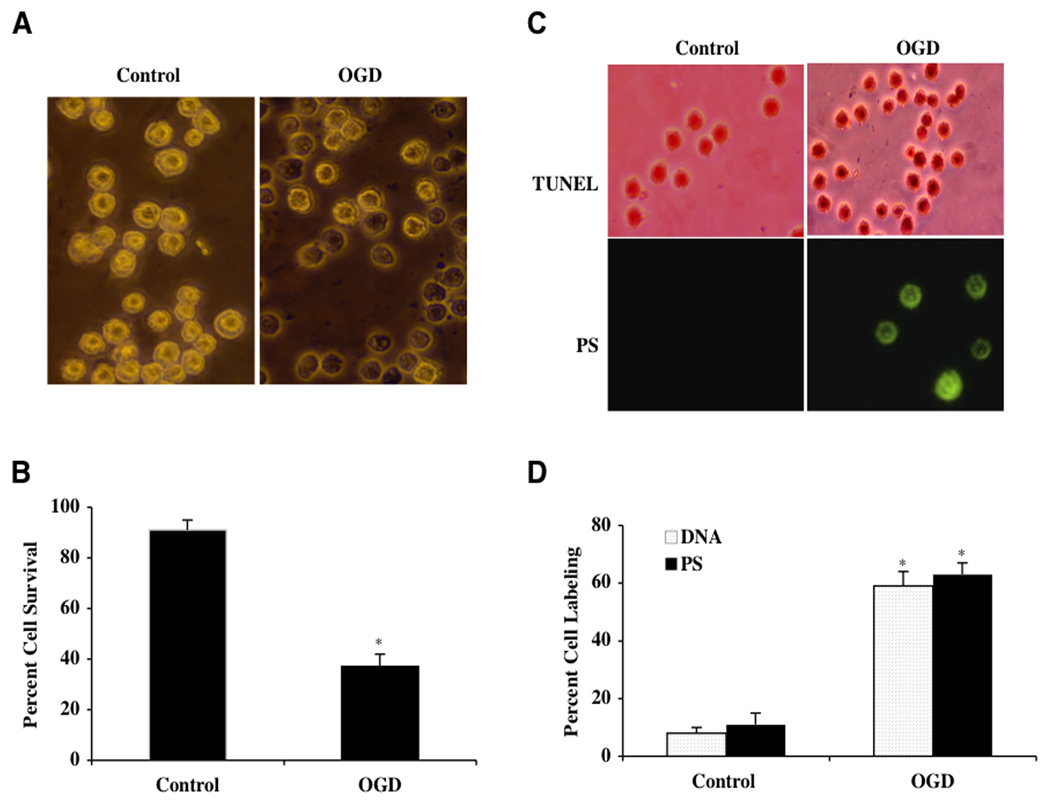
In order to examine the effect of OGD on microglial viability, we determined microglial survival 24 hours following OGD that was applied for a 6 hour period. As shown in Fig. (1A), representative pictures indicate that OGD lead to trypan blue staining in microglia, but no significant staining was found in control cells. In Fig. (1B), the quantification of data demonstrated that microglial survival was significantly reduced (37 ± 5%) 24 hours following a 6 hour period of OGD when compared to untreated control microglia (91 ± 4%, p<0.01). Since OGD exposure for a period of 6 hours resulted in survival rate of approximately 40% (a 60% microglial cell loss), this duration of OGD was used for the reminder of the experimental paradigms.
Fig. (1). Oxygen-glucose deprivation (OGD) results in microglial injury with early and late apoptotic damage.
(A) Microglial cells were exposed to OGD and cell survival was determined 24 hours after OGD with the trypan blue exclusion method. Representative images show that OGD leads to significant trypan blue uptake. (B) Microglial survival was decreased with OGD exposure (*P<0.01 vs. untreated control). Each data point represents the mean and SEM from 6 experiments. (C) Representative images show that OGD leads to apoptotic DNA fragmentation (dark nuclei) with TUNEL stain and membrane PS externalization (green fluorescence) with annexin V twenty-four hours following OGD. (D) OGD exposure significantly increased DNA fragmentation and PS exposure in microglia (*P<0.01 vs. untreated control). Each data point represents the mean and SEM from 6 experiments.
Early Phosphatidylserine (PS) Exposure and Subsequent Nuclear DNA Degradation Occurs During OGD Exposure
Oxidative stress, such as during OGD, leads to the destruction of cells through both apoptotic (Chong et al., 2006a; De Felice et al., 2007; Lin and Maiese, 2001) and autophagic pathways (Lee et al., 2009). Furthermore, apoptosis is a dynamic process that consists of both the early exposure of membrane phosphatidylserine (PS) residues and the later destruction of genomic DNA (Chong et al., 2005b; Maiese et al., 2008f). Following a 6 hour period of OGD, apoptotic DNA fragmentation was determined by TUNEL and cell membrane PS exposure was assessed by annexin V 24 hours later. In Fig. (1C), untreated control cells were without DNA fragmentation or PS externalization. In cells exposed to OGD, significant DNA fragmentation (TUNEL) and membrane PS exposure (annexin V) was present. In Fig. (1D), OGD lead to a significant increase in percent DNA fragmentation (59 ± 5%) and membrane PS exposure (61 ± 4%) in microglia 24 hours after OGD compared to untreated control cultures for DNA (8 ± 2%) and for PS (11 ± 4%) respectively.
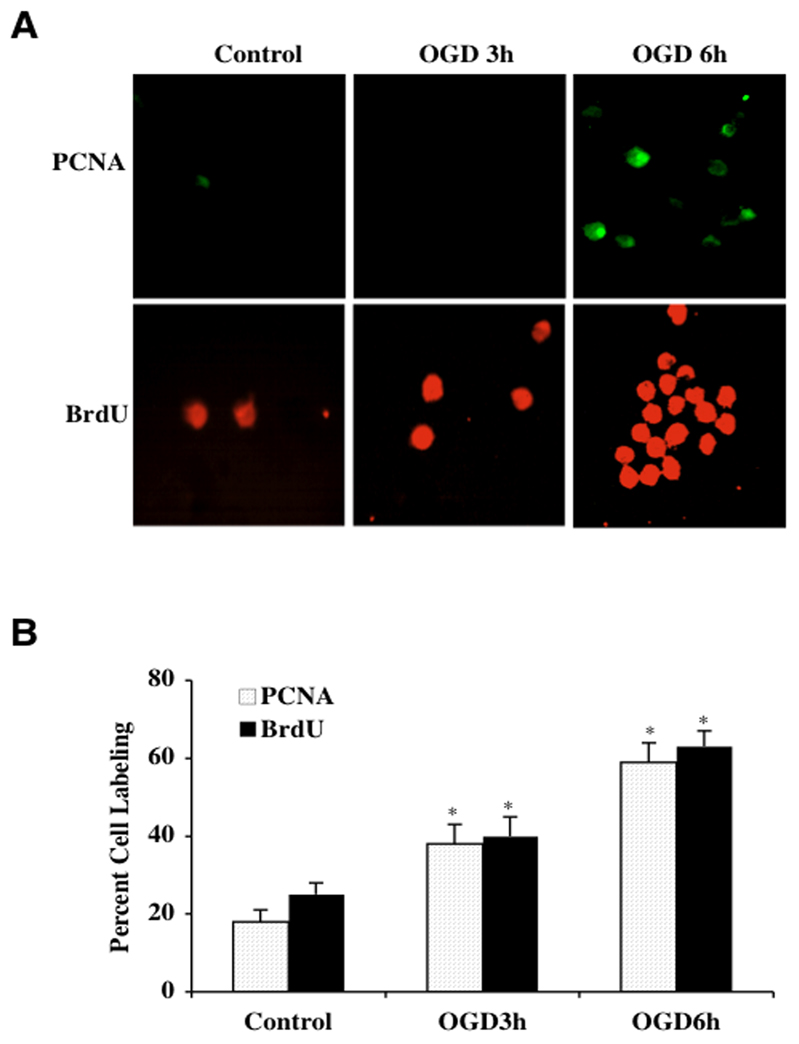
OGD Leads to Activation and Proliferation of Microglia
Externalization of membrane PS residues is an early event during cell apoptosis (Maiese et al., 2000; Mari et al., 2004) that can become a signal for the phagocytosis of cells (Chong et al., 2005a; Li et al., 2006b; Lin and Maiese, 2001). The subsequent loss of membrane phospholipid asymmetry leads to the exposure of membrane PS residues on the cell surface and assists microglia to target cells for phagocytosis (Chong et al., 2003c; Kang et al., 2003a, b; Maiese and Chong, 2003; Mallat et al., 2005). We therefore examined whether OGD results in the activation and proliferation of microglia given that OGD can lead to membrane PS exposure in microglia (Fig. 1). In Fig. (2A), representative microglial cultures illustrate a significant increase in microglial activation and proliferation following OGD at 3 hours and 6 hours as evidenced by increased PCNA expression and BrdU uptake. Untreated control cells are without significant PCNA expression or BrdU uptake. In Fig. (2B), quantification of PCNA labeling demonstrates significant expression in PCNA at 3 hours (38 ± 8%, p<0.01) and 6 hours (58 ± 5%, p<0.01) compared to control microglia (18 ± 5%). Similarly, BrdU uptake was significantly increased to 42 ± 6% at 3 hours and 61 ± 5% at 6 hours following OGD compared to untreated control cells (26 ± 4%).
Fig. (2). Early microglial activation and proliferation is present with OGD.
(A) Representative images and quantitative analysis illustrate that PCNA and BrdU expression is significantly and rapidly increased in microglia at 3 hours and 6 hours after OGD (*P<0.01 vs. control). (B) Quantification of data demonstrate that PCNA and BrdU were significantly increased following OGD (*p<0.01 vs. control). In all cases, control = untreated cells. Each data point represents the mean and SEM from 6 experiments.
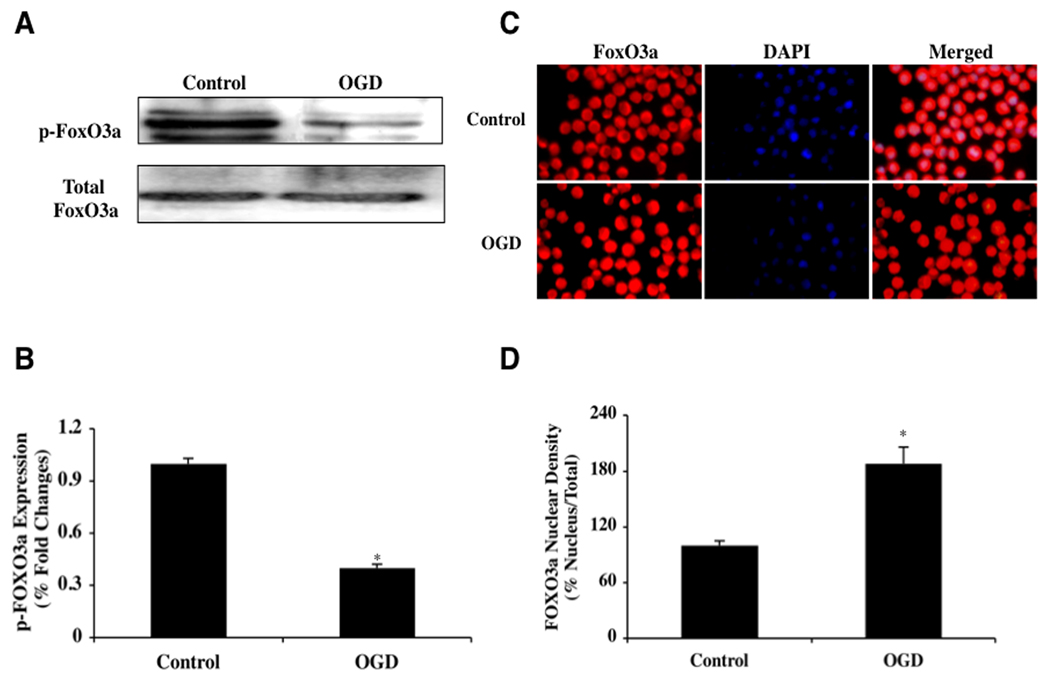
During OGD, Phosphorylation of FoxO3a is Reduced to Allow for Nuclear Translocation
Loss of post-translational phosphorylation of FoxO proteins can prevent association of FoxOs with 14-3-3 proteins (Chong and Maiese, 2007; Maiese et al., 2005b) and allow FoxOs to translocate to the cell nucleus to initiate a “pro-apoptotic” program (Maiese et al., 2009a; Maiese et al., 2009c, d; Shang et al., 2009). We therefore examined the role of OGD during this process. Since unphosphorylated FoxO3a can translocate to the cell nucleus (Chong and Maiese, 2007; Maiese et al., 2008c; Maiese et al., 2008e, 2009d), we initially investigated the phosphorylation of FoxO3a 6 hours following OGD by using western blot analysis. Western blot assay was performed for phosphorylated FoxO3a (p-FoxO3a) at the preferential phosphorylation site for protein kinase B (Akt) of Ser253 as well as for the expression of total FoxO3a at 6 hours following OGD (Figs. 3A, 3B). After 6 hours following OGD, expression of phosphorylated (inactive) p-FoxO3a was significantly decreased but expression of total FoxO3a remained unchanged, suggesting that the unphosphorylated and active FoxO3a form was present and that the total FoxO3a protein was not degraded (Figs. 3A, 3B).
Fig. (3). OGD leads to loss of phosphorylation of FoxO3a with subsequent subcellular trafficking to the nucleus.
In A and B, microglial protein extracts (50 μ/lane) were immunoblotted with anti-phosphorylated-FoxO3a (p-FoxO3a, Ser253) or anti-total FoxO3a at 6 hours following OGD. Phosphorylated (inactive) FoxO3a (p-FoxO3a) expression is significantly decreased 6 hours following OGD but total FoxO3a is not affected (*P<0.01 vs. control). In C and D, microglia were imaged 6 hours following OGD with immunofluorescent staining for FoxO3a (Texas-red streptavidin). Nuclei of microglia were counterstained with DAPI. In merged images, untreated control microglia have readily visible nuclei (dark white in color) that illustrate absence of FoxO3a in the nucleus. In contrast, merged images after OGD have completely red cytoplasm and nucleus with minimal visibility of the nucleus with DAPI illustrating translocation of FoxO3a to the nucleus. Quantification of the intensity of FoxO3a nuclear staining was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) (*P<0.01 vs. control). Control = untreated microglia.
These observations were supported by our studies that show that the active FoxO3a transcription factor translocates from the cell cytosol to the nucleus during this 6 hour period following OGD. In microglia, we next performed immunofluorescent staining for FoxO3a and DAPI nuclear staining to follow the subcellular translocation of FoxO3a 6 hours following OGD (Figs. 3C, 3D). Significant immunofluorescent staining for FoxO3a in the nucleus of microglia is present during OGD. This is evident by the inability to detect significant DAPI nuclear staining (blue in color) in cells during merged OGD images since prominent FoxO3a staining is present in the nucleus (Figs. 3C, 3D). In contrast, in untreated control cells, FoxO3a is maintained in the cytoplasm with minimal nuclear staining as shown with DAPI staining (white in color) in the nucleus in cells in merged images. In Fig. (3D), data analysis illustrated that microglial nuclear staining area is significantly increased (189 ± 5%) 24 hours following a 6 hour period after OGD when compared to untreated control cells (107 ± 7%).
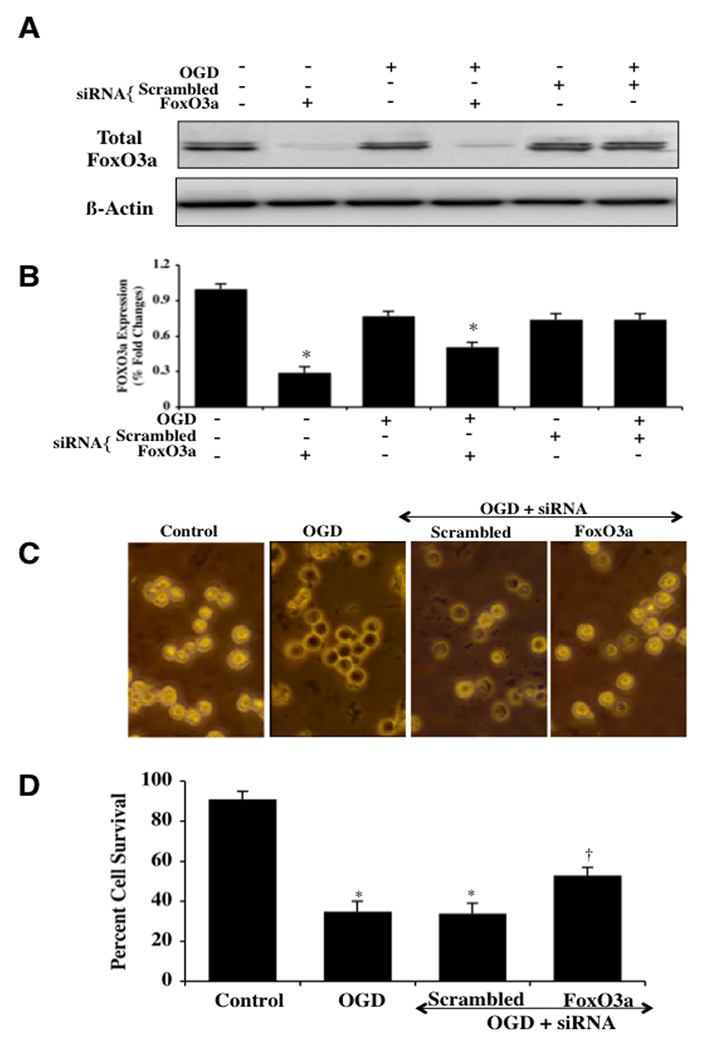
Transient Gene Knockdown of FoxO3a Prevents Microglial Injury During OGD
Microglia were transfected with FoxO3a siRNA and the expression of total FoxO3a protein was documented with Western blot analysis (Figs. 4A, 4B). Transient gene knockdown of FoxO3a in either untreated control cells or in cells exposed to OGD alone resulted in markedly reduced or absent expression of total FoxO3a 6 hours after OGD (Figs. 4A, 4B). As a control, non-specific scrambled FoxO3a siRNA did not alter total FoxO3a expression in untreated control cells or cells exposed to OGD, illustrating the specificity for FoxO3a siRNA to block protein expression of total FoxO3a (Figs. 4A, 4B). Representative figures illustrate significant trypan blue staining in microglial cells 24 hours after OGD administration alone or with OGD during scrambled (non-specific) siRNA (Fig. 4C). In contrast, markedly reduced trypan blue uptake is present in microglia following OGD with FoxO3a siRNA transfection for 3 days (Fig. 4C), demonstrating that the presence of FoxO3a contributes to microglial injury during OGD. On further analysis in Fig. (4D), percent microglial survival was increased from 37 ± 5% during OGD administration alone to 59 ± 4% (p<0.01) with OGD and FoxO3a siRNA 24 hours after OGD administration. Transfection with scrambled siRNA did not prevent microglial injury during OGD.
Fig. (4). Transient gene knockdown of FoxO3a increases microglial survival during OGD.
In A and B, microglial protein extracts (50 μ/lane) were immunoblotted with anti-phosphorylated-FoxO3a (p-FoxO3a, Ser253) or anti-total FoxO3a at 6 hours following OGD. Transient gene knockdown of FoxO3a was performed with transfection of FoxO3a siRNA (siRNA). FoxO3a siRNA significantly reduced expression of total FoxO3a alone or following OGD but non-specific scrambled siRNA did not alter total FoxO3a expression (*P<l0.01 vs. control). In C and D, gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) significantly increased microglial survival and decreased microglial membrane injury assessed by trypan blue staining 24 hours after OGD (*P<0.01 vs. Control; †P <0.01 vs. OGD). FoxO3a siRNA alone was not toxic and non-specific scrambled siRNA did not protect cells during OGD (*P<0.01 vs. untreated control cells). In all cases, each data point represents the mean and SEM from 6 experiments.
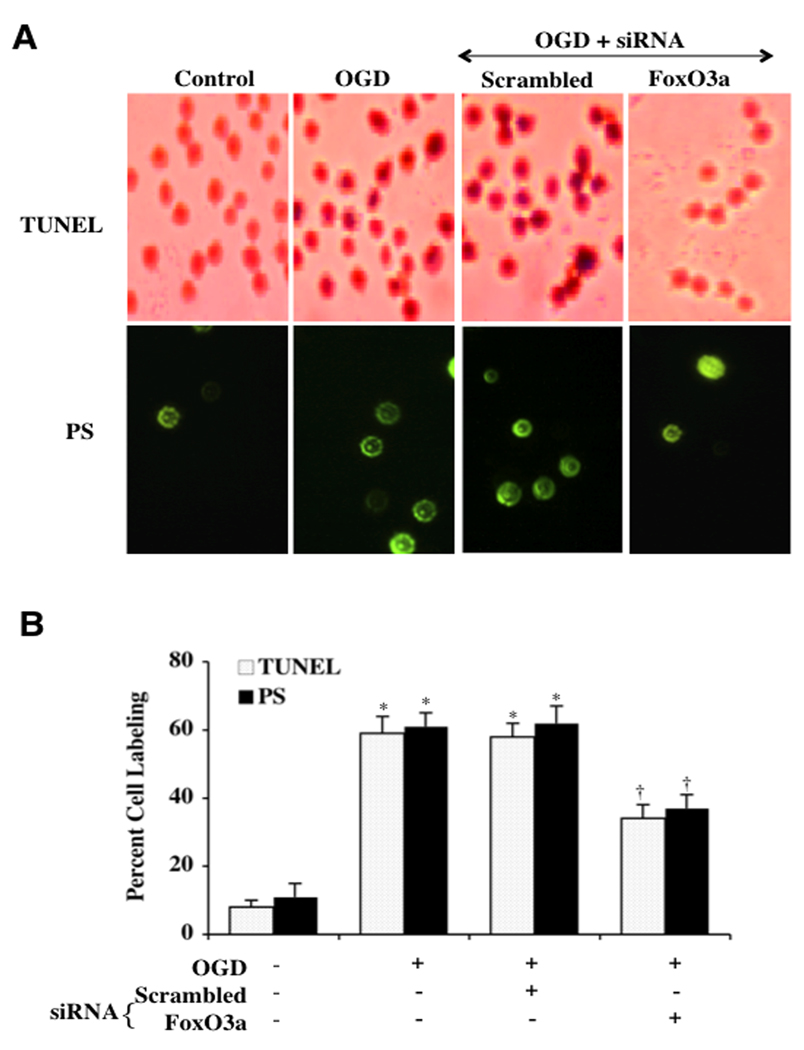
Absence of FoxO3a Protein can Limit Apoptotic DNA Fragmentation and Phosphatidylserine (PS) Exposure and Block the Early Activation and Proliferation of Microglia
We further examined the role of FoxO3a during OGD with apoptotic genomic DNA fragmentation and membrane PS exposure in microglia through TUNEL and annexin V analysis. In Fig. (5A), representative figures show a significant decrease in DNA fragmentation and PS exposure during OGD in microglia transfected with FoxO3a siRNA. Transfection with non-specific scrambled siRNA did not prevent DNA fragmentation or PS exposure in microglial cells. Quantification of these observations illustrate that transfection of microglia with FoxO3a siRNA during OGD decreased DNA fragmentation from 59 ± 5% (OGD alone) to 35 ± 2% with FoxO3a siRNA and decreased apoptotic PS exposure from 62 ± 5% (OGD alone) to 38 ± 4% with FoxO3a siRNA (Fig. 5B).
Fig. (5). Transient gene knockdown of FoxO3a limits apoptotic DNA fragmentation and phosphatidylserine (PS) exposure.
In A and B, representative images and quantitative analysis illustrate transient gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) significantly blocked microglial genomic DNA degradation assessed by TUNEL and membrane PS externalization assessed by annexin V (green fluorescence) 24 hours after OGD (*P<0.01 vs. untreated cells; †P <0.01 vs. OGD). FoxO3a siRNA alone was not toxic and non-specific scrambled siRNA did not protect cells during OGD (*P<0.01 vs. untreated cells). Each data point represents the mean and SEM from 6 experiments.
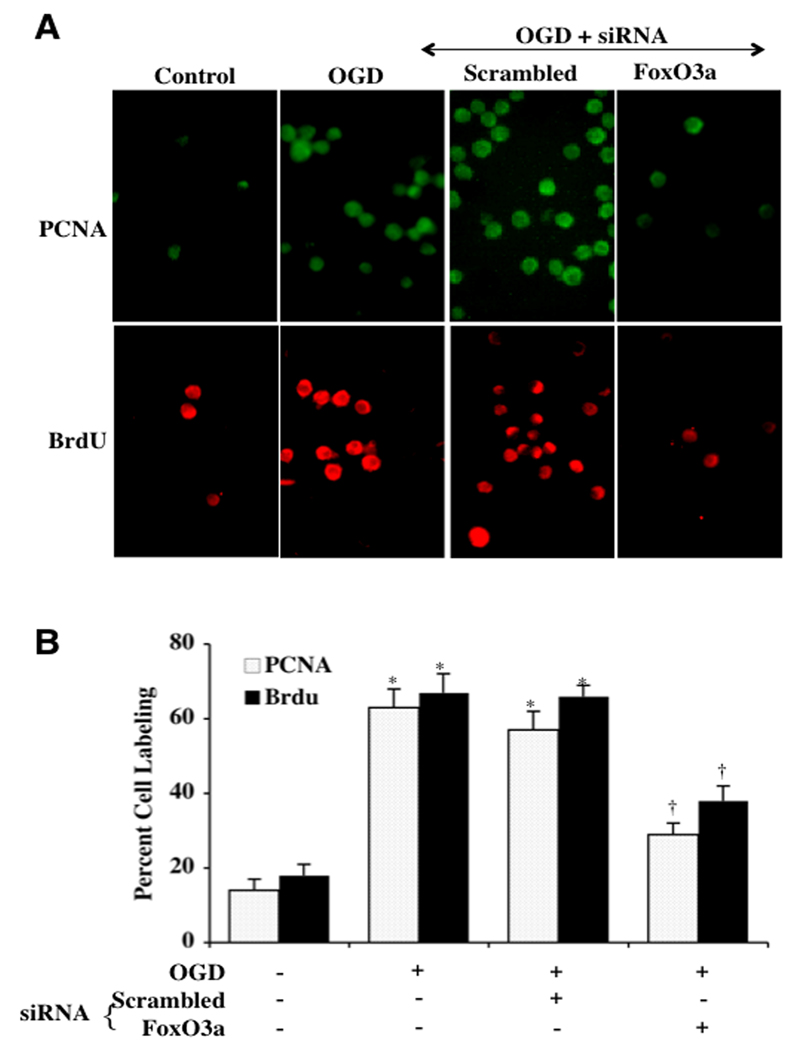
In Fig. (6A), representative microglial cells demonstrate a significant increase in the activation (PCNA expression) and proliferation (BrdU uptake) of microglia at 6 hours after OGD when compared to untreated control microglia. In contrast, microglial transfection with scrambled siRNA during OGD also led to a marked increase in PCNA expression and BrdU uptake during OGD. In contrast, the expression of PCNA and BrdU uptake was significantly reduced in cells with FoxO3a siRNA transfection. In Fig. (6B), transfection of microglia with FoxO3a siRNA that was assessed 6 hours following OGD decreased PCNA expression from 64 ± 5% (OGD alone) to 32 ± 5% during OGD with FoxO3a siRNA and decreased BrdU uptake from 68 ± 5% (OGD alone) to 37 ± 4% during OGD with FoxO3a siRNA. Non-specific scrambled siRNA transfection did not prevent the increase in PCNA expression or BrdU uptake during OGD, illustrating the ability of FoxO3a to lead to early activation and proliferation in microglia.
Fig. (6). Absence of FoxO3a protein prevents the early activation and proliferation of microglia.
In A and B, representative images and quantitative analysis demonstrate that transient gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) decreases the expression of PCNA and the uptake of BrdU at 6 hours after OGD (*P<0.01 vs. untreated cells; †P <0.01 vs. OGD). FoxO3a siRNA alone was not toxic and non-specific scrambled siRNA did not alter PCNA expression or BrdU uptake during OGD (*P<0.01 vs. untreated cells). In all cases, control = untreated cells. Each data point represents the mean and SEM from 6 experiments.
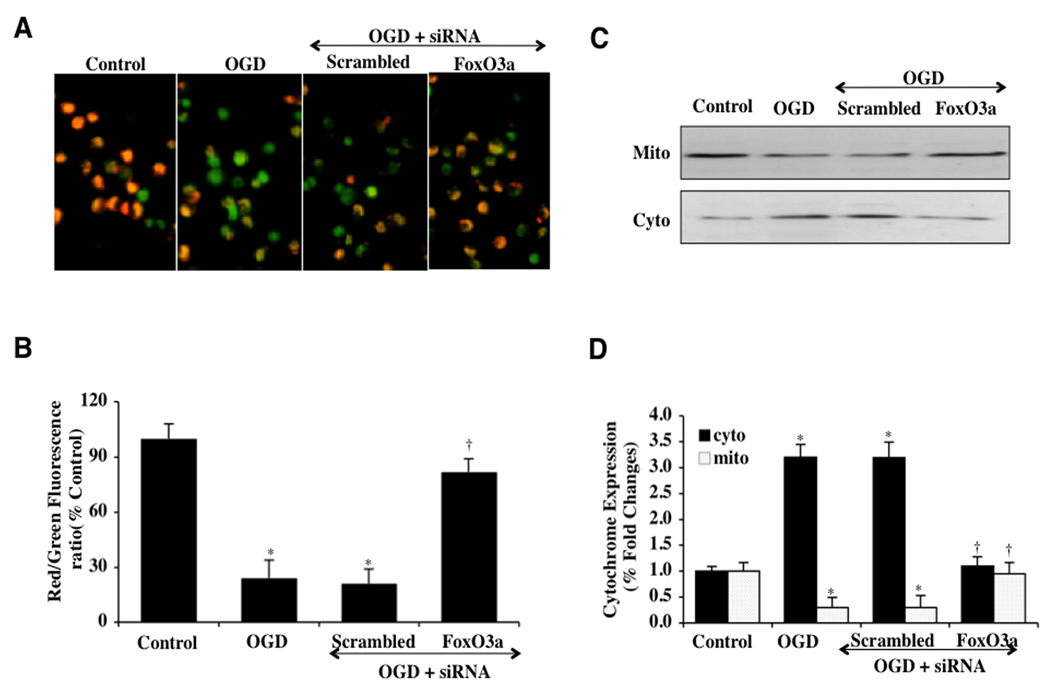
Transient Gene Knockdown of FoxO3a Blocks Mitochondrial Depolarization and the Release of Mitochondrial Cytochrome c
OGD yielded a significant decrease in the mitochondrial red/green fluorescence intensity ratio within 6 hours (27 ± 5%) with labeling of microglial mitochondria by the cationic membrane potential indicator JC-1 when compared to untreated control mitochondria (97 ± 4%) (Figs. 7A, 7B), demonstrating that OGD produces mitochondrial membrane depolarization. Transfection of FoxO3a siRNA in microglia prior to OGD exposure significantly increased the red/green fluorescence intensity of the mitochondria (82 ± 5%), indicating that mitochondrial permeability transition pore membrane potential was markedly improved (Figs. 7A, 7B). In contrast, non-specific scrambled siRNA during OGD did not prevent mitochondrial membrane depolarization, suggesting that FoxO3a is necessary, at least in part, for OGD to lead to the depolarization of the mitochondrial membrane.
Fig. (7). Transient gene knockdown of FoxO3a prevents mitochondrial depolarization and the release of mitochondrial cytochrome c.
(A) OGD produced a significant decrease in the red/green fluorescence intensity ratio of mitochondria using a cationic membrane potential indicator JC-1 within 6 hours when compared with untreated control cultures, demonstrating that OGD results in mitochondrial membrane depolarization. Transient gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) during OGD significantly increased the red/green fluorescence intensity of mitochondria in microglia, indicating that membrane potential was restored. Non-specific scrambled siRNA did not prevent mitochondrial membrane depolarization. (B) The relative ratio of red/green fluorescent intensity of mitochondrial staining in both untreated (control) microglia and microglia exposed to OGD or transfected with FoxO3a siRNA was measured in 6 independent experiments with analysis performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) (Control vs. OGD, *P<0.01; OGD vs. FoxO3a siRNA, †P<0.01). (C and D) A representative Western blot with equal amounts of mitochondrial (mito) or cytosol (cyto) protein extracts (50 µg/lane) were immunoblotted demonstrating that transfection of FoxO3a siRNA significantly prevented cytochrome c release from mitochondria during OGD (*P<0.01 vs. Control; †P <0.01 vs. OGD). Non-specific scrambled siRNA did not prevent mitochondrial depolarization. Each data point represents the mean and SEM from 6 experiments.
In regards to cytochrome c release, OGD within 6 hours resulted in a marked release of cytochrome c from the mitochondria to a 3.3 ± 0.3% fold increase when compared to untreated control mitochondria (Figs. 7C, 7D) using western analysis. Non-specific scrambled siRNA did not alter this release of cytochrome c during OGD exposure, but transfection of mitochondria with FoxO3a siRNA prevented cytochrome c release to a similar degree that occurs with untreated control microglial mitochondria (Figs. 7C, 7D).
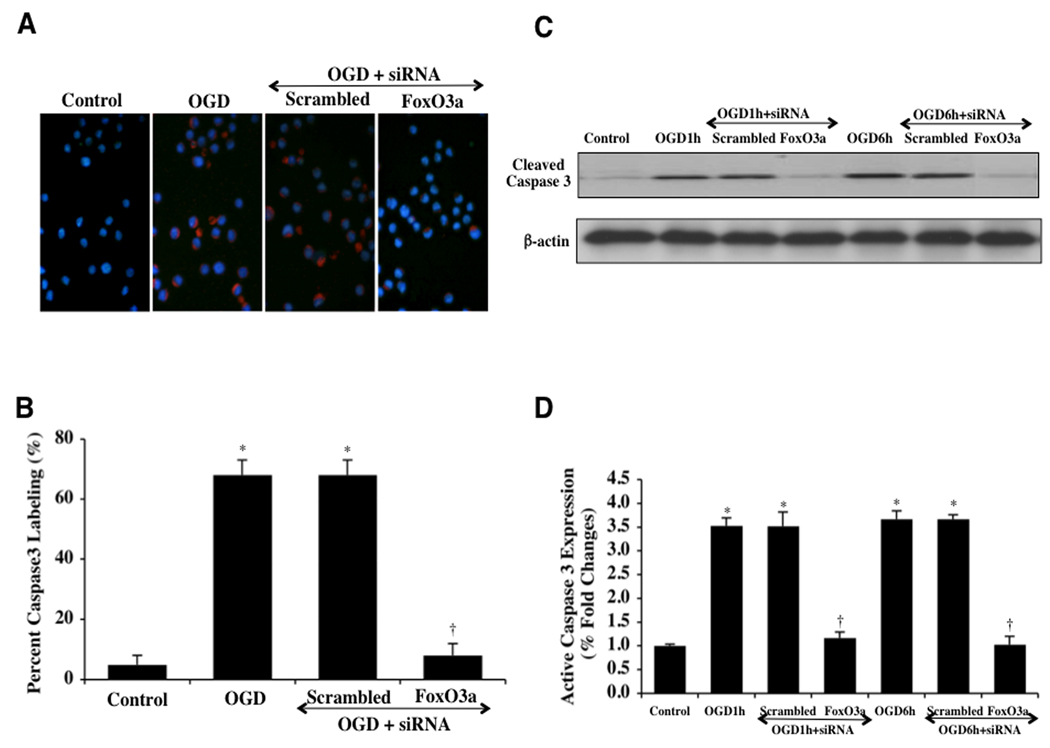
Activities of Caspase 3, 8, and 9 are Controlled by FoxO3a During OGD
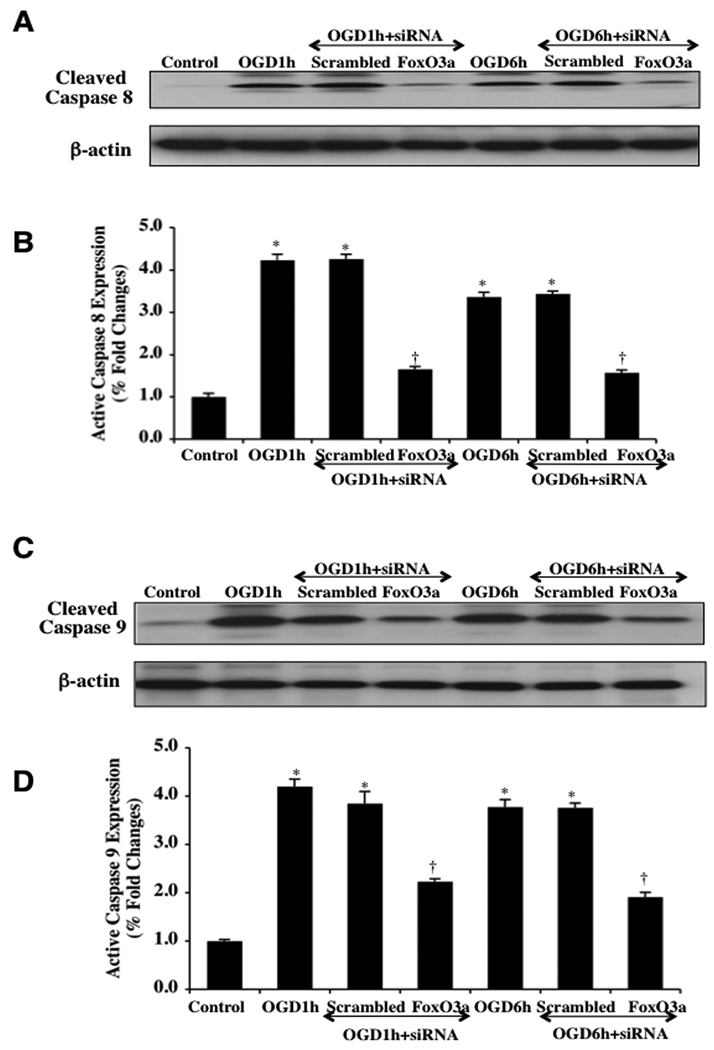
Caspases are a family of cysteine proteases that are synthesized as inactive zymogens and are proteolytically cleaved into subunits during loss of mitochondrial membrane permeability (Li et al., 2006a; Maiese et al., 2005a; Okouchi et al., 2007). We therefore elected to investigate the ability of FoxO3a to modulate caspase 3, 8, and 9 activity. In Figs. (8A, 8B), cleaved caspase 3 immunocytochemistry reveals significant active caspase 3 (blue/red staining) within 6 hours following OGD exposure and during transfection of nonspecific scrambled siRNA. However, transfection of FoxO3a siRNA in microglia significantly blocks caspase 3 activity as evidenced by primarily blue immunocytochemical staining and by reducing the percentage of cleaved caspase 3 labeling to 8 ± 3% from 68 ± 4% in microglia exposed to OGD alone (Figs. 8A, 8B). In addition, Figs. (8C, 8D) demonstrate that on western analysis expression of cleaved active caspase 3 is not present or at minimal levels similar to untreated controls during FoxO3a transfection at very early time periods of either 1 hour or 6 hours following OGD exposure. Almost paralleling the observations with active caspase 3 during FoxO3a transfection, expression of cleaved active caspase 8 (Figs. 9A, 9B) and expression of cleaved active caspase 9 are elevated approximately 4 fold over untreated control microglia levels at 1 hour and 6 hours following OGD, but transfection with FoxO3a siRNA significantly blocks cleaved active caspase 8 activity (Figs. 9A, 9B) and markedly reduces, although to a lesser extent, cleaved active caspase 9 activity (Figs. 9C, 9D). Further supporting the ability of FoxO3a to lead to the activation of caspase 8 and caspase 9 are the observations that non-specific scrambled siRNA was ineffective in reducing caspase 8 or caspase 9 activity during OGD at 1 hour and at 6 hours (Figs. 9A, 9B, 9C, 9D).
Fig. (8). FoxO3a modulates caspase 3 activity during OGD.
In A and B, microglial cells were exposed to OGD and caspase 3 activation was determined 6 hours after OGD exposure through immunocytochemistry with antibodies against cleaved active caspase 3 (17 kDa). Representative images illustrate active caspase 3 staining (red) in cells following OGD in which red staining is almost absent during transfection with FoxO3a siRNA. Non-specific scrambled siRNA does not eliminate caspase 3 activity. Quantification of data demonstrates that OGD significantly increased the expression of cleaved active caspase 3 when compared to untreated control cells (*P<0.01 vs. control). Yet, the expression of cleaved active caspase 3 was significantly decreased in cells with transfection of FoxO3a siRNA for 3 days prior to the exposure to OGD (*P <0.01 vs. Control; †P<0.01 vs. OGD). In C and D, microglial protein extracts (50 µg/lane) were immunoblotted with anti-cleaved caspase 3 product (active caspase 3, 17 kDA) at 1 hour (1h) and 6 hours (6h) following OGD. OGD significantly increased cleaved caspase 3 expression, but transient gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) significantly prevented cleaved caspase 3 expression 1 hour and 6 hours after OGD. FoxO3a siRNA alone was not toxic and non-specific scrambled siRNA did not reduce cleaved caspase 3 expression during OGD (*P <0.01 vs. Control; †P<0.01 vs. OGD). In all cases, each data point represents the mean and SEM from 6 experiments.
Fig. (9). Caspase 8 and 9 activities are controlled by FoxO3a during OGD.
In A and B, microglial protein extracts (50 µg/lane) were immunoblotted with anti-cleaved caspase 8 product (active caspase 8, 18 kDA) at 1 hour (1h) and 6 hours (6h) following OGD. OGD markedly increased cleaved caspase 8 expression, but transient gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) significantly blocked cleaved caspase 8 expression 1 hour and 6 hours after OGD. FoxO3a siRNA alone was not toxic and non-specific scrambled siRNA did not reduce cleaved caspase 8 expression during OGD (*P <0.01 vs. Control; †P<0.01 vs. OGD). In all cases, each data point represents the mean and SEM from 6 experiments. In C and D, microglial protein extracts (50 µg/lane) were immunoblotted with anti-cleaved caspase 9 product (active caspase 9, 37 kDA) at 1 hour (1h) and 6 hours (6h) following OGD. OGD markedly increased cleaved caspase 9 expression, but transient gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) limited cleaved caspase 9 expression 1 hour and 6 hours after OGD. FoxO3a siRNA alone was not toxic and non-specific scrambled siRNA did not reduce cleaved caspase 9 expression during OGD (*P <0.01 vs. Control; †P<0.01 vs. OGD). In all cases, each data point represents the mean and SEM from 6 experiments.
DISCUSSION
Oxidative stress and apoptotic injury involves several cell types that include neurons, endothelial cells, cardiomyocytes, and smooth muscle cells (Chong et al., 2004a; Chong et al., 2007b; Harris et al., 2007; Kang et al., 2003b; Karunakaran et al., 2007; Verdaguer et al., 2007). In addition, inflammatory cells of the brain are no exception to this list of cells affected by oxidative stress (Bureau et al., 2008; Chong et al., 2005a; Chong et al., 2003b, 2004a; Chong et al., 2007b; Chu et al., 2008; Denes et al., 2008; Li et al., 2006b; Park et al., 2009; Power et al., 2008; Sanchez et al., 2009; Shang et al., 2009; Zhao et al., 2009). We show that OGD exposure for a period of 6 hours results in a significantly reduced survival rate for microglia with DNA fragmentation and early apoptotic changes associated with membrane PS exposure over a 24 hour course. However, cell injury assessed by trypan blue exclusion is significantly limited in microglia following OGD exposure during transient gene knockdown of FoxO3a, illustrating that the presence of FoxO3a is a necessary component for microglial injury during OGD. Furthermore, a significant decrease in apoptotic DNA fragmentation and membrane PS exposure during OGD in microglia transfected with FoxO3a siRNA occurs. In studies with both cell survival and apoptotic DNA degradation and PS exposure, transfection with non-specific scrambled siRNA did not prevent injury in microglial cells, supporting the specificity of FoxO3a to control cell injury and apoptotic early and late programs in microglia. Our studies are consistent with prior work that demonstrate FoxO3a must be present for oxidant stress – induced apoptosis (Nakamura and Sakamoto, 2007), that FoxO3a controls an apoptotic ligand activating a Fas-mediated death pathway in motoneurons (Barthelemy et al., 2004), and that FoxO3a in conjunction with tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL) and BH3-only proteins results in apoptotic injury in neuroblastoma cells (Obexer et al., 2007). In addition, removal of FoxO expression during oxidative stress has been shown to be cytoprotective. Protein inhibition or gene knockdown of FoxO3a can reduce cerebral ischemia (Won et al., 2006), mediate protection of metabotropic glutamate receptors during vascular injury (Chong et al., 2006b), enhance pancreatic β-cell or neuronal survival through NAD+ precursors during oxidative stress (Chong et al., 2004b), provide trophic factor protection with erythropoietin (Chong and Maiese, 2007) and neurotrophins (Caporali et al., 2008), and protect microglia against amyloid toxicity (Shang et al., 2009).
However, prior to the onset of cell injury and apoptosis in microglia during oxidative stress, FoxO3a also controls a novel function of microglia. During initial OGD exposure within 3 and 6 hours, microglia have a significant increase in activation (PCNA expression) and proliferation (BrdU uptake) when compared to untreated control microglia. In contrast, the expression of PCNA and BrdU uptake in microglia was significantly reduced in cells with FoxO3a siRNA transfection while microglial transfection with scrambled siRNA during OGD resulted in a marked increase in PCNA expression and BrdU uptake during OGD. During these early time periods of 3 and 6 hours following OGD exposure, we also we investigated the phosphorylation of FoxO3a. After 6 hours following OGD, expression of phosphorylated (inactive) p-FoxO3a was significantly decreased but expression of total FoxO3a remained unchanged, suggesting that the unphosphorylated and active post-translational form of FoxO3a was most likely present and that the total FoxO3a protein was not destroyed. These observations were further supported by our work that demonstrates that the active FoxO3a transcription factor translocates from the cell cytosol to the nucleus during this same time period (within 6 hours following OGD). Post-translational modification of FoxO3a that yields an unphosphorylated (active) state allows FoxO3a to not associate with 14-3-3 proteins in the cytosol and to shuttle to the nucleus to allow for transcriptional activity (Chong and Maiese, 2007; Maiese et al., 2009a; Maiese et al., 2009b; Maiese et al., 2009e).
The protection of mitochondrial function also may be an important factor for FoxO3a to regulate microglial cell survival. Loss of mitochondrial membrane potential (ΔΨm ) through the opening of the mitochondrial permeability transition pore and the release of cytochrome c represents a significant determinant for cell injury (Leuner et al., 2007; Maiese and Chong, 2004) and the induction of apoptosis (Chong et al., 2002a; Chong et al., 2003d; Miki et al., 2006). In HCT116 cells, FoxO3a has been shown to interact with the mitochondrial sirtuin SIRT3 (Jacobs et al., 2008), suggesting that FoxO3a can control mitochondrial signal transduction pathways. In addition, FoxO3a has been shown to lead to cytochrome c release in neuroblastoma cells and neurons (Chong et al., 2004b; Obexer et al., 2007) and initiate caspase activation in microglial cells (Shang et al., 2009). In our current studies, we show that OGD in microglia leads to mitochondrial membrane depolarization and cytochrome c release within 6 hours after OGD exposure. However, transient knockdown of FoxO3a in microglia prevents mitochondrial membrane depolarization as well as the release of cytochrome c during OGD, illustrating that FoxO3a is required, at least in part, for the initiation of mitochondrial pathways that can lead to apoptotic cell injury in microglia.
Since mitochondrial function is closely linked to caspase activity, we next investigated the role of specific caspases with FoxO3a. Although initiator caspases, such as caspase 8 and 9, are necessary to activate downstream effector caspases, it is the downstream effector caspases, such as caspase 3, that can directly lead to DNA destruction (Chong et al., 2005d, e; Maiese et al., 2005a; Maiese et al., 2008f) and cellular membrane PS exposure (Chong et al., 2003a; Chong et al., 2003d; Takahashi et al., 1999). In addition, caspase 3 is tied to a unique regulatory mechanism that leads to proteolytic degradation of phosphorylated FoxO3a that potentially can enhance the vulnerability of cells to apoptotic injury (Charvet et al., 2003). Prior work also has shown that FoxO3a activity promotes caspase-induced apoptotic death (Chong et al., 2006b; Chong et al., 2004b; Chong and Maiese, 2007; Obexer et al., 2007), but inhibition of caspase 3 also can maintain the phosphorylated “inactive” state of FoxO3a to prevent cell injury (Chong et al., 2006b; Chong et al., 2004b; Chong and Maiese, 2007). Additional studies have shown that caspase 3 activity and cleavage is promoted during transfection of a triple mutant FoxO3a expression in which three phosphorylation sites have been altered to prevent inactivation of FoxO3a (Gomez-Gutierrez et al., 2006). Furthermore, FoxO3a may control early activation and subsequent apoptotic injury in microglia during amyloid exposure through caspase 3 (Shang et al., 2009).
We show that cleaved caspase 3 activity through immunocytochemistry is significantly increased within 6 hours after OGD. In contrast, transfection of FoxO3a siRNA in microglia significantly blocked caspase 3 activity. Furthermore, FoxO3a was able to lead to a rapid and marked increase in cleaved caspase 3 expression on western analysis with 1 and 6 hours following OGD, since loss of FoxO3a during transient gene knockdown abrogated increases in caspase 3 activity during OGD. Similar to our observations with caspase 3 during FoxO3a transfection, expression of cleaved active caspase 8 was increased almost 4 fold over untreated control microglia levels at 1 hour and 6 hours following OGD, but transient gene knockdown of FoxO3a also significantly prevented cleaved active caspase 8 activity.
Of interest is our observation that loss of FoxO3a reduced caspase 9 activity to a lesser extent than that noted with caspase 3 and 8 activities, suggesting that FoxO3a in relation to caspase 9 may be more reliant upon other signal transduction pathways potentially independent from caspase 3 and 8. One potential pathway that comes to mind is protein kinase B (Akt). Activation of Akt is usually cytoprotective, such as during cell proliferation (Gayer et al., 2009), ischemia/stress (An et al., 2008; Tsolakidou et al., 2008), hypoxia (Chong et al., 2002a), β-amyloid toxicity (Chong et al., 2005c), cardiomyopathy (Kim et al., 2008), cellular aging (Tajes et al., 2009), neurodegeneration (Morissette et al., 2008a; Morissette et al., 2008b), and oxidative stress (Chong et al., 2004a; Kang et al., 2003a, b). In relation to the modulation of FoxO3a activity, Akt can prevent cellular apoptosis through the phosphorylation of FoxO3a (Maiese et al., 2008e, 2009c) and maintain FoxO3a in the cytoplasm by association with 14-3-3 proteins (Chong and Maiese, 2007; Maiese et al., 2005b). In addition, cytoprotection through Akt can involve the maintenance of mitochondrial membrane potential, prevention of cytochrome c release, and blockade of caspase activity including caspase 9 (Chong et al., 2005a; Chong et al., 2002a; Kang et al., 2003a, b), raising the possibility that Akt may be more influential in limiting the effects of FoxO3a on caspase 9 activity during oxidative stress.
ACKNOWLEDGEMENTS
This research was supported by the following grants (KM): American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), NIH NIA, and NIH NINDS.
REFERENCES
- An J, Zhang C, Polavarapu R, Zhang X, Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein induce Akt phosphorylation in the ischemic brain. Blood. 2008;112:2787–2794. doi: 10.1182/blood-2008-02-141630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi P, Margenthaler E, Laporte V, Crawford F, Mullan M. Novel role of CXCR2 in regulation of gamma-secretase activity. ACS Chem Biol. 2008;3:777–789. doi: 10.1021/cb800167a. [DOI] [PubMed] [Google Scholar]
- Barbosa NB, Oliveira C, Araldi D, Folmer V, Rocha JB, Nogueira CW. Acute diphenyl diselenide treatment reduces hyperglycemia but does not change delta-aminolevulinate dehydratase activity in alloxan-induced diabetes in rats. Biol Pharm Bull. 2008;31:2200–2204. doi: 10.1248/bpb.31.2200. [DOI] [PubMed] [Google Scholar]
- Barthelemy C, Henderson CE, Pettmann B. Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci. 2004;5:48. doi: 10.1186/1471-2202-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau G, Longpre F, Martinoli MG. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J Neurosci Res. 2008;86:403–410. doi: 10.1002/jnr.21503. [DOI] [PubMed] [Google Scholar]
- Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B, Newby AC, Madeddu P, Emanueli C. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet C, Alberti I, Luciano F, Jacquel A, Bernard A, Auberger P, Deckert M. Proteolytic regulation of Forkhead transcription factor FOXO3a by caspase-3-like proteases. Oncogene. 2003;22:4557–4568. doi: 10.1038/sj.onc.1206778. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang J, Li F, Maiese K. mGluRI Targets Microglial Activation and Selectively Prevents Neuronal Cell Engulfment Through Akt and Caspase Dependent Pathways. Curr Neurovasc Res. 2005a;2:197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002a;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003a;23:320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003b;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Metabotropic glutamate receptors promote neuronal and vascular plasticity through novel intracellular pathways. Histol Histopathol. 2003c;18:173–189. doi: 10.14670/HH-18.173. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L)and caspase 1, 3, and 9. Exp Cell Res. 2004a;296:196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Employing new cellular therapeutic targets for Alzheimer's disease: a change for the better? Curr Neurovasc Res. 2005b;2:55–72. doi: 10.2174/1567202052773508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005c;2:387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005d;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer's disease. Brain Res Brain Res Rev. 2005e;49:1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Attempted Cell Cycle Induction in Post-Mitotic Neurons Occurs in Early and Late Apoptotic Programs Through Rb, E2F1, and Caspase 3. Curr Neurovasc Res. 2006a;3:25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim, and beta-Catenin During Oxidative Stress. Curr Neurovasc Res. 2006b;3:107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007a;19:1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007b;19:263–272. [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003d;71:659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associated "anti-apoptotic" pathways. Curr Neurovasc Res. 2005f;2:271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. Nicotinamide Modulates Mitochondrial Membrane Potential and Cysteine Protease Activity during Cerebral Vascular Endothelial Cell Injury. J Vasc Res. 2002b;39:131–147. doi: 10.1159/000057762. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004b;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150:839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Jung KH, Lee ST, Kim JH, Kang KM, Kim HK, Lim JS, Park HK, Kim M, Lee SK, Roh JK. Erythropoietin reduces epileptogenic processes following status epilepticus. Epilepsia. 2008;49:1723–1732. doi: 10.1111/j.1528-1167.2008.01644.x. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28:1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- Dombroski D, Balasubramanian K, Schroit AJ. Phosphatidylserine expression on cell surfaces promotes antibody- dependent aggregation and thrombosis in beta2-glycoprotein I-immune mice. J Autoimmun. 2000;14:221–229. doi: 10.1006/jaut.2000.0365. [DOI] [PubMed] [Google Scholar]
- Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal. 2005;7:1223–1233. doi: 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- Duarte AI, Santos P, Oliveira CR, Santos MS, Rego AC. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3beta signaling pathways and changes in protein expression. Biochim Biophys Acta. 2008;1783:994–1002. doi: 10.1016/j.bbamcr.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Erol A. Unraveling the Molecular Mechanisms Behind the Metabolic Basis of Sporadic Alzheimer's Disease. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2009-1047. [ Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Fei M, Lu M, Wang Y, Zhao Y, He S, Gao S, Ke Q, Liu Y, Li P, Cui X, Shen A, Cheng C. Arsenic trioxide-induced growth arrest of human hepatocellular carcinoma cells involving FOXO3a expression and localization. Med Oncol. 2009;26:178–185. doi: 10.1007/s12032-008-9105-8. [DOI] [PubMed] [Google Scholar]
- Gayer CP, Chaturvedi LS, Wang S, Craig DH, Flanigan T, Basson MD. Strain-induced proliferation requires the phosphatidylinositol 3-kinase/AKT/glycogen synthase kinase pathway. J Biol Chem. 2009;284:2001–2011. doi: 10.1074/jbc.M804576200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gutierrez JG, Souza V, Hao HY, Montes de Oca-Luna R, Dong YB, Zhou HS, McMasters KM. Adenovirus-mediated gene transfer of FKHRL1 triple mutant efficiently induces apoptosis in melanoma cells. Cancer Biol Ther. 2006;5:875–883. doi: 10.4161/cbt.5.7.2911. [DOI] [PubMed] [Google Scholar]
- Gossai D, Lau-Cam CA. The effects of taurine, taurine homologs and hypotaurine on cell and membrane antioxidative system alterations caused by type 2 diabetes in rat erythrocytes. Adv Exp Med Biol. 2009;643:359–368. doi: 10.1007/978-0-387-75681-3_37. [DOI] [PubMed] [Google Scholar]
- Guarnieri G, Zanetti M, Vinci P, Cattin MR, Barazzoni R. Insulin resistance in chronic uremia. J Ren Nutr. 2009;19:20–24. doi: 10.1053/j.jrn.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Hao J, Shen W, Tian C, Liu Z, Ren J, Luo C, Long J, Sharman E, Liu J. Mitochondrial nutrients improve immune dysfunction in the type 2 diabetic Goto-Kakizaki rats. J Cell Mol Med. 2009;13:701–711. doi: 10.1111/j.1582-4934.2008.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. A genetic association analysis of cognitive ability and cognitive ageing using 325 markers for 109 genes associated with oxidative stress or cognition. BMC Genet. 2007;8:43. doi: 10.1186/1471-2156-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Pennington JD, Bisht KS, Aykin-Burns N, Kim HS, Mishra M, Sun L, Nguyen P, Ahn BH, Leclerc J, Deng CX, Spitz DR, Gius D. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int J Biol Sci. 2008;4:291–299. doi: 10.7150/ijbs.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessel R, Haertel S, Socaciu C, Tykhonova S, Diehl HA. Kinetics of apoptotic markers in exogeneously induced apoptosis of EL4 cells. J Cell Mol Med. 2002;6:82–92. doi: 10.1111/j.1582-4934.2002.tb00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson H, Allen P, Peng SL. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat Med. 2005;11:666–671. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003a;74:37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003b;64:557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- Karunakaran S, Diwakar L, Saeed U, Agarwal V, Ramakrishnan S, Iyengar S, Ravindranath V. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson's disease: protection by alpha-lipoic acid. FASEB J. 2007;21:2226–2236. doi: 10.1096/fj.06-7580com. [DOI] [PubMed] [Google Scholar]
- Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther. 2008;324:160–169. doi: 10.1124/jpet.107.125773. [DOI] [PubMed] [Google Scholar]
- Lappas M, Lim R, Riley C, Rice GE, Permezel M. Localisation and expression of FoxO1 proteins in human gestational tissues. Placenta. 2009;30:256–262. doi: 10.1016/j.placenta.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Cho KS, Koh JY. Oxidative injury triggers autophagy in astrocytes: the role of endogenous zinc. Glia. 2009;57:1351–1361. doi: 10.1002/glia.20854. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Tegelberg S, Schipper H, Su H, Zukor H, Manninen O, Kopra O, Joensuu T, Hakala P, Bonni A, Lehesjoki AE. Cystatin B deficiency sensitizes neurons to oxidative stress in progressive myoclonus epilepsy, EPM1. J Neurosci. 2009;29:5910–5915. doi: 10.1523/JNEUROSCI.0682-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner K, Hauptmann S, Abdel-Kader R, Scherping I, Keil U, Strosznajder JB, Eckert A, Muller WE. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer's disease? Antioxid Redox Signal. 2007;9:1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Vital elements of the wnt-frizzled signaling pathway in the nervous system. Curr Neurovasc Res. 2005;2:331–340. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006a;13:883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006b;3:187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21:262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu DM, Zheng YL, Sun DX, Hu B, Shan Q, Zhang ZF, Fan SH. Trace amounts of copper exacerbate beta amyloid-induced neurotoxicity in the cholesterol-fed mice through TNF-mediated inflammatory pathway. Brain Behav Immun. 2009;23:193–203. doi: 10.1016/j.bbi.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Ludikhuize J, de Launay D, Groot D, Smeets TJ, Vinkenoog M, Sanders ME, Tas SW, Tak PP, Reedquist KA. Inhibition of forkhead box class O family member transcription factors in rheumatoid synovial tissue. Arthritis Rheum. 2007;56:2180–2191. doi: 10.1002/art.22653. [DOI] [PubMed] [Google Scholar]
- Maiese K. Diabetic stress: new triumphs and challenges to maintain vascular longevity. Expert Rev Cardiovasc Ther. 2008a;6:281–284. doi: 10.1586/14779072.6.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K. Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008b;62:218–232. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K. High anxiety: Recognizing stress as the stressor. Oxid Med Cell Longev. 2009a;2:61–62. doi: 10.4161/oxim.2.2.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K. Marking the onset of oxidative stress: Biomarkers and novel strategies. Oxid Med Cell Longev. 2009b;2:1. doi: 10.4161/oxim.2.1.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Ahmad I, TenBroeke M, Gallant J. Metabotropic glutamate receptor subtypes independently modulate neuronal intracellular calcium. J Neurosci Res. 1999;55:472–485. doi: 10.1002/(SICI)1097-4547(19990215)55:4<472::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong Z, Hou J, Shang Y. The “O” Class: Crafting clinical care with FoxO transcription factors. In: Maiese K, editor. Forkhead Transcription Factors: Vital Elements in Biology and Medicine. vol 665. Austin, TX: Landes Bioscience; 2009a. ISBN 978- 1-4419-1598-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong Z, Li F. Reducing oxidative stress and enhancing neurovascular longevity during diabetes mellitus. In: Maiese K, editor. Neurovascular Medicine: Pursuing Cellular Longevity for Healthy Aging. New York, NY: Oxford University Press; 2009b. pp. 540–564. [Google Scholar]
- Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24:228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ. Insights into oxidative stress and potential novel therapeutic targets for Alzheimer disease. Restor Neurol Neurosci. 2004;22:87–104. [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008a;5:125–142. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Li F. Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr Neurovasc Res. 2005a;2:425–446. doi: 10.2174/156720205774962692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: Elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008b;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007a;14:1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. "Sly as a FOXO": New paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007b;4:295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008c;14:219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008d;19:145–155. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, Hou J. Clever cancer strategies with FoxO transcription factors. Cell Cycle. 2008e;7:3829–3839. doi: 10.4161/cc.7.24.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, Hou J. Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling? Expert Opin Ther Targets. 2008f;12:905–916. doi: 10.1517/14728222.12.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, Hou J. A "FOXO" in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009c;29:395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci Lond. 2009d;116:191–203. doi: 10.1042/CS20080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Hou J, Chong ZZ, Shang YC. A fork in the path: Developing therapeutic inroads with FoxO proteins. Oxid Med Cell Longev. 2009e;2:119–126. doi: 10.4161/oxim.2.3.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005b;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007c;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Vincent A, Lin SH, Shaw T. Group I and Group III metabotropic glutamate receptor subtypes provide enhanced neuroprotection. J Neurosci Res. 2000;62:257–272. doi: 10.1002/1097-4547(20001015)62:2<257::AID-JNR10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Maiese K, Vincent AM. Critical temporal modulation of neuronal programmed cell injury. Cell Mol Neurobiol. 2000a;20:383–400. doi: 10.1023/A:1007070311203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res. 2000b;59:568–580. doi: 10.1002/(SICI)1097-4547(20000215)59:4<568::AID-JNR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Mari C, Karabiyikoglu M, Goris ML, Tait JF, Yenari MA, Blankenberg FG. Detection of focal hypoxic-ischemic injury and neuronal stress in a rodent model of unilateral MCA occlusion/reperfusion using radiolabeled annexin V. Eur J Nucl Med Mol Imaging. 2004;31:733–739. doi: 10.1007/s00259-004-1473-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Contreras A, Huerta M, Lopez-Perez S, Garcia-Estrada J, Luquin S, Beas Zarate C. Astrocytic and microglia cells reactivity induced by neonatal administration of glutamate in cerebral cortex of the adult rats. J Neurosci Res. 2002;67:200–210. doi: 10.1002/jnr.10093. [DOI] [PubMed] [Google Scholar]
- Miki T, Miura T, Yano T, Takahashi A, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Nishihara M, Naitoh K, Ohori K, Shimamoto K. Alteration in erythropoietin-induced cardioprotective signaling by postinfarct ventricular remodeling. J Pharmacol Exp Ther. 2006;317:68–75. doi: 10.1124/jpet.105.095745. [DOI] [PubMed] [Google Scholar]
- Morissette M, Al Sweidi S, Callier S, Di Paolo T. Estrogen and SERM neuroprotection in animal models of Parkinson's disease. Mol Cell Endocrinol. 2008a;290:60–69. doi: 10.1016/j.mce.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Morissette M, Le Saux M, D'Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008b;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sakamoto K. Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol. 2007;281(1–2):47–55. doi: 10.1016/j.mce.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Newman M, Musgrave IF, Lardelli M. Alzheimer disease: amyloidogenesis, the presenilins and animal models. Biochim Biophys Acta. 2007;1772:285–297. doi: 10.1016/j.bbadis.2006.12.001. [DOI] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007;14:534–547. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- Okouchi M, Ekshyyan O, Maracine M, Aw TY. Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal. 2007;9:1059–1096. doi: 10.1089/ars.2007.1511. [DOI] [PubMed] [Google Scholar]
- Park SJ, Kim HY, Kim H, Park SM, Joe EH, Jou I, Choi YH. Oxidative stress induces lipid-raft-mediated activation of Src homology 2 domain-containing protein-tyrosine phosphatase 2 in astrocytes. Free Radic Biol Med. 2009;46:1694–1702. doi: 10.1016/j.freeradbiomed.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Power JH, Asad S, Chataway TK, Chegini F, Manavis J, Temlett JA, Jensen PH, Blumbergs PC, Gai WP. Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer's disease pathology. Acta Neuropathol. 2008;115:611–622. doi: 10.1007/s00401-008-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa RM, Hoch NC, Furtado GV, Saffi J, Henriques JA. DNA damage in tissues and organs of mice treated with diphenyl diselenide. Mutat Res. 2007;633:35–45. doi: 10.1016/j.mrgentox.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Ruf TF, Quintes S, Sternik P, Gottmann U. Atorvastatin reduces the expression of aldo-keto reductases in HUVEC and PTEC. A new approach to influence the polyol pathway. Clin Invest Med. 2009;32:E219–E228. doi: 10.25011/cim.v32i3.6111. [DOI] [PubMed] [Google Scholar]
- Sales Santos I, da Rocha Tomé A, Saldanha G, Ferreira P, Militão G, de Freitas R. Oxidative stress in the hippocampus during experimental seizures can be ameliorated with the antioxidant ascorbic acid. Oxid Med Cell Longev. 2009;2:23–30. doi: 10.4161/oxim.2.4.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K. Siglec receptors and hiding plaques in Alzheimer's disease. J Mol Med. 2009;87:697–701. doi: 10.1007/s00109-009-0472-1. [DOI] [PubMed] [Google Scholar]
- Sanchez PE, Navarro FP, Fares RP, Nadam J, Georges B, Moulin C, Le Cavorsin M, Bonnet C, Ryvlin P, Belmeguenai A, Bodennec J, Morales A, Bezin L. Erythropoietin receptor expression is concordant with erythropoietin but not with common beta chain expression in the rat brain throughout the life span. J Comp Neurol. 2009;514:403–414. doi: 10.1002/cne.22020. [DOI] [PubMed] [Google Scholar]
- Sela U, Dayan M, Hershkoviz R, Cahalon L, Lider O, Mozes E. The negative regulators Foxj1 and Foxo3a are up-regulated by a peptide that inhibits systemic lupus erythematosus-associated T cell responses. Eur J Immunol. 2006;36:2971–2980. doi: 10.1002/eji.200636137. [DOI] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Hou J, Maiese K. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009;6:20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol. 2009;156:713–727. doi: 10.1111/j.1476-5381.2008.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajes M, Yeste-Velasco M, Zhu X, Chou SP, Smith MA, Pallas M, Camins A, Casadesus G. Activation of Akt by lithium: Pro-survival pathways in aging. Mech Ageing Dev. 2009;130:253–261. doi: 10.1016/j.mad.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Nakamura S, Asano K, Kinouchi M, Ishida-Yamamoto A, Iizuka H. Fas antigen modulates ultraviolet B-induced apoptosis of SVHK cells: sequential activation of caspases 8, 3, and 1 in the apoptotic process. Exp Cell Res. 1999;249:291–298. doi: 10.1006/excr.1999.4476. [DOI] [PubMed] [Google Scholar]
- Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano A, Alvarez MI, Caballero I, Carmona P, De Miguel E. Immunohistochemical increase in cyclooxygenase-2 without apoptosis in different brain areas of subchronic nicotine- and Damphetamine-treated rats. J Neural Transm. 2008;115:1093–1108. doi: 10.1007/s00702-008-0040-9. [DOI] [PubMed] [Google Scholar]
- Tsolakidou A, Trumbach D, Panhuysen M, Putz B, Deussing J, Wurst W, Sillaber I, Holsboer F, Rein T. Acute stress regulation of neuroplasticity genes in mouse hippocampus CA3 area--possible novel signalling pathways. Mol Cell Neurosci. 2008;38:444–452. doi: 10.1016/j.mcn.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Verdaguer E, Susana Gde A, Clemens A, Pallas M, Camins A. Implication of the transcription factor E2F-1 in the modulation of neuronal apoptosis. Biomed Pharmacother. 2007;61:390–399. doi: 10.1016/j.biopha.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Williams K, Schwartz A, Corey S, Orandle M, Kennedy W, Thompson B, Alvarez X, Brown C, Gartner S, Lackner A. Proliferating cellular nuclear antigen expression as a marker of perivascular macrophages in simian immunodeficiency virus encephalitis. Am J Pathol. 2002;161:575–585. doi: 10.1016/S0002-9440(10)64213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, Chebloune Y, Davis RL, Buch SJ. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia. 2009;57:734–743. doi: 10.1002/glia.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won CK, Ji HH, Koh PO. Estradiol prevents the focal cerebral ischemic injury-induced decrease of forkhead transcription factors phosphorylation. Neurosci Lett. 2006;398:39–43. doi: 10.1016/j.neulet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- Wu SY, Wang GF, Liu ZQ, Rao JJ, Lu L, Xu W, Wu SG, Zhang JJ. Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol Sin. 2009;30:202–208. doi: 10.1038/aps.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ma W, Fariss RN, Wong WT. Retinal vascular repair and neovascularization are not dependent on CX3CR1 signaling in a model of ischemic retinopathy. Exp Eye Res. 2009;88:1004–1013. doi: 10.1016/j.exer.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng WH, Kar S, Quirion R. FKHRL1 and its homologs are new targets of nerve growth factor Trk receptor signaling. J Neurochem. 2002;80:1049–1061. doi: 10.1046/j.0022-3042.2002.00783.x. [DOI] [PubMed] [Google Scholar]
- Zhou M, Xu W, Liao G, Bi X, Baudry M. Neuroprotection against neonatal hypoxia/ischemia-induced cerebral cell death by prevention of calpain-mediated mGluR1alpha truncation. Exp Neurol. 2009;218:75–82. doi: 10.1016/j.expneurol.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]