Abstract
Background
Chronic nonhealing wounds are difficult to manage. Various substances are being used to heal these wounds. We sought to observe the effects of autologous epidermal cell suspension dressings on chronic nonhealing ulcers.
Methods
We enrolled patients of the wound clinic at University Hospital, Varanasi, India, with nonhealing wounds of more than 6 weeks’ duration. We treated the wound beds with sterile dressings and antibiotics until the swab cultures became sterile. We prepared autologous epidermal cell suspensions from skin grafts and used them on the ulcer beds along with Vaseline gauze dressings. Follow-up visits with patients occurred weekly for assessment of wound healing and other changes.
Results
Fifteen patients enrolled in our study. Of these, 6 patients had completely healed at 12 weeks, 1 patient at 16 weeks and 2 patients at 20 weeks after treatment. We observed a slow healing response in 6 patients, of whom 1 patient had healed completely at 32 weeks and another at 48 weeks. One patient needed skin grafting, and 3 patients were lost to follow-up.
Conclusion
Autologous noncultured epidermal cell suspension transplantation seems to be an effective, simple and time-saving method to treat chronic nonhealing wounds.
Abstract
Contexte
La prise en charge des plaies chroniques qui ne guérissent pas pose un défi. Diverses substances existent pour favoriser la cicatrisation de ces plaies. Nous avons voulu observer les effets de pansements à base de suspension de cellules épidermiques autologues sur des ulcères chroniques.
Méthodes
Pour cette étude, nous avons recruté les participants parmi les patients de la clinique de traitement des plaies de l’Hôpital universitaire de Varanasi, en Inde; les patients choisis avaient des plaies qui persistaient depuis plus de 6 semaines. Nous avons traité les plaies au moyen de pansements stériles et d’antibiotiques jusqu’à ce que les cultures de spécimens soient négatives. Nous avons préparé des suspensions de cellules épidermiques autologues à partir de greffons cutanés et les avons appliquées sur les ulcères avec des pansements de gaze imprégnés de Vaseline. Nous avons ensuite procédé à un suivi hebdomadaire des patients pour vérifier la cicatrisation de leurs plaies et autres changements.
Résultats
Nous avons inscrit 15 patients à notre étude. Parmi eux, 6 ont présenté une cicatrisation complète à 12 semaines, 1, à 16 semaines, et 2, à 20 semaines suivant le traitement. Nous avons observé une cicatrisation lente chez 6 patients, dont un a connu une guérison complète à 32 semaines et un autre, à 48 semaines. Un patient a dû subir une greffe de peau et 3 n’ont pu être retracés au suivi.
Conclusion
L’inoculation d’une suspension de cellules épidermiques autologues non cultivées semble une méthode efficace, simple et rapide de traitement des plaies chroniques qui ne guérissent pas.
Wound healing is a dynamic biologic process to restore normal anatomy and physiology of the integumentary system and includes overlapping phases of inflammation, proliferation and maturation.1 A prolonged and persistent proliferative phase owing to systemic and local factors may lead to chronic nonhealing wounds. Comorbid conditions such as diabetes, leprosy and peripheral vascular disease may be associated with chronic nonhealing wounds.2
Complete removal of necrotic tissue and its replacement with a skin graft change the repair process from wound healing to graft-take.3 The efficacy of various surgical procedures such as pinch graft, split thickness graft, cultured epidermal autograft and cultured keratinocyte allograft have been assessed as treatment methods for chronic nonhealing wounds.4–6
Cultured epidermis has been used for grafting, but the early methods had limited proliferation, and it was not possible to expand the epidermis in large amounts.7 Cultured keratinocytes grafted on full-thickness wounds have not yielded satisfactory results owing to poor graft-take and epidermal fragility.8
Allograft of cultured keratinocytes seems to act as a potent stimulus for wound healing but does not survive permanently on the wound bed. Multiple cytokines are released that stimulate epithelialization from the wound periphery and the wound bed.9 A marked decrease in the size of nonhealing wounds has been reported with the use of cultured allogenic keratinocytes.10
Epidermal cell suspension and cultured and noncultured keratinocytes have not fulfilled the criteria for ideal skin substitute, but have generated interest owing to their effects on wound healing. Activated keratinocytes secrete many growth factors, which have effects on wound healing apart from graft-take.8 An experimental study using noncultured and cultured keratinocytes in a porcine model has revealed that wounds transplanted with keratinocyte suspension showed accelerated re-epithelializaiton and rapid healing.11
We transplated epidermal cell suspensions of autologus keratinocytes to chronic nonhealing wounds, as cultured epithelium has been proven to be a potent stimulus for wound healing. The cost and time taken to prepare cultured keratinocytes prompted us to use noncultured autologous epidermal cell suspension, which is prepared easily and economically in a short time. We sought to assess the effect of autologous epidermal cell suspension on chronic nonhealing wounds.
Methods
We enrolled patients of the wound clinic at University Hospital, Varanasi, India, with nonhealing wounds of more than 6 weeks’ duration. Our exclusion criteria were malignant disease and osteomyelitis. We obtained informed consent from the patients and ethical approval from the institute’s ethics committee before initiating the study.
We recorded patients’ demographic and clinical characteristics, including age, sex, comorbidities, size of ulcer, duration and treatment. Patients were admitted to hospital, and we administered proper antibiotics and wound care until the swab culture became sterile. Healthy granulation tissue at the base of the ulcer was required for patients to proceed in the study.
After part preparation and maintaining an aseptic and sterile environment, we obtained skin grafts from the medial aspect of the thigh while the patients were under local anesthesia (2% lignoaine). Using a Humby knife, we obtained thin shavings of skin (0.8 mm) with even thickness, which we incubated overnight at 4°C in screw-capped bottles containing 0.25% trypsin (Loba Chemie) and glucose phosphate buffer. We dressed the donor site with supra tullae dressing.
We rinsed the tissue graft thoroughly in normal saline and then prepared a thin film of 0.8% sodium citrate solution in normal saline to further soak the tissue graft for 20 minutes. Using tweezers, we separated the dermis from the epidermis. Vigorous agitation with a pipette took place to separate the epidermal cells further after putting the suspension in a glass tube.
Under aseptic and sterile conditions, we transplanted an adequate amount of the autologous noncultured epidermal cell suspension to the prepared ulcer bed. We used a disposable syringe (Dispovan) to transplant and evenly distribute the suspension and then used a Vaseline gauze dressing to cover the wound. We advised patients to keep their activity levels to a minimum.
The first inspection of the wound took place after 1 week. We dressed ulcers producing slough or discharge with antibiotic ointment. We recorded swab culture and sensitivity. At first, patients were assessed weekly for healing, and thereafter follow-up occurred at 1 week, 4 weeks and every subsequent 4 weeks until the ulcer healed or 48 weeks, whichever came first.
We performed our statistical analysis using SPSS version 11.0 software (SPSS Inc.). We used Student t tests and Fisher exact tests to assess healing, and we considered results to be significant at p < 0.05 (2-tailed).
Results
Patient characteristics
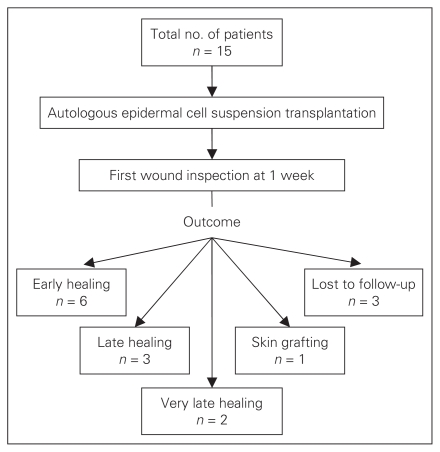
A total of 15 patients, 10 male and 5 female, ranging in age from 10 to 70 years (mean 43, standard deviation [SD] 22.5 yr) enrolled in the study. The mean age of male patients was 48 (SD 22.4) years and that of female patients was 37 (SD 25.0) years (Table 1). Tobacco addiction was present in 48% (6 male, 1 female) of patients. Duration of intake of tobacco varied from 5 to 58 years with a mean of 30 (SD 16.4) years. The outcomes for all patients are summarized in Figure 1.
Table 1.
Age and sex distribution of 15 patients with chronic nonhealing wounds who underwent autologous epidermal cell suspension transplantation
| Age, yr | Sex, no. of patients* | Total no. (%) of patients* | |
|---|---|---|---|
| Male | Female | ||
| 10–20 | 1 | — | 1 (7) |
| 21–30 | — | 2 | 2 (13) |
| 31–40 | 2 | 2 | 4 (27) |
| 41–50 | 3 | — | 3 (20) |
| 51–60 | 2 | — | 2 (13) |
| 61–70 | 2 | 1 | 3 (20) |
| Total | 10 | 5 | 15 (100) |
| Mean age (SD) | 48 (22.4) | 37 (25.0) | 43 (22.5) |
SD = standard deviation.
Unless otherwise indicated.
Fig. 1.
Outcomes of 15 patients who underwent autologous epidermal cell suspension transplantation to treat chronic nonhealing wounds.
Wound characteristics
Trauma was the most common underlying cause of non-healing ulcers (53%) in our study, followed by diabetes (27%), venous ulceration (13%) and leprosy (7%; Table 2). Duration of the nonhealing wound ranged from 18 to 84 weeks (Table 3). The ulcer was on a lower limb in 80% of patients; the upper limb was affected in 13% and the trunk in 7%. We assessed wound size weekly by tracing on graph paper and clinical photographs.
Table 2.
Etiology of the wounds of 15 patients who underwent autologous epidermal cell suspension transplantation
| Etiology | Sex, no. of patients | Total no. (%) of patients | |
|---|---|---|---|
| Male | Female | ||
| Trauma | 6 | 2 | 8 (53) |
| Diabetes | 2 | 2 | 4 (27) |
| Venous ulcer | 1 | 1 | 2 (13) |
| Leprosy | 1 | 0 | 1 (7) |
Table 3.
Duration of the wounds of 15 patients before autologous epidermal cell suspension transplantation
| Duration of ulcer, wk | Sex, no. of patients | Total no. (%) of patients | |
|---|---|---|---|
| Male | Female | ||
| 18–24 | 3 | 2 | 5 (33.3) |
| 25–44 | 5 | 2 | 7 (46.7) |
| 45–64 | — | — | — |
| 65–84 | 2 | 1 | 3 (20.0) |
| Total | 10 | 5 | 15 (100.0) |
Wound swabs revealed bacteria in 33% of patients (n = 5), with Staphylococcus aureus in 26% (n = 4) and Pseudomonas aeruginosa in 7% (n = 1). Infection was monomicrobial in all 5 patients, and all 5 had sterile swab cultures at the time of epidermal cell suspension transplantation. We obtained swab cultures only if there was a foul smell or discharge during follow-up. We obtained a total of 10 swabs for culture and sensitivity: 5 at 4 weeks, 3 at 8 weeks and 2 at 12 weeks. Six patients (40%) had completely healed at 12 weeks (Appendix 1, available at www.cma.ca/cjs). One patient (7%) improved at 16 weeks and 2 (13%) responded at 20 weeks. The wound area in all patients decreased by 10%–16% at 1 week and by 30%–62% at 4 weeks. The decrease in area was 56%–83% at 8 weeks and 93%–94% at 16 weeks (Table 4).
Table 4.
Size of ulcer and healing of wounds during follow-up in 15 patients who underwent autologous epidermal cell suspension transplantation
| Patient | Duration of disease, wk | Follow-up; ulcer size, cm3 | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 (baseline) | 1 wk | 4 wk | 8 wk | 12 wk | 16 wk | 20 wk | ||
| 1 | 32 | 31.2 | 26.2 | 12.6 | 5.5 | Healed | — | — |
| 2 | 18 | 148.6 | 133.6 | 124.0 | 107.6 | 89.7 | 768.0 | 66.6* |
| 3 | 84 | 25.8 | 22.5 | 19.7 | 16.5 | 14.0 | 12.6 | 11.3† |
| 4 | 30 | 47.6 | 40.2 | 22.0 | 9.0 | Healed | — | — |
| 5 | 32 | 45.0 | 40.4 | 20.5 | 8.8 | Healed | — | — |
| 6 | 40 | 86.8 | 74.3 | 42.1 | 23.4 | 9.3 | Healed | — |
| 7 | 80 | 36.0 | 30.2 | 25.5 | 20.2 | 18.4 | 14.0 | 11.5‡ |
| 8 | 24 | 358.8 | 320.4 | 202.0 | 126.6 | 71.3 | 22.0 | Healed |
| 9 | 72 | 24.6 | 22.1 | 18.7 | 14.0 | 11.7 | 10.0 | 9.3† |
| 10 | 42 | 89.0 | 81.2 | 74.4 | 65.0 | 56.0 | 50.3 | 43.9‡ |
| 11 | 36 | 109.0 | 100.0 | 93.7 | 87.2 | 76.0 | 65.0 | 58.5† |
| 12 | 24 | 14.0 | 12.0 | 7.2 | 2.9 | Healed | — | — |
| 13 | 20 | 38.0 | 31.8 | 18.2 | 6.4 | Healed | — | — |
| 14 | 22 | 37.8 | 32.7 | 18.2 | 6.0 | Healed | — | — |
| 15 | 36 | 122.2 | 110.0 | 85.4 | 54.0 | 29.6 | 9.0 | Healed |
Needed skin grafting.
Lost to follow-up.
Delayed healing.
Six patients (40%) showed delayed response. The decrease in wound area in these patients was 8%–16% at 1 week and 14%–29% at 4 weeks. The size further decreased by 30%–51% at 8 weeks, 40%–61% at 16 weeks and 50%–68% at 20 weeks. Reduction in size of the ulcer was statistically significant (p = 0.043). There was a significant change in the size of the ulcer at 8 weeks (p = 0.008) and at 1–12 weeks (p = 0.009) compared with baseline.
At 20 weeks, the wounds had healed completely in 9 patients, whereas the wounds were 50%–68% healed in the remaining 6 patients. Two patients achieved complete healing by 32 weeks and 48 weeks, and 1 patient needed skin grafting after 48 weeks. Three patients in whom healing was delayed were lost to follow-up at 24, 28 and 36 weeks, respectively.
We detected diabetes in 4 patients with delayed healing, and we detected venous ulcers in 2 patients. In 5 patients, tobacco addiction was associated with delayed healing. All traumatic ulcers, the largest of which was 358.8 cm2 had completely healed by 20 weeks. The wound on the donor site in all patients healed within 2 weeks, leaving pigmentary changes. No patients had scarring at the donor site.
Discussion
Chronic nonhealing wounds pose serious problems for patients and clinicians alike. Despite treatment, the wounds are difficult to manage and thus have significant negative physical, economic and social effects on patients.12 Changed lifestyle is contributing tremendously to the increasing number of nonhealing ulcers in developing countries. Resources in these countries are not able to cope with expenditures in the treatment of nonhealing wounds. Skin substitutes, including autologous cultured keratinonocytes, are markedly expensive.
Cutaneous wound repair is a multifaceted process involving clot formation, cell migration and proliferation, extracellular matrix synthesis and deposition, contraction and finally dermal and epidermal reconstitution.11 Our study was designed to assess whether epidermal cell suspension was effective in epidermal re-epithelialization and healing of chronic nonhealing wounds.
Nine of 15 patients healed completely within 20 weeks, which constitutes a 60% success rate among our study participants. Six patients (40%) had incomplete but greater than 50% healing (50%–68%) by week 20. Delayed healing (by 32 weeks and 48 weeks) occurred in 2 patients, and the therapy failed in 1 patient (7%) who needed skin grafting.
In an earlier study,13 we observed 100% healing by 16 weeks in patients whose wounds were managed with debridement and infection control, whereas all patients in the present study did not heal after debridement and treatment of infection for more than 18 weeks. The healing of the wounds within 20 weeks in these patients is remarkable.
A previous study14 using an animal model demonstrated the re-epithelialization capacity of noncultured keratinocytes that was 4 times greater in the treatment group than in the control group, and our study design confirms the findings of previous studies.10,11,14–16 The stimulatory effect on epithelialization from the wound base or periphery is considered to be mediated by growth factors and cytokines released from keratinocytes.9,17 Although epidermal cell suspension containing keratinocytes does not cover the wound, it accelerates wound healing.
Most patients (60%) in our study were older than 40 years, which is in accordance with a previous report by Knighton and colleagues.18 Most of the patients (67%) were male, which might be explained by more frequent outdoor activities, which predisposed them to traumatic ulcers. The duration of nonhealing ulcers ranged from 8 to 84 weeks.
Trauma was the most common (53%) cause of the ulcers, followed by diabetes (27%). Staphylococcus aureus was the most common organism isolated in 4 patients (27%) and P. aeruginosa was isolated in 1 patient (7%). Swartz and Weinberg19 reported that S. aureus and group A streptococcus were the most common organisms.
We did not observe complete covering of the wound with the epithelial sheet in any of the patients at 1 week; ulcers healed starting from the periphery. The first inspection after 7 days showed cream-coloured and dome-shaped kerationcyte colonies measuring about 1–3 mm (Appendix 2, available at www.cjs.ca/cjs) scattered throughout the floor of the ulcer. We observed thin epithelial sheets as the wound area shrunk from the periphery during subsequent follow-up. We observed complete epithelialization in 6 (40%) patients at 12 weeks, 1 patient at 16 weeks and 2 patients at 20 weeks. Healing time was directly proportional to the size of the ulcer. One patient who had healed at 20 weeks had an ulcer 358.8 cm2 in size and was taking steroids. This finding suggests that systemic corticosteroids delay wound healing.
Limited studies are available on noncultured keratinocytes grafting. Autologous epidermal cell suspension was used for wound healing for the first time in rabbits,7 and a comparative study was performed in pigs.11 The pig study concluded that there were significantly fewer keratinocyte colonies associated with noncultured keratinocyte transplantation than with cultured kerationocyte transplantation.11 Various studies using pure epidermal sheet grafting,20 cultured keratinocyte grafting21,22 and engineered skin products have emphasized the pivotal role of keratinocytes in wound healing.
It may be concluded that, like cultured keratinocyte allograft, autologous noncultured epidermal cell suspension might stimulate the growth factors and extracellular matrix proteins to help wound healing or stimulate the migration or multiplication of acceptor keratinocytes.
This new technique of transplanting noncultured autologous epidermal cell suspension to nonhealing ulcers is a pilot study, but it seems to be an effective and simple method. Cost, technique, laboratory set-up and preparation time are less demanding compared with cultured keratinocytes. A minimum of 3–4 weeks is required to prepare cultured keratinocytes for grafting,23 whereas epidermal cell suspension is easily trypsinized and prepared in only 24 hours.
Although the number of patients enrolled in our study was small and there was no comparative control data, our results are encouraging. The healing of the chronic wounds definitely accelerated with epidermal cell suspension transplantation.
Footnotes
Competing interests: None declared.
Contributors: Drs. Shukla and Gulati designed the study. Drs. Barnwal, Gulati and Pandey acquired the data, which all authors analyzed. Drs. Tiwari and Barnwal wrote the article, which Drs. Shukla, Barnwal, Gulati and Pandey reviewed. All authors approved the paper for publication.
References
- 1.Clark RAF. Basics of cutaneous wound repair. J Dermatol Surg Oncol. 1993;19:693–706. doi: 10.1111/j.1524-4725.1993.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 2.Shukla VK, Rasheed MA, Kumar M, et al. A trial to determine the role of placental extract in the treatment of chronic non-healing wounds. J Wound Care. 2004;13:177–9. doi: 10.12968/jowc.2004.13.5.26668. [DOI] [PubMed] [Google Scholar]
- 3.Ehrlich HP. Understanding experimental biology of skin equivalent: from laboratory to clinical use in patients with burns and chronic wounds. Am J Surg. 2004;187(5A):29S–33S. doi: 10.1016/S0002-9610(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 4.Oien RF, Håkansson A, Hansen BU, et al. Pinch grafting of chronic leg ulcers in primary care: fourteen years’ experience. Acta Derm Venereol. 2002;82:275–8. doi: 10.1080/000155502320323243. [DOI] [PubMed] [Google Scholar]
- 5.Límová M, Mauro T. Treatment of leg ulcers with cultured epithelial autografts: clinical study and case reports. Ostomy Wound Manage. 1995;41:48–50. 52, 54–60. [PubMed] [Google Scholar]
- 6.Leigh IM, Purkis PE, Navsaria HA, et al. Treatment of chronic venous ulcers with sheets of cultured allogenic keratinocytes. Br J Dermatol. 1987;117:591–7. doi: 10.1111/j.1365-2133.1987.tb07491.x. [DOI] [PubMed] [Google Scholar]
- 7.Billingham RE, Reynolds J. Transplantation studies on sheets of pure epidermal epithelium and on epidermal cell suspension. Br J Plast Surg. 1952;5:25–36. doi: 10.1016/s0007-1226(52)80004-9. [DOI] [PubMed] [Google Scholar]
- 8.Myers S, Navsariya H, Sanders R, et al. Transplantation of keratinocytes in the treatment of wounds. Am J Surg. 1995;170:75–83. doi: 10.1016/s0002-9610(99)80258-x. [DOI] [PubMed] [Google Scholar]
- 9.Phillipe TJ, Gilchrest BA. Clinical applications of cultured epithelium. Epithelial Cell Biol. 1992;1:39–46. [PubMed] [Google Scholar]
- 10.Beele H, Naeyaert JM, Goetren M, et al. Repeated cultured epidermal autografts in the treatment of chronic leg ulcers of various origins. Dermatologica. 1991;183:31–5. doi: 10.1159/000247628. [DOI] [PubMed] [Google Scholar]
- 11.Svensjo T, Yao F, Pomahac B, et al. Autologus keratinocyte suspensions accelerate wound healing in pigs. J Surg Res. 2001;99:211–21. doi: 10.1006/jsre.2001.6197. [DOI] [PubMed] [Google Scholar]
- 12.Tiwary SK, Shukla D, Tripathi AK, et al. Effect of placental extract gel and cream on non-healing wound. J Wound Care. 2006;15:325–8. doi: 10.12968/jowc.2006.15.7.26937. [DOI] [PubMed] [Google Scholar]
- 13.Saraf SK, Shukla VK, Kaur P, et al. A clinico-epidemiological profile of non-healing wounds in an Indian hospital. J Wound Care. 2000;9:247–50. doi: 10.12968/jowc.2000.9.5.25987. [DOI] [PubMed] [Google Scholar]
- 14.Regauer S, Compton C. Cultured porcine epithelial groups: an improved method. J Invest Dermatol. 1990;94:230–4. doi: 10.1111/1523-1747.ep12874554. [DOI] [PubMed] [Google Scholar]
- 15.Green H. Cultured cells for treatment of disease. Sci Am. 1991;265:96–102. doi: 10.1038/scientificamerican1191-96. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Díaz C, Cuenca-Pardo J, Sosa-Serrano A, et al. Controlled clinical study of deep partial-thickness burns treated with frozen cultured human allogeneic epidermal sheets. J Burn Care Rehabil. 2000;21:291–9. doi: 10.1067/mbc.2000.106393. [DOI] [PubMed] [Google Scholar]
- 17.Falanga V, Sabolinki M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1994;7:201–7. doi: 10.1046/j.1524-475x.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- 18.Knighton DR, Ciresi KF, Fiegel YD, et al. Classification and treatment of chronic non healing wounds. Successful treatment with autologus platelet derived wound healing factors. Ann Surg. 1986;204:322–30. doi: 10.1097/00000658-198609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swartz MN, Weinberg AN. Infections due to gram positive bacteria in non-healing wounds. In: Fitzpatrick TB, editor. Dermatology in general medicine. 4th ed. New York (NY): McGraw Hill; 1993. pp. 2309–34. [Google Scholar]
- 20.Yamaguchi Y, Kubo T, Tarutani M, et al. Treatment of palmoplantar wounds by nonpalmoplantar pure epidermal sheet grafts. Arch Dermatol. 2001;137:621–8. [PubMed] [Google Scholar]
- 21.Harding KG, Kreig T, Eming SA, et al. Efficacy and safety of the freeze-dried cultured human keratinocyte lysate LyphoDermTM 0.9% in the treatment of hard to heal venous leg ulcers. Wound Repair Regen. 2005;13:138–47. doi: 10.1111/j.1067-1927.2005.130204.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuroyanagi Y. Advances in regenerative medicine for skin. Nippon Ronan Igakaki Zasshi. 2005;42:609–15. doi: 10.3143/geriatrics.42.609. [DOI] [PubMed] [Google Scholar]
- 23.Rouabhia M. Permanent skin replacement using chronic epithelial cultured sheets comprising xenogensic and syngeneic keratinocyte. Transplantation. 1996;61:1290–300. doi: 10.1097/00007890-199605150-00002. [DOI] [PubMed] [Google Scholar]