Abstract
Background
Spasm through the internal anal sphincter is one of the supposed causes for pain after hemorrhoidectomy, a common and distressing experience. We hypothesized that the addition of topical nifedipine to lidocaine would improve pain control by causing a relaxation of the smooth muscle of the internal anal sphincter.
Methods
We conducted a multicentre randomized, double-blind trial to compare the efficacy of 0.3% nifedipine and 1.5% lidocaine ointment versus 1.5% lidocaine ointment alone in reducing pain after hemorrhoidectomy. A physician unaware of the treatment arm measured pain by use of the Analogue Chromatic Continuous Scale (ACCS) at baseline; soon after surgery; at 2, 4, 6, 8 and 24 hours after surgery; on day 7 after surgery; and at a final visit 14 days after surgery. The physician also noted the time to first analgesic administration within 24 hours after surgery.
Results
In all, 135 patients per group participated (270 total). Evaluation of the delta ACCS score versus basal value, a covariate for rescue analgesic administration time, revealed better pain control in the group that received nifedipine with lidocaine at 6 hours after surgery and on day 7 (p < 0.011 and p < 0.054, respectively). We noticed no difference between groups for time of administration of rescue analgesic, blood pressure, heart rate or frequency of headache.
Conclusion
Although there was no difference between groups for time of administration of rescue analgesic after open hemorrhoidectomy, the patients’ assessment of pain using ACCS showed that the use of topical nifedipine with lidocaine may provide a slight significant difference in favour of the study group at 6 hours and at day 7 after surgery. Narcotic analgesics and nonsteroidal anti-inflammatory drug administration should continue to be recommended. Further research focusing on these outcomes is warranted.
Abstract
Contexte
Le spasme dans le sphincter anal interne constitue une des causes supposées de douleur après une hémorrhoïdectomie, expérience courante et pénible. Nous avons posé l’hypothèse que l’ajout de nifédipine à la lidocaïne améliorerait le contrôle de la douleur et détendrait le muscle lisse du sphincter anal interne.
Méthodes
Nous avons procédé à une étude à double insu, randomisée et multicentrique pour comparer l’efficacité d’un onguent contenant 0,3 % de nifédipine et 1,5 % de lidocaïne par rapport à un onguent contenant 1,5 % de lidocaïne seulement pour atténuer la douleur après une hémorrhoïdectomie. Un médecin ne connaissant pas le volet de traitement a mesuré la douleur à l’aide de l’échelle analogique chromatique continue (ACCS) au départ; peu après l’intervention chirurgicale; 2, 4, 6, 8 et 24 heures après l’intervention; 7 jours après l’intervention; et lors d’une dernière visite 14 jours après l’intervention. Le médecin a aussi noté l’heure de l’administration de la première dose d’analgésique dans les 24 heures suivant l’intervention chirurgicale.
Résultats
Au total, 135 patients par groupe ont participé (270 en tout). L’évaluation du score différentiel selon l’échelle ACCS par rapport à la valeur de base, covariable du moment d’administration de l’analgésique de soulagement, a révélé un meilleur contrôle de la douleur chez les patients qui ont reçu la combinaison nifédipine et lidocaïne 6 heures après et 7 jours (p < 0,011 et p < 0,054, respectivement) après l’intervention chirurgicale. Nous n’avons constaté aucune différence entre les groupes quant à l’heure de l’administration de l’analgésique de soulagement ou aux effets sur la tension artérielle, la fréquence cardiaque ou la fréquence des maux de tête.
Conclusion
Même s’il n’y avait pas de différences entre les groupes quant à l’heure de l’administration de l’analgésique de soulagement après une hémorrhoïdectomie ouverte, l’évaluation de la douleur par le patient en fonction de l’échelle ACCS a montré que l’utilisation de la nifédipine topique et de la lidocaïne pourrait offrir une légère différence significative en faveur du groupe d’étude, 6 heures et 7 jours après l’intervention chirurgicale. Il faudrait continuer de recommander l’administration d’analgésiques narcotiques et d’anti-inflammatoires non stéroïdiens. Une recherche plus poussée sur ces résultats est justifiée.
Treatment of hemorrhoidal disease depends on the stage of the disorder and the symptoms.1 Surgical hemorrhoidectomy is indicated for the treatment of third- and fourth-degree symptomatic hemorrhoids.2 However, surgery is associated with severe postoperative pain that is a source of such anxiety that some patients decide not to undergo the operation. Nonsteroidal anti-inflammatory drugs (NSAIDs) and opiates have often been used to control pain, but their use is confined to a short period of time and is associated with frequent side effects.3 Consequently, the introduction of novel methods for the control of pain after hemorrhoidectomy is required.
When pain is adequately controlled, hemorrhoidectomy may be conducted in an outpatient setting, saving a lot of money.4,5 Anorectal manometry has shown that anal pressure is greater in patients with hemorrhoids than in the healthy population.6–8 Posthemorrhoidectomy pain seems to be significantly associated with a spasm of the internal anal sphincter.5,9 Various invasive and noninvasive methods, including sphincterotomy,10 anal dilation,11 application of topical preparations, such as trimebutine,12 nitrates,5 botulinum toxin,13 metronidazole,4,14 topical administration and infiltration of local anesthetics such as lidocaine and prilocaine15–17 and flavonoids,18 have been suggested to relieve internal sphincter spasm and solve posthemorrhoidectomy pain. All these methods have yielded promising results in terms of postoperative pain control, but most are associated with invasive procedures and nitrates with frequent side effects.19,20
Within this context, recent evidence suggests that nifedipine, a calcium channel blocker, is effective in reducing anal resting pressure21 and in healing chronic anal fissure and acute thrombosed hemorrhoids.22–24 Therefore, it seems likely that a new ointment containing nifedipine and lidocaine (Antrolin; Bracco S.p.A.) may be considered as a treatment that affects one of the supposed causes for pain after hemorrhoidectomy. Lidocaine and nifedipine have complementary actions. Lidocaine is a local anesthetic usually used to relieve pain of anal fissures and symptomatic hemorrhoids.25,26 Nifedipine may interfere with the ethiopathogenesis of pain after hemorrhoidectomy according to the findings of in vitro tests, which show that it relaxes anal sphincter smooth muscle,27,28 and the results of clinical trials in patients with anal fissures and hemorrhoidal thrombosis, which show that it is safe and effective for anorectal application.29,30 The aim of our study was to determine whether the addition of the smooth muscle relaxing property of nifedipine to the anesthetic action of lidocaine would improve pain control better than topical lidocaine alone in a large population of patients undergoing Milligan–Morgan hemorrhoidectomy.
Methods
We recruited patients undergoing 1-day procedures between February and November 2006 to participate in our study. To be included in the study, patients had to be 18 years or older, have third- or fourth-degree hemorrhoids according to the American Society of Colon and Rectum Surgery classification and had to be scheduled for Milligan–Morgan hemorrhoidectomy with subarachnoid spinal anesthesia, without anal sphincter dilatation or sphincterotomy.
Exclusion criteria were pregnancy or lactation, allergy to nifedipine or lidocaine, associated complications warranting surgery (e.g., abscess, fistula, anal fissure and cancer) and poor general condition (i.e., American Society of Anesthesiologists classification 4 or 5).
We performed a multicentre, prospective, randomized, double-blind study comparing the use of topical nifedipine with lidocaine ointment versus active treatment control. Before the start of the study, we informed all patients about the aims and procedures of the clinical trial, and they gave their written consent. The ethics committee of the A. Cardarelli Hospital in Naples, Italy, and the ethics committees of the 17 Italian institutions that participated in the trial approved our study protocol. This clinical trial was regarded as a phase-3 study of a new pharmacological preparation of nifedipine and lidocaine ointment and was registered in the EMEA public registry of European clinical trials (Antro 01–05 study, EdraCT: 2005–003514–15, http://eudract.emea.europa.eu/).
We randomly assigned patients to either the investigational product (0.3% nifedipine and 1.5% lidocaine ointment) or to the active control (1.5% lidocaine alone) group. In each group, treatment involved 3 g applied topically twice daily for 14 days, including the day of hemorrhoidectomy, according to our previous trials with patients22,24,29,30 and volunteers.31 The investigational product and the active control were manufactured as 2 indistinguishable white ointments, stored in similar disposable microenemas containing 3 g of preparation each and labelled with the randomization code. We provided each patient with the exact number of microenemas for a 2-week-long treatment and instructed them to apply 3 g of ointment circumferentially 1 cm inside the anus. We generated a randomization list in blocks of 4 using a software program by the Biometrics and Biostatistics Unit of Bracco SpA. Blinding was ensured by the fact that each patient pack contained microenemas with the ointment specified by the randomization list for the patient’s consecutive number. The label had a particular randomization number, of which the physician was unaware, that corresponded with 2 sealed envelopes containing the code break (one at the biostatistics service and the other at the centre).
A logical alternative would have been placebo ointment for the control group; however, we chose lidocaine ointment to make the 2 treatments comparable, with the only difference being the inclusion of nifedipine in 1 group. The use of lidocaine ointment postoperatively is not standard in hemorrhoidectomies, but some studies have reported that lidocaine (2.5%) with prilocaine (2.5%) cream decreased pain intensity and the number of requests for additional meperidine after hemorrhoidectomy.16,17 We included lidocaine as a treatment in the control arm to conform with the requirements of the ethics committee and to ensure a slight active pain treatment for control patients that would be indistinguishable in appearance from the investigational product.
The primary efficacy variable for the assessment of postoperative pain was the time elapsed between surgery and the time at which an additional analgesic (30 mg of ketorolac tromethamine, intramuscular or intravenous) was administered for the first time in the first 24 hours after surgery. Patients were to be discharged the day after surgery and evaluated at 24 hours. During the convalescence period, patients underwent clinical evaluations performed by the investigator, who was unaware of the treatment arm, on days 7 and 14 after surgery. In addition, we asked the patients to note the severity of the pain they experienced during the first postoperative week in a study diary. We recommended they take 2 teaspoons of lactulose syrup 3 times daily to keep stools soft during the first week after surgery.
The secondary efficacy variable for the assessment of pain was the interpretation of the Analogue Chromatic Continuous Scale (ACCS),32 which is a version of the classic visual analog scale (VAS), on which patients indicated their perception of pain severity based on a colour scale where white (0 mm) indicated no pain and deep red (100 mm) indicated the worst pain. An investigator unaware of the treatment arm assessed the patients’ perception of pain at baseline; soon after surgery; at 2, 4, 6, 8 and 24 hours after surgery; at day 7 after surgery; and at day 14 after surgery. The patients recorded their pain measurements at home on days 2, 3, 4, 5 and 6 after surgery, but we did not use these measurements for our statistical evaluation of the secondary outcome. Additional information to be recorded in patients’ study diaries were the occurrence of symptoms and disturbances other than pain and the length of administration of oral analgesics (150 mg of ketoprofen by mouth every 12 h), which was to be assessed on day 7 after surgery. Safety analysis was based on the occurrence of adverse events, on the measurement of blood pressure and heart rate.
After a preliminary consistency assessment, we analyzed the results obtained at the various centres together. We assessed the primary objective parameter, the comparison of the percentages of patients requiring another analgesic in the first 24 hours, using the log-rank test. A sample size of 128 patients per treatment group was needed to detect the equality of the survival curves with an α significance level of 0.05 for a 2-tailed test and a β power of 90%. This constitutes a 20% difference between groups in terms of the percentage of patients who needed an analgesic in the first 24 hours, assuming that such a percentage would be 70% in the group receiving only lidocaine and 50% in the group receiving nifedipine and lidocaine. We made no allowance for drop-outs in the first 24 hours.
We performed a descriptive analysis of all secondary variables related to pain and safety variables. Then we compared, time by time, changes in VAS values of mean daily severity of pain using repeated-measure analysis of covariance, adjusting for the time of first analgesic administration. We analyzed blood pressure and heart rate using the Wilcoxon rank-sum test.
Additional efficacy data were the presence or absence of continuous administration of oral analgesics at day 7 after surgery and signs and symptoms that investigators were to note using a χ2 test with continuity or a Student t test for independent samples. We analyzed all variables using 2-tailed tests with a significance level of α = 0.05.
Results
Two groups of 135 patients participated in our study. The mean age was 49.8 (standard deviation [SD] 13.1) years in the study group and 48.1 (SD 12.0) years in the control group. The groups were matched for age, sex, hemorrhoid degree, general condition and other baseline features that are summarized in Table 1. There were no significant differences between the 2 treatment groups at baseline. However, there was a trend toward worse general condition and greater pain in the study group.
Table 1.
Demographic and clinical characteristics of patients undergoing hemorrhoidectomy, by treatment group
| Characteristic | Group, no. (%) of patients* | p value | |
|---|---|---|---|
| Nifedipine and lidocaine, n = 135 | Lidocaine, n = 135 | ||
| Age, mean (SD) [range] yr | 49.8 (13.1) [21–80] | 48.1 (12.0) [23–77] | 0.70 |
| Male sex | 79 (58.5) | 77 (57.0) | 0.80 |
| Hemorrhoid degree | 0.89 | ||
| III | 93 (68.9) | 92 (68.1) | |
| IV | 42 (31.1) | 43 (31.9) | |
| General condition | 0.15 | ||
| ASA 1 | 61 (45.2) | 77 (57.0) | |
| ASA 2 | 67 (49.6) | 53 (39.3) | |
| ASA 3 | 7 (5.2) | 5 (3.7) | |
| Rectal examination | |||
| Hyperemia | 47 (35.3) | 43 (31.9) | 0.49 |
| Inflammation | 26 (19.6) | 21 (15.6) | 0.40 |
| Concomitant diseases | 77 (57.0) | 61 (45.2) | 0.30 |
| VAS, mean (SD) mm | 7.3 (20.4) | 4.3 (15.7) | 0.17 |
ASA = American Society of Anesthesiologists; SD = standard deviation; VAS = visual analog scale.
Unless otherwise indicated.
In all, 251 patients completed the study; 133 in the treatment group and 131 in the control group were available for the assessment of the primary end-point after 24 hours, and 125 in the treatment group and 126 in the control group were available after 1 and 2 weeks. Thus, 10 patients receiving study nifedipine and lidocaine (7.4%) and 9 patients receiving lidocaine only (6.7%) dropped out of the study (Table 2).
Table 2.
Summary of the reasons patients dropped out of the study, by treatment group
| Reason | Group, no. (%) of patients | |
|---|---|---|
| Nifedipine and lidocaine, n = 135 | Lidocaine, n = 135 | |
| Withdrew informed consent | 5 (3.7) | 7 (5.3) |
| Adverse events | 2 (1.5) | 1 (0.7) |
| Lost to follow-up | 1 (0.7) | 1 (0.7) |
| Other | 2 (1.5) | — |
| Total | 10 (7.4) | 9 (6.7) |
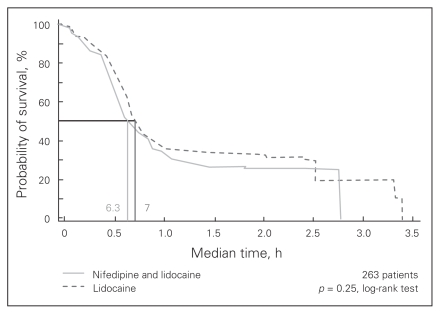
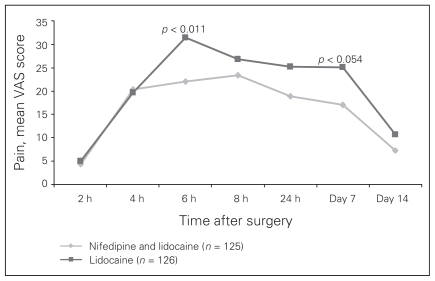
Compliance with at-home treatment was satisfactory. At week 1, compliance was 87.9% in the study group and 85.5% in the control group. The corresponding values at week 2 were 96.8% and 97.6%, respectively. Survival analysis with a log-rank test showed that the median time elapsed from surgery to the first administration of an analgesic did not differ significantly between the groups (6.3 h in the treatment group v. 7 h in the control group, p = 0.25; Fig. 1). The mean VAS for pain at first administration of an analgesic was 38.9 mm in the study group and 41.6 mm in the control group (p = 0.53). The only significant difference observed between groups in a subgroup analysis accounting for hemorrhoid degree, sex and need for the analgesic was between patients with third- and fourth-degree hemorrhoids: the median time to administration of analgesic in patients with third-degree hemorrhoids was 7.7 hours, whereas it was 6.0 hours for in patients with fourth-degree hemorrhoids (p = 0.001). The overall proportion of patients who asked for additional analgesia was similar in the 2 treatment groups (104 of 135 [77.04%] in the study group v. 100 of 135 [74.07%] in the control group), but this was not statistically significant. The ACCS score related to pain increased much less in the study group than in the control group starting at 6 hours after surgery. Table 3 and Figure 2 show that the only times where there was a significant difference between the study group and the control group was at 6 hours after surgery and at day 7 after surgery. Evaluation of the delta ACCS score versus basal value (0 mm), a covariate for first rescue analgesic administration time, was significant at 6 hours (22 mm in the treatment group v. 31.5 mm in the control group, p < 0.011) and at the end of the first week (17 mm in the treatment group v. 25 mm in the control group, p < 0.054). Covariance analysis showed that both treatment and time elapsed from surgery contributed to these differences. Pain measurements at 8 and 24 hours after surgery and at day 14 after surgery were lower in the treatment group, but the difference was not significant. The occurrence of symptoms other than pain in each group is summarized in Table 4. Continuous administration of oral analgesics after 7 days of therapy was required in 43 of 135 (31.85%) patients in the treatment group compared with 48 of 135 (35.55%) in the control group, but this difference was not significant.
Fig. 1.
Survival analysis for the time elapsed from surgery to the first administration of an analgesic during the first 24 hours after surgery.
Table 3.
Change of mean pain scores from baseline in the 2 treatment groups
| Time point after surgery | Group; mean change in pain score (SD) | |
|---|---|---|
| Nifedipine and lidocaine, n = 135 | Lidocaine, n = 135 | |
| 2 h | 4.37 (17.58) | 5.02 (15.57) |
| 4 h | 20.39 (30.04) | 19.73 (29.15) |
| 6 h | 22.09* (27.39) | 31.47 (30.86) |
| 8 h | 23.34 (28.32) | 26.84 (28.38) |
| 24 h | 18.93 (26.84) | 25.19 (28.79) |
| 7 d | 17.04* (27.33) | 25.05 (25.79) |
| 14 d | 7.33 ( 23.18) | 10.66 (20.58) |
SD = standard deviation.
Delta visual analog scale covariate for analgesic administration time versus baseline in study and control groups at 6 hours (p < 0.011) and 7 days (p < 0.054) after surgery.
Fig. 2.
Evaluation of the delta Analogue Chromatic Continuous Scale score versus basal value, which was used as a covariate for the time to analgesic administration. VAS = visual analog scale.
Table 4.
Occurrence of symptoms other than pain, by treatment group
| Symptom; time point | Group, no. (%) of patients | |
|---|---|---|
| Nifedipine and lidocaine, n = 125 | Lidocaine, n = 126 | |
| Evacuation check | ||
| 24 h | 8 (6.4) | 9 (7.1) |
| 1 wk | 46 (36.8) | 42 (33.3) |
| 2 wk | 122 (97.6) | 124 (98.4) |
| Solid stools | ||
| 24 h | 9 (7.2) | 12 (9.5) |
| 1 wk | 88 (70.4) | 86 (68.2) |
| 2 wk | 103 (82.4) | 108 (85.7) |
| Bleeding | ||
| 24 h | 10 (8.0) | 7 (5.5) |
| 1 wk | 39 (31.2) | 33 (26.1) |
| 2 wk | 19 (15.2) | 16 (12.6) |
| Fecal incontinence | ||
| 24 h | 0 | 0 |
| 1 wk | 3 (2.4) | 2 (1.5) |
| 2 wk | 2 (1.6) | 1 (0.8) |
| Nausea | ||
| 24 h | 9 (7.2) | 5 (3.9) |
| 1 wk | 5 (4.0) | 4 (3.1) |
| 2 wk | 1 (0.8) | 1 (0.8) |
| Vomiting | ||
| 24 h | 3 (2.4) | 0 |
| 1 wk | 3 (2.4) | 1 (0.8) |
| 2 wk | 1 (0.8) | 0 |
| Headache | ||
| 24 h | 4 (3.2) | 7 (4.3) |
| 1 wk | 6 (4.8) | 11 (8.7) |
| 2 wk | 2 (1.6) | 1 (0.8) |
We included the data of all 270 patients who received treatment at least once during the trial in the safety analysis. Adverse events are showed in Table 5; there were no significant differences between the 2 groups. Results of blood pressure and heart rate measurements are shown in Table 6. We observed no statistically significant decrease in blood pressure or increasing in heart rate. Mean systolic and diastolic values were lower in the control group.
Table 5.
Occurrence of adverse events, by treatment group
| Adverse event | Group, no. of patients | |
|---|---|---|
| Nifedipine and lidocaine, n = 135 | Lidocaine, n = 135 | |
| Headache | 5 | 4 |
| Sphincter pain | 1 | 1 |
| Hypertension | 1 | 0 |
| Fecal incontinence | 1 | 0 |
| Nausea | 1 | 2 |
| Vomiting | 1 | 0 |
| Bleeding upon defecation | 1 | 3 |
| Anal burning | 2 | 0 |
| Perianal edema | 2 | 0 |
| Edema of the wound | 1 | 0 |
| Anal itching | 1 | 0 |
| Fever | 0 | 1 |
| Dizziness | 0 | 1 |
| Asthenia | 0 | 1 |
| Total | 17 | 13 |
Table 6.
Average blood pressure and heart rate, by treatment group
| Variable; treatment group | Time after surgery, mean | |||||
|---|---|---|---|---|---|---|
| Baseline | 2 h | 8 h | 24 h | Day 7 | Day 14 | |
| Systolic pressure, mm Hg* | ||||||
| Nifedipine and lidocaine | 126.95 | 124.23 | 125.75 | 124.16 | 125.38 | 126.94 |
| Lidocaine | 124.53 | 122.17 | 122.24 | 122.56 | 124.64 | 125.47 |
| Diastolic pressure, mm Hg* | ||||||
| Nifedipine and lidocaine | 76.85 | 76.24 | 76.09 | 76.13 | 77.3 | 76.26 |
| Lidocaine | 76.54 | 75.77 | 75.34 | 74.89 | 77.99 | 77.76 |
| Heart rate, beats/min* | ||||||
| Nifedipine and lidocaine | 72 | 71.26 | 72.93 | 72.91 | 72.09 | 71.74 |
| Lidocaine | 72.35 | 71.73 | 72.87 | 72.63 | 71.58 | 70.83 |
Not statistically significant.
Discussion
Pain after hemorrhoidectomy is a common and distressing experience for patients. It may delay discharge, recovery and return to work. Several invasive and noninvasive attempts have been made to reduce or alleviate pain after hemorrhoidectomy.4,5,10–20,33 The origin of such pain is undetermined and seems to be multifactorial. Current theories propose that hypertonia of the internal anal sphincter is associated with ethiopathogenesis of anal fissure and pain after hemorrhoidectomy.5,9 Topical nifedipine causes a relaxation of the smooth muscle of the internal anal sphincter27,28 and may be effective in managing anal fissure and acute thrombosed hemorrhoids.22–24,30,34 Pain in patients with anal fissures is reported to be substantially reduced after the application of nifedipine and lidocaine ointment.29 This led us to verify the hypothesis that the addition of nifedipine to lidocaine would improve pain control in a large population of patients undergoing open hemorrhoidectomy, because a previous study revealed that the anorectal application of ointment containing nifedipine (0.3%) and lidocaine (1.5%) did not show any pharmacologically relevant serum levels of the active ingredients and any hemodynamic effects in healthy volunteers.31
Analyzing the efficacy of nifedipine and lidocaine on postoperative hemorrhoidectomy pain raises some issues to consider. The VAS documented the patients’ perception of pain. Differences in administration of oral analgesics were not significant in both groups, and differences in mean pain scores at the 7 time points in our study also seemed to be minimal if they were measured as VAS values. However, perception of pain was less in the treatment group, and these differences were significant when we determined the delta ACCS value. The addition of nifedipine to lidocaine was of benefit later on, beginning 6 hours after surgery, when the evaluation of the delta ACCS score versus basal value (a covariate for rescue analgesic administration time) revealed significantly better control of pain in the treatment group. Furthermore, pain was reported to be significantly worse in the control group than in the treatment group on day 7. Pain scores between and after these times (i.e., 8 h, 24 h and 14 days after surgery) were lower in the study group, but there was no significant difference between the groups.
Further survival analysis showed that the median time to first administration of an analgesic did not differ significantly between the 2 treatment groups in the first 24 hours after traditional hemorrhoidectomy, with the exception of a significant difference between patients with third- or fourth-degree hemorrhoids. Surgical procedure, the extent of the excision and perioperative analgesia may explain this significant delay. All patients underwent spinal anesthesia, and it is possible that there was some residual anesthesia that remained during the first 12 hours after surgery, which could have affected the pain measurements of primary outcome in both groups. We cannot determine the specific role of spinal anesthesia based on the present data, so further research is warranted.
Furthermore, various noninvasive methods suggested to control posthemorrhoidectomy pain, including application of topical preparations such as nitrates,5,19,20,35 botulinum toxin13,36 and metronidazole,4,14, do not seem to offer any benefit in terms of rapid pain relief in the first 24 hours after open hemorrhoidectomy. The rationale for the addition of nifedipine was that, by relaxing the sphincter smooth muscle, it would provide additional pain control by reducing anal pressure, which is significantly higher in individuals with hemorrhoids than in controls.7,8 Data reported in the present study cannot prove this assumption. The question of sphincter tone reduction could be addressed by manometric studies, but these studies are difficult to perform in a postoperative patient population. It has been shown that hemorrhoidectomy normalizes anal pressure in the long term,37 but it was believed that anal pressure was not resolved and was greater immediately after surgery. A recent study by Patti and colleagues36 has shown that maximum resting anal pressure significantly increases on the fifth day after hemorrhoidectomy compared with that before surgery. This is consistent with the achievement of a significant difference in pain control at 6 hours and 7 days after surgery, and a slight difference in favour of the treatment group at 8 hours, 24 hours and 14 days after surgery. We suggest that the temporary reduction of postoperative sphincter tone seems to be useful because it accelerates wound healing and induces postoperative pain reduction when resting and during defecation. A study by Silverman and colleagues38 supports our findings, but according to their data any reduction of postoperative sphincter tone would be achieved around the fifth postoperative day rather than the first postoperative day, when NSAIDs and narcotic drug administration should continue to be recommended.
Most of the adverse events that patients experienced were well-known complications of hemorrhoidectomy: local burning, edema, irritation, bleeding, fecal incontinence, urinary disorders such as urinary retention, formation of protrusions, infections and related symptoms such as fever. Overall, these adverse events accounted for about 50% of those reported both in the treatment and control groups. In addition, patients experienced nausea and vomiting, which are well-known adverse reactions to anesthesia. Fewer adverse events were clearly related to the use of nifedipine with lidocaine. It is possible that allergic dermatoses and reactions to lidocaine ointment were not observed in the present study, because the length of treatment was short and lidocaine is a rare contact allergen compared with other local anesthetics such as benzocaine, which commonly sensitizes.39,40
Fecal incontinence occurred more frequently in the treatment arm, but this difference was not significant. However, incontinence is a complex phenomenon, and it may occur with significant frequency after hemorrhoidectomy (2%–12%).41 Variation and overlap in test results and patient-, instrument- or operator-dependent factors require cautious interpretation.42 The overall incidence of headache in the treatment group (5 of 135 patients, 3.7%) was similar to that in the control group (4 of 135 patients, 2.9%), but for 1 patient, the headache was severe enough to cause discontinuation of treatment. Furthermore, headache is a substantial problem in patients using glyceryl trinitrate, which has been used in the treatment of pain after hemorrhoidectomy,5,20 especially with higher drug concentrations, in 2 or more administrations. In our study, headache incidence was far less frequent than in other reports using glyceryl trinitrate, and the monitoring of vital signs did not reveal any hemodynamic changes. Because nifedipine is a calcium antagonist with a well-known hypotensive effect, it was relevant to determine whether the local administration of nifedipine and lidocaine ointment would cause systemic effects. The results of our study seem to confirm in a large population that patients who received nifedipine and lidocaine ointment do not experience any systemic hemodynamic effects and that blood pressure values do not diminish.
Conclusion
Pain after open hemorrhoidectomy remains a common problem in surgery. Narcotic analgesics and NSAIDs are generally required. Although there was no difference between groups in the time of administration of rescue analgesic after open hemorrhoidectomy, our results suggest that an ointment containing nifedipine and lidocaine may provide better pain control than lidocaine alone at 6 hours and 7 days after surgery, and further research focusing on these secondary outcomes is warranted. In addition, although topical application of an ointment in patients who recently had perianal surgery, particularly hemorrhoid surgery, is usually not easy, our results show that topical combination of nifedipine and lidocaine is well tolerated in patients who have undergone open hemorrhoidectomy.
Acknowledgements
Bracco S.p.A., Milan, Italy, provided financial support to the phase-3 level of clinical study under the Good Clinical Practice rules and Italian law. The authors thank the surgeons from the Italian institutions who participated in the trial and obtained approval for the study from their local ethics committees.
Footnotes
A poster presentation of the study was discussed at the 15th United European Gastroenterology Week “UEGW 2007,” Paris, France, Oct. 27–31, 2007.
Competing interests: None declared for Drs. Perrotti and Antropoli. Drs. Dominici, Grossi and Cerutti are employed by Bracco. S.p.A., Milan.
Contributors: Drs. Perrotti, Grossi and Antropoli designed the study. Drs. Perrotti and Antropoli acquired the data; they analyzed it with Drs. Dominici, Grossi and Cerutti. Dr. Perrotti wrote the article. All authors reviewed the article and approved its publication.
References
- 1.Holzheimer RG. Hemorrhoidectomy: indications and risks. Eur J Med Res. 2004;9:18–36. [PubMed] [Google Scholar]
- 2.Nisar PJ, Scholefield JH. Managing haemorrhoids. BMJ. 2003;327:847–51. doi: 10.1136/bmj.327.7419.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein ET, Williamson PR, Larach SW. Subcutaneous morphine pump for postoperative hemorrhoidectomy pain management. Dis Colon Rectum. 1993;36:439–46. doi: 10.1007/BF02050008. [DOI] [PubMed] [Google Scholar]
- 4.Carapeti EA, Kamm MA, McDonald PJ, et al. Double-blind randomised controlled trial of effect of metronidazole on pain after day-case haemorrhoidectomy. Lancet. 1998;351:169–72. doi: 10.1016/S0140-6736(97)09003-X. [DOI] [PubMed] [Google Scholar]
- 5.Wasvary HJ, Hain J, Mosed-Vogel M, et al. A randomized, prospective, double-blind, placebo-controlled trial of effect of nitroglycerin ointment on pain after hemorrhoidectomy. Dis Colon Rectum. 2001;44:1069–73. doi: 10.1007/BF02234622. [DOI] [PubMed] [Google Scholar]
- 6.Hiltunen KM, Matikainen M. Anal manometric findings in symptomatic hemorrhoids. Dis Colon Rectum. 1985;28:807–9. doi: 10.1007/BF02555482. [DOI] [PubMed] [Google Scholar]
- 7.Arabi Y, Alexander-Williams J, Keighley MR. Anal pressures in hemorrhoids and anal fissure. Am J Surg. 1977;134:608–10. doi: 10.1016/0002-9610(77)90445-7. [DOI] [PubMed] [Google Scholar]
- 8.Deutsch AA, Moshkovitz M, Nudelman I, et al. Anal pressure measurements in the study of hemorrhoid etiology and their relation to treatment. Dis Colon Rectum. 1987;30:855–7. doi: 10.1007/BF02555423. [DOI] [PubMed] [Google Scholar]
- 9.Roe AM, Bartolo DC, Vellacott KD, et al. Submucosal versus ligation excision haemorrhoidectomy: a comparison of anal sensation, anal sphincter manometry and postoperative pain and function. Br J Surg. 1987;74:948–51. doi: 10.1002/bjs.1800741022. [DOI] [PubMed] [Google Scholar]
- 10.Kanellos I, Zacharakis E, Christoforidis E, et al. Usefulness of lateral internal sphincterotomy in reducing postoperative pain after open hemorrhoidectomy. World J Surg. 2005;29:464–8. doi: 10.1007/s00268-004-7432-2. [DOI] [PubMed] [Google Scholar]
- 11.Goligher JC, Graham NG, De Dombal FT, et al. The value of stretching of anal sphincters in the relief of pain after haemorrhoidectomy. Br J Surg. 1969;56:390. [PubMed] [Google Scholar]
- 12.Ho YH, Seow-Choen F, Low JY, et al. Randomised controlled trial of trimebutine (anal sphincter relaxant) for pain after haemorrhoidectomy. Br J Surg. 1997;84:377–9. [PubMed] [Google Scholar]
- 13.Davies J, Duffy D, Boyt N, et al. Botulinum toxin (botox) reduces pain after hemorrhoidectomy: results of a double-blind, randomized study. Dis Colon Rectum. 2003;46:1097–102. doi: 10.1007/s10350-004-7286-6. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson TJ, Armstrong D. Topical metronidazole (10 percent) decreases posthemorrhoidectomy pain and improves healing. Dis Colon Rectum. 2004;47:711–6. doi: 10.1007/s10350-003-0129-z. [DOI] [PubMed] [Google Scholar]
- 15.Smith SL, Simon R. Viscous lidocaine as a posthemorrhoidectomy analgesic. Dis Colon Rectum. 1979;22:40–1. doi: 10.1007/BF02586755. [DOI] [PubMed] [Google Scholar]
- 16.Shiau JM, Su HP, Chen HS, et al. Use of a topical anesthetic cream (EMLA) to reduce pain after hemorrhoidectomy. Reg Anesth Pain Med. 2008;33:30–5. doi: 10.1016/j.rapm.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Shiau JM, Hung KC, Chen HH, et al. Combination of topical EMLA with local injection of lidocaine: superior pain relief after Ferguson hemorrhoidectomy. Clin J Pain. 2007;23:586–90. doi: 10.1097/AJP.0b013e3180e00d31. [DOI] [PubMed] [Google Scholar]
- 18.Colak T, Akca T, Dirlik M, et al. Micronized flavonoids in pain control after hemorrhoidectomy: a prospective randomized controlled study. Surg Today. 2003;33:828–32. doi: 10.1007/s00595-003-2604-5. [DOI] [PubMed] [Google Scholar]
- 19.Coskun A, Duzgun SA, Uzunkoy A, et al. Nitroderm TTS band application for pain after hemorrhoidectomy. Dis Colon Rectum. 2001;44:680–5. doi: 10.1007/BF02234566. [DOI] [PubMed] [Google Scholar]
- 20.Elton C, Sen P, Montgomery AC. Initial study to assess the effects of topical glyceryl trinitrate for pain after haemorrhoidectomy. Int J Surg Investig. 2001;2:353–7. [PubMed] [Google Scholar]
- 21.Chrysos E, Xynos E, Tzovaras G, et al. Effect of nifedipine on rectoanal motility. Dis Colon Rectum. 1996;39:212–6. doi: 10.1007/BF02068078. [DOI] [PubMed] [Google Scholar]
- 22.Antropoli C, Perrotti P, Rubino M, et al. Nifedipine for local use in conservative treatment of anal fissures: preliminary results of a multicenter study. Dis Colon Rectum. 1999;42:1011–5. doi: 10.1007/BF02236693. [DOI] [PubMed] [Google Scholar]
- 23.Ezri T, Susmallian S. Topical nifedipine vs. topical glyceryl trinitrate for treatment of chronic anal fissure. Dis Colon Rectum. 2003;46:805–8. doi: 10.1007/s10350-004-6660-8. [DOI] [PubMed] [Google Scholar]
- 24.Perrotti P, Antropoli C, Noschese G, et al. Topical nifedipine for conservative treatment of acute hemorrhoidal thrombosis. Colorectal Dis. 2000;2:18–21. doi: 10.1046/j.1463-1318.2000.00130.x. [DOI] [PubMed] [Google Scholar]
- 25.Gough MJ, Lewis A. The conservative treatment of fissure-in-ano. Br J Surg. 1983;70:175–6. doi: 10.1002/bjs.1800700312. [DOI] [PubMed] [Google Scholar]
- 26.Neiger A, Herms E. The symptomatic therapy of hemorrhoids and anal eczema — a report of experiences from proctology practice. Schweiz Rundsch Med Prax. 1990;79:918–20. [PubMed] [Google Scholar]
- 27.Cook TA, Brading AF, Mortensen NJ. Effects of nifedipine on anorectal smooth muscle in vitro. Dis Colon Rectum. 1999;42:782–7. doi: 10.1007/BF02236936. [DOI] [PubMed] [Google Scholar]
- 28.Cook TA, Brading AF, Mortensen NJ. Differences in contractile properties of anorectal smooth muscle and the effects of calcium channel blockade. Br J Surg. 1999;86:70–5. doi: 10.1046/j.1365-2168.1999.00998.x. [DOI] [PubMed] [Google Scholar]
- 29.Perrotti P, Bove A, Antropoli C, et al. Topical nifedipine with lidocaine ointment vs. active control for treatment of chronic anal fissure: results of a prospective, randomized, double-blind study. Dis Colon Rectum. 2002;45:1468–75. doi: 10.1007/s10350-004-6452-1. [DOI] [PubMed] [Google Scholar]
- 30.Perrotti P, Antropoli C, Molino D, et al. Conservative treatment of acute thrombosed external hemorrhoids with topical nifedipine. Dis Colon Rectum. 2001;44:405–9. doi: 10.1007/BF02234741. [DOI] [PubMed] [Google Scholar]
- 31.Perrotti P, Grumetto L, Barbato F, et al. Serum levels and possible haemodynamic effects following anorectal application of an ointment containing nifedipine and lignocaine. A study in healthy volunteers. Clin Drug Investig. 2006;26:459–67. doi: 10.2165/00044011-200626080-00004. [DOI] [PubMed] [Google Scholar]
- 32.Grossi E, Borghi C, Cerchiari EL, et al. Analogue chromatic continuous scale (ACCS): a new method for pain assessment. Clin Exp Rheumatol. 1983;1:337–40. [PubMed] [Google Scholar]
- 33.Asfar SK, Juma TH, Ala-Edeen T. Hemorrhoidectomy and sphincterotomy: a prospective study comparing the effectiveness of anal stretch and sphincterotomy in reducing pain after hemorrhoidectomy. Dis Colon Rectum. 1988;31:181–5. doi: 10.1007/BF02552543. [DOI] [PubMed] [Google Scholar]
- 34.Tranqui P, Trottier DC, Victor JC, et al. Nonsurgical treatment of chronic anal fissure: nitroglycerin and dilatation versus nifedipine and botulinum toxin. Can J Surg. 2006;49:41–5. [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang do Y, Yoon SG, Kim HS, et al. Effect of 0.2 percent glyceryl trinitrate ointment on wound healing after a hemorrhoidectomy: results of a randomized, prospective, double-blind, placebo-controlled trial. Dis Colon Rectum. 2003;46:950–4. doi: 10.1007/s10350-004-6692-0. [DOI] [PubMed] [Google Scholar]
- 36.Patti R, Almasio PL, Muggeo VM, et al. Improvement of wound healing after hemorrhoidectomy: a double-blind, randomized study of botulinum toxin injection. Dis Colon Rectum. 2005;48:2173–9. doi: 10.1007/s10350-005-0179-5. [DOI] [PubMed] [Google Scholar]
- 37.Read MG, Read NW, Haynes WG, et al. A prospective study of the effect of haemorrhoidectomy on sphincter function and faecal continence. Br J Surg. 1982;69:396–8. doi: 10.1002/bjs.1800690713. [DOI] [PubMed] [Google Scholar]
- 38.Silverman R, Phillip J, Bendick, et al. A randomized, prospective, double-blind, placebo-controlled trial of the effect of a calcium channel blocker ointment on pain after hemorrhoidectomy. Dis Colon Rectum. 2005;48:1913–6. doi: 10.1007/s10350-005-0135-4. [DOI] [PubMed] [Google Scholar]
- 39.Handfield–Jones SE, Cronin E. Contact sensitivity to lignocaine. Clin Exp Dermatol. 1993;18:342–3. doi: 10.1111/j.1365-2230.1993.tb02213.x. [DOI] [PubMed] [Google Scholar]
- 40.Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81–5. [PubMed] [Google Scholar]
- 41.Madoff RD, Fleshman JW. American Gastroenterological Association technical review on the diagnosis and treatment of hemorrhoids. Gastroenterology. 2004;126:1463–73. doi: 10.1053/j.gastro.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Holzheimer RG. Hemorrhoidectomy: indications and risks. Eur J Med Res. 2004;9:18–36. [PubMed] [Google Scholar]