Abstract
Objective. To report the feasibility, safety and efficacy of percutaneous aortic valve implantation (PAVI) with the CoreValve self-expanding aortic valve bioprosthesis in elderly patients with aortic valve stenosis who are rejected for surgery or have a high surgical risk.
Methods. PAVI using the CoreValve ReValving System was performed under general anaesthesia in 30 high-risk (surgical) patients with a symptomatic severe aortic valve stenosis.
Results. The patients had a mean age of 80.5±7.7 years, a mean aortic valve area of 0.71±0.19 cm2, a peak transvalvular aortic gradient of 79±25 mmHg, as measured with echo Doppler, a logistic EuroSCORE of 15±10% and a Society of Thoracic Surgeons (STS) score of 5.2±2.9%. Device success was achieved in all patients and acute procedural success in 27 patients (90%). In the surviving patients, there was in a reduction of the peak aortic pressure gradient from 76±24 mmHg to 22±7 mmHg (n=24, p<0.00001) 30 days after successful device implantation. At 30 days, major adverse cardiovascular and cerebral events had occurred in seven patients (23%). This included mortality in six patients (20%), of which one death was cardiovascular. The other five non-cardiovascular deaths involved two patients who died of an exacerbation of severe pre-existent pulmonary disease and three of infectious complications.
Conclusions. Percutaneous aortic valve implantation was successfully performed in our centre in highrisk patients, with a 30-day mortality of 20%. When successful, marked haemodynamic improvement and relief of symptoms was achieved. (Neth Heart J 2010;18:18-24.)
Keywords: aortic valve stenosis, valvuloplasty, transcatheter valve therapy
Currently, the standard therapy of symptomatic aortic valve stenosis (AS) is open chest aortic valve replacement. However, since symptomatic AS usually occurs in the elderly, a high prevalence of comorbidities is present in these patients. The common comorbidities in these patients, such as advanced age, previous cardiac surgery, reduced systolic left ventricular function, pulmonary disease and renal insufficiency, are known to be associated with a high periprocedural and postprocedural risk of mortality and morbidity. Therefore almost one third of AS patients with these comorbidities are not referred or are rejected for surgery.1,2 Furthermore, the long revalidation period after open chest surgery may be a reason to withhold surgery from high-risk patients.
Therefore, the development of a less invasive, percutaneous approach to aortic valve replacement was required. Currently, two Crédit Européen (CE) certified aortic bioprostheses are available for percutaneous retrograde implantation: the CoreValve and the Cribier-Edwards valve.3-6 Previous reports have shown that percutaneous retrograde implantation of aortic bioprosthetic valves is feasible but that mortality and morbidity remain high in these patients.
This report is an evaluation of the feasibility, safety and efficacy of percutaneous aortic valve implantation (PAVI) with the CoreValve ReValving™ System in the first 30 patients in our centre.
Patients and methods
From October 2007 to June 2009 percutaneous aortic valve implantation was performed in 30 patients. A carefully designed clinical protocol was approved by the institutional research and ethics committee. This protocol included an independent data safety and monitoring board including an experienced interventional cardiologist.
After evaluation in a team of two interventional cardiologists, a cardiac surgeon, an echocardiographist and a cardioanaesthesiologist, 30 patients with severe symptomatic native aortic valve stenosis and a high surgical risk were selected to undergo PAVI. The patients were all considered poor surgical candidates with a high surgical risk: 21 patients were considered inoperable. All patients gave written informed consent.
Inclusion and exclusion criteria
Patients were considered candidates for PAVI if the AS was severe, i.e. aortic valve area <1 cm2 and symptomatic, the aortic annulus diameter was between 20 and 27 mm, with a sinotubular junction diameter ≤43 mm, and either patient age ≥80 years or logistic EuroSCORE ≥15% or one or more of the following complicating factors: previous cardiac surgery, right ventricular insufficiency, pulmonary insufficiency, pulmonary hypertension, history of mediastinal radiotherapy, burning thoracic sequelae, severe connective tissue disease, liver cirrhosis, cachexia, morbid overweight, porcelain aorta and patients (n=9) who refused aortic valve surgery.
Patients were considered not suitable for PAVI in case of known hypersensitivity or contraindication for aspirin, heparin, ticlopidine, clopidogrel or Nitinol, refusal of rescue aortic valve surgery by the patient if considered possible by the surgeon, sepsis (including active endocarditis), recent (<30 days) myocardial infarction, ventricular or atrial thrombus, previous surgical aortic valve replacement, evolutive or recent cerebrovascular accident, severe femoral, iliac or aortic stenosis, tortuosity or aneurysm (not applicable for PAVI via subclavian route), uncontrolled bleeding diathesis or coagulopathy, refusal of blood transfusion, and enrolment in another investigational study.
Transcatheter aortic valve procedure
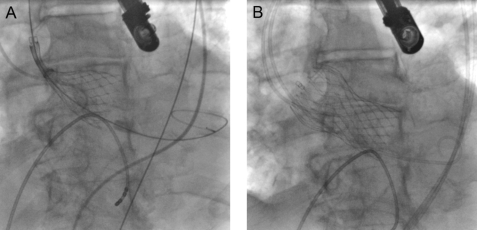
The technique of PAVI with the CoreValve ReValving™ System has been described in previous studies.3,4,6 Procedures were performed in the catheterisation laboratory, with the patient under general anaesthesia. Vascular access was obtained via the femoral artery (n=29) or left subclavian artery (n=1) and femoral vein. The procedure was initiated with a balloon valvuloplasty under rapid pacing using an Amplatz superstiff guidewire placed in the left ventricle (LV). Next, the CoreValve delivery system was advanced through the femoral artery or the subclavian artery (n=1) to the aortic annulus under fluoroscopic guidance. When the delivery system was in the correct position, the aortic valve prosthesis was deployed (figures 1A and B). After complete deployment of the prosthesis, valve position and function were assessed with angiography and transoesophageal echocardiography and if necessary a postdilatation of the valve was performed (n=2).
Figure 1.
Implantation of the CoreValve prosthesis under fluoroscopic guidance (patient no. 7). A) Outer sheath is pulled back as a result of which the prosthesis can deploy. B) Maximally deployed prosthesis.
Follow-up and endpoints
Clinical follow-up, blood analysis and transthoracic echocardiography were obtained before discharge and at one month after discharge.
Three feasibility endpoints were defined: (1) device success, (2) acute procedural success and (3) the occurrence of major adverse cardiovascular and cerebral events (MACCEs) within 30-day follow-up. Device success was defined as stable device placement and adequate function as assessed by angiography and echocardiography. Acute procedural success was defined as device success with the absence of periprocedural MACCEs in the first 48 hours after device implantation. The combined endpoint of MACCEs includes death from any cause, myocardial infarction, cardiac tamponade, stroke, urgent or emergent conversion to surgery or balloon valvuloplasty, emergent percutaneous coronary intervention, cardiogenic shock, endocarditis, aortic dissection or major bleeding.
Other clinical endpoints were the presence of symptoms, New York Heart Association (NYHA) class and cardiac function, and valve performance measured with echocardiography.
Statistical analysis
Statistical analysis was performed using SPSS 16.0.1. Descriptive summaries of the distributions of continuous baseline variables are presented in terms of frequencies and percentages. Categorical variables are presented as frequencies and compared by a binomial test or a Fisher's exact probability test. Continuous variables are presented as mean ± standard deviation (SD). A paired Student's t test for within-group comparison of continuous variables was used. Values of p<0.05 were considered statistically significant.
Results
Patient population
Between October 2007 and April 2009, 30 patients (15 men, 15 women; mean age 80.5 years; range 55 to 89 years) underwent a PAVI. Baseline patient characteristics are shown in table 1.
Table 1.
Baseline patient characteristics (n=30).
| Male gender, n (%) | 15 (50.0) |
| Age, years, mean ± SD | 80.5±7.7 |
| Hypertension, n (%) | 17 (56.7) |
| Diabetes mellitus, n (%) | 11 (36.7) |
| Chronic renal insufficiency,a n (%) | 10 (33.3) |
| Peripheral vascular disease,b n (%) | 5 (16.7) |
| Coronary artery disease, n (%) | 13 (43.3) |
| Congestive heart failure, n (%) | 10 (33.3) |
| Prior myocardial infarction, n (%) | 2 (6.7) |
| Prior stroke, n (%) | 2 (6.7) |
| Prior bypass graft surgery, n (%) | 3 (10.0) |
| Prior percutaneous coronary intervention, n (%) | 7 (23.3) |
| Chronic atrial fibrillation, n (%) | 12 (40.0) |
| Chronic pulmonary disease,c n (%) | 6 (20.0) |
| Pulmonary hypertension,d n (%) | 23 (76.7) |
| Permanent pacemaker, n (%) | 2 (6.7) |
| NYHA class, n (%) | |
| - I | 0 (0.0) |
| - II | 7 (23.3) |
| - III | 13 (43.3) |
| - IV | 10 (33.3) |
| Left ventricular function, n (%) | |
| - Poor | 2 (6.7) |
| - Moderate | 5 (16.7) |
| - Good | 23 (76.7) |
| Logistic EuroSCORE, mean ± SD | 15±10 |
| STS Risk score, mean ± SD | 5.2±2.9 |
| Peak pressure gradient, mm Hg, mean ± SD | 79±25 |
| Mean pressure gradient, mm Hg, mean ± SD | 52±20 |
| Aortic valve area, cm2, mean ± SD | 0.71±0.19 |
a Renal insufficiency=estimated glomerular filtration rate <60 using the four-variable modified diet in renal disease (MDRD) equation : eGFR (ml/min/1.73 m2) = 32788 × serum creatinine −1.154 × age −0.203 × 0.742 [if the patient is female] × 1.210 [if the patient is black]. Where serum creatinine is in μ;g/dl, and age is in years. b Peripheral vascular disease is defined by a history of symptomatic claudication, previous or planned intervention on abdominal aorta or limb arteries and/or evident peripheral arterial disease on angiogram. c Chronic pulmonary disease=a history of respiratory problems associated with maintenance inhaled bronchodilator therapy. d Pulmonary hypertension=pulmonary artery systolic pressure >30 mmHg.
All patients had a severe symptomatic AS with an echocardiographic peak transvalvular aortic gradient of 79±25 mmHg, a mean gradient of 52±20 mmHg and a mean calculated aortic valve area of 0.71±0.19 cm2. Twenty-three patients were in NYHA functional class III or IV. The predicted inhospital mortality rates were 15±10% according to the logistic EuroSCORE and 5.2±2.9% according to the Society of Thoracic Surgeons (STS) score in case of cardiac surgery. Table 2 shows the comorbidity and contraindications for heart surgery of the individual patients.
Table 2.
Patient comorbidities and contraindications for surgery.
| Patient | Gender | Age | Rejected for surgery | Comorbidity | Logistic EuroScore | STS risk |
|---|---|---|---|---|---|---|
| 1 | M | 76 | Yes | Prior CABG; PVD | 20.0 | 6.2 |
| 2 | F | 85 | No | CAD | 10.7 | 3.3 |
| 3 | M | 70 | Yes | Pulmonary fibrosis (FEV1: 47%), renal failure, obesity | 5.0 | 4.6 |
| 4 | M | 76 | Yes | Prior CABG; poor LVF; 3VD; TMLR; PVD; renal failure | 42.7 | 5.8 |
| 5 | M | 75 | Yes | Severe COPD (FEV1: 33%) | 16.4 | 3.3 |
| 6 | F | 81 | Yes | Prior stroke, obesity (BMI > 30 kg/m2) | 8.4 | 9.0 |
| 7 | M | 84 | No | SSS with cRBBB | 7.5 | 1.8 |
| 8 | M | 81 | Yes | Pectus excavatum | 6.2 | 1.9 |
| 9 | F | 77 | Yes | Severe COPD (FEV1: 37%) | 10.4 | 8.4 |
| 10 | F | 88 | Yes | DDD pacemaker (bradycardia) | 12.8 | 5.5 |
| 11 | F | 74 | Yes | Previous MVR (mechanical), moderate COPD (FEV1: 64%) | 35.7 | 5.4 |
| 12 | M | 76 | Yes | Severe COPD (FEV1: 23%) | 7.2 | 3.7 |
| 13 | M | 78 | Yes | Prior CABG, porcelain aorta | 24.1 | 3.0 |
| 14 | F | 87 | No | No important comorbidity | 17.3 | 4.8 |
| 15 | F | 84 | Yes | Obesity (BMI > 30 kg/m2) | 19.5 | 3.6 |
| 16 | F | 87 | Yes | Bifascicular block | 12.1 | 4.9 |
| 17 | M | 86 | Yes | Prior stroke, anaemia | 13.2 | 2.2 |
| 18 | F | 84 | No | Renal failure | 10.1 | 4.1 |
| 19 | M | 88 | Yes | Renal failure | 16.9 | 7.6 |
| 20 | M | 85 | No | Moderate LVF, PVD | 11.6 | 8.8 |
| 21 | F | 89 | Yes | Prior chest radiation | 13.6 | 7.0 |
| 22 | F | 82 | No | No important comorbidity | 9.0 | 3.8 |
| 23 | M | 72 | Yes | Poor LVF, PVD | 9.8 | 3.9 |
| 24 | F | 55 | Yes | Obesity (BMI=45 kg/m2) | 2.1 | 1.5 |
| 25 | M | 67 | Yes | Severe LVH, T-cell lymphoma | 2.5 | 9.8 |
| 26 | F | 89 | No | Mild COPD | 13.6 | 4.7 |
| 27 | F | 87 | No | No important comorbidity | 12.1 | 3.8 |
| 28 | M | 85 | Yes | Severe cachexia | 8.0 | 3.5 |
| 29 | F | 77 | No | Mild COPD | 16.9 | 4.7 |
| 30 | F | 88 | Yes | Moderate LVF, PVD | 45.8 | 15.1 |
STS=Society of Thoracic Surgeons, CABG=coronary artery bypass grafting, PVD=peripheral vascular disease, CAD=coronary artery diesase, LVF=left ventricular function, 3VD=trivascular coronary artery disease, TMLR=mitral valve replacement, COPD=chronic obstructive pulmonary disease, BMI=body mass index, SSS=sick sinus syndrome, cRBBB=complete right bundle branch block, MVR=mitral valve replacement, LVH=left ventricular hypertrophy.
Clinical outcomes
Procedural data (table 3): Device success was achieved in all 30 PAVI patients. Acute procedural success rate was 90% (27 patients), due to MACCEs in three patients within 48 hours after device implantation (described hereafter).
Table 3.
Procedural data and 30-day MACCEs and outcome (n=30).
| Device success, n (%) | 30 (100.0) |
| Acute procedural success, n (%) | 27 (90.0) |
| Predilatation balloon diameter, mm, mean±SD | 22.9±2.4 |
| Median procedure time, min, mean±SD | 90±29 |
| Postdilatation, n (%) | 2 (6.7) |
| MACCEs within 30 days, n (%) | |
| - Death | 6 (20.0)a-f |
| - Major arrhythmia | 0 (0.0) |
| - Myocardial infarction | 0 (0.0) |
| - Cardiac tamponade | 2 (6.7)d,g |
| - Cardiogenic shock | 1 (3.3) b |
| - Respiratory failure | 3 (10.0)a,c,f |
| - Stroke | 0 (0.0) |
| - Conversion to surgery | 0 (0.0) |
| - Conversion to valvuloplasty | 0 (0.0) |
| - Emergent PCI | 0 (0.0) |
| - Endocarditis | 0 (0.0) |
| - Aortic dissection | 0 (0.0) |
| - Major bleeding | 1 (3.3) b |
| Other events within 30 days, n (%) | |
| - Bradyarrhythmia | 9 (30.0) |
| - New permanent pacemaker | 7 (23.3) |
| - New left bundle branch block | 18 (60.0) |
| Median duration of admission, days, mean±SD | |
| - Intensive care unit | 2±6 |
| - Hospital | 10±6 |
MACCEs=major adverse cardiovascular and cerebral events, PCI=per-cutaneous coronary intervention. a Patient no. 3, b Patient no. 4, c Patient no. 9, d Patient no. 16, e Patient no. 21, f Patient no. 25, g Patient no. 2.
Thirty-day mortality (table 3): At 30-day follow-up, the mortality rate was 20% (6 patients), which included one cardiovascular death and five non-cardiovascular deaths. The cardiovascular death involved a 76-yearold man (patient 4) with a prior CABG, a poor LV function, three-vessel coronary artery disease with only one functioning jump-graft, severe peripheral arterial disease and renal insufficiency, who was therefore rejected for surgery. During the procedure the patient developed a retroperitoneal haematoma after injury of the right iliac artery, which was treated with a covered stent and blood transfusion. A few hours after PAVI he died, however, of hypovolaemic and cardiogenic shock on the intensive care unit (ICU). Autopsy showed extensive myocardial fibrosis due to previous transmyocardial laser therapy and multiple myocardial infarctions. The cause of death was deemed to be acute heart failure due to hypotension caused by bleeding in a patient with a preprocedural poor left ventricular function. The valve position was good with no obstruction of the coronary ostia or venous bypass graft ostium.
Of the five non-cardiovascular related deaths, two patients (patients 3 and 9) died of respiratory failure due to an exacerbation of their pre-existent chronic pulmonary disease (severe pulmonary fibrosis and severe COPD, respectively). The other non-cardiovascular deaths involved three patients (no. 16, 21 and 25) who died of infectious complications: one patient died one week after PAVI on the ICU as a direct consequence of sepsis, probably caused by an infection of the central venous line or external pacemaker wire. Two patients died eventually of an aspiration pneumonia, one week and three weeks after PAVI, respectively. Both patients were in a poor clinical condition, due to renal failure in one patient and severe left ventricular hypertrophy with obliteration and advanced stage T-cell lymphoma in the other patient. No autopsy was performed in the three patients who died of infectious causes.
Other 30-day MACCEs (table 3): An 85-year-old lady (patient 2) developed a cardiac tamponade one day after PAVI caused by an incorrect removal of the right ventricular external pacemaker wire. This was initially treated with pericardial drainage but required surgical repair. She recovered uneventfully and resumed her former activities.
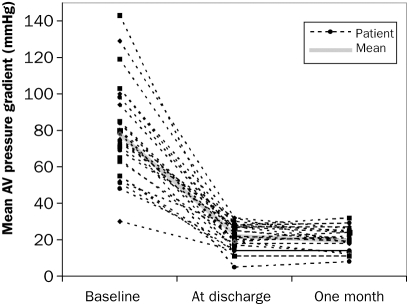
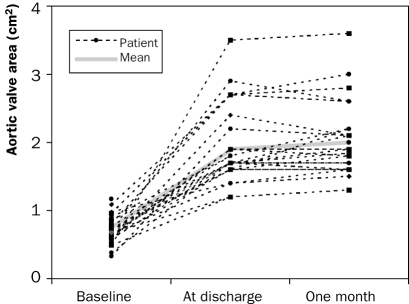
Echocardiographic evaluation (table 4): The peak transvalvular aortic pressure from the patients who were alive after 30 days decreased from 76±24 mmHg preprocedurally to 22±7 mmHg (n=24, <0.00001) a few days after the procedure (figure 2) and the aortic valve area increased from 0.69±0.18 cm2 to 2.0±0.6 cm2 (n=24, p<0.00001; figure 3).
Table 4.
Postprocedure haemodynamic valve performance in patients with immediate procedural success (see also figures 2 and 3).
| Before implantation (n=30) | At discharge (n=24) | At 30-day follow-up (n=24) | |
|---|---|---|---|
| Peak pressure gradient, mmHg, mean±SD | 79±25 | 22±7 | 19±7 |
| Mean pressure gradient, mmHg, mean±SD | 52±20 | 13±5 | 11±4 |
| Aortic valve area, cm2, mean±SD | 0.71±0.19 | 2.0±0.6 | 2.0±0.6 |
| Aortic regurgitation | |||
| - None | 9 | 2 | 1 |
| - Mild | 17 | 14 | 15 |
| - Moderate | 4 | 8 | 8 |
| - Severe | 0 | 0 | 0 |
Figure 2.
Improvements in aortic valve mean pressure before and after percutaneous aortic valve implantation (n=30).
Figure 3.
Improvements in aortic valve area before and after percutaneous aortic valve implantation (n=30).
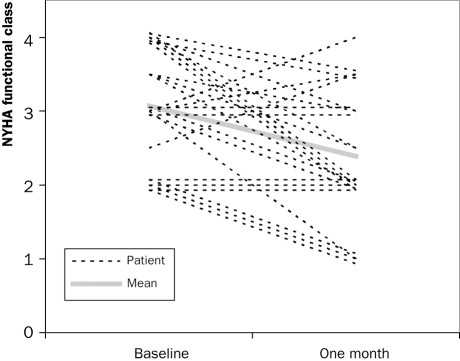
Other clinical outcomes: Of the 24 patients who were alive after 30-day follow-up, 23 were clinically improved, as partially expressed by the improvement in NYHA class (figure 4). One patient, who was treated with PAVI via a subclavian access route, did not improve clinically after the procedure, even though postprocedural echocardiography showed a good function of the prosthetic valve.
Figure 4.
Improvements in New York Heart Association (NYHA) functional class before and one month after percutaneous aortic valve implantation (n=24, p=0.002).
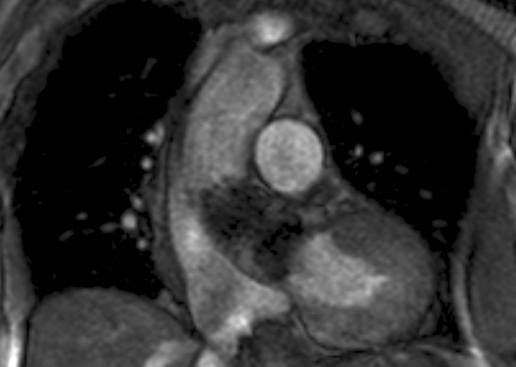
None of the surviving 24 patients had any major adverse events within 30-day follow-up after PAVI. Repeat echocardiography one month after discharge showed the device in a stable position with sustained performance of the prosthetic valve compared with the immediate postprocedural echo. Figure 5 shows the stable position of the CoreValve prosthesis on a cardiac MRI, performed in one of the patients six months after PAVI.
Figure 5.
Coronal MRI image of the heart with the CoreValve prosthesis in situ, six months after implantation (patient no. 7).
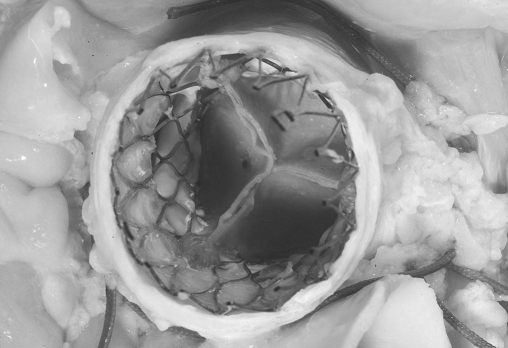
Postmortem device assessment: Autopsy was performed in three deceased patients and showed a good position of the prosthetic valve device and no device-related complications as cause of the death (figure 6). All coronary and bypass graft ostia were patent and no structural damages were observed.
Figure 6.
Postmortem with a caudal view of the aortic root and the CoreValve prosthesis in situ (patient no. 9).
Discussion
This study shows that percutaneous aortic valve implantation performed in patients with a high risk for conventional treatment is feasible in our centre. Direct device success was achieved in all patients. Proper and fixed device position without obstruction of coronary ostia was reached in all patients directly after implantation, demonstrated by means of angiography. This was confirmed in the postmortem assessments of three patients. Maintenance of a stable device position at 30 days after PAVI was shown in the other 24 patients by means of transthoracic echocardiography. Feasibility of PAVI has also been demonstrated by the instantaneous improvement of aortic valve performance with a marked reduction of the transvalvular pressure gradient, which sustained after 30-day follow-up. Haemodynamic improvement translated in the relief of symptoms after PAVI in at least 18 of the 24 patients at 30-day follow-up.
The postprocedural 30-day mortality rate of 20% (6 patients) is considerably higher than the predicted mortality rate according to the logistic EuroSCORE. However, there was only one cardiovascular death, which involved an extremely high-risk patient (patient 4). The other five deaths, which were not cardiovascular, occurred between one week and one month after the procedure and were caused by an exacerbation of pre-existing pulmonary disease and infectious complications. It is of importance to note that all six deceased patients were highly symptomatic, had a poor prognosis and were declined for conventional aortic valve replacement.
Patient characteristics, device success and acute procedural success rates and occurrences of postprocedural MACCEs of our study are comparable with those reported by Grube et al. and Webb et al.4,5,7
An important lesson that can be learned from this study is that the mortality risk of PAVI is substantial in patients with comorbidity who do not tolerate general anaesthesia or temporary hypotension. A critical patient selection, in which appropriate risk stratification and proper outweighing of the expected benefits against the risks of PAVI is essential, and may reduce the mortality rate.
The decision to perform PAVI as a ‘last resort treatment’ on this high-risk patient group should only be made after the risks of the procedure have been properly discussed with and accepted by the patient. Performing PAVI in the six patients who died in the 30-day follow-up period of this study had been a wellconsidered choice of both patient and relatives.
Another lesson from this study is that PAVIs can be performed with good results in patients who are not rejected for surgery and/or have an intermediate risk for surgical treatment. Such an approach would expand the indication to perform PAVI in patients with lower risks for a surgical valve replacement, i.e. patients, specifically at older age who prefer a less invasive treatment.
The costs of these novel treatment techniques are mainly determined by the high cost of the new devices. However, the shorter ICU and hospital stay as well as the shorter rehabilitation may eventually result in a more cost-effective treatment.
Conclusion
We report the successful initiation of our percutaneous aortic valve implantation programme. Before initiation we drew up a specific protocol, in which the feasibility and safety parameters were defined. Feasibility and safety of this procedure by means of the CoreValve self-expandable device is shown by the high rates of direct and acute device success and a direct and longterm haemodynamic improvement in the majority of the patients. However, the postprocedural mortality and morbidity rates remain high in patients who have severe comorbidities. When successful, PAVI can reduce symptoms and improve quality of life in patients with severe symptomatic aortic stenosis who are considered at high risk for conventional aortic valve surgery.
Acknowledgments
The authors acknowledge our nursing staff of the cardiac catheterisation laboratory for their skilled assistance, especially W.B.J. Zwiers, RN, M.S. Gorlewska, RN, L. Venckus-Venckiene, RN, W.J. Rohling, RN, and S. van Gilst, RN.
References
- 1.Bouma BJ, van Den Brink RB, van Der Meulen JH, Verheul HA, Cheriex EC, Hamer HP, et al. To operate or not on elderly patients with aortic stenosis: the decision and its consequences. Heart. 1999;82:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–43. [DOI] [PubMed] [Google Scholar]
- 3.Grube E, Laborde JC, Zickmann B, Gerckens U, Felderhoff T, Sauren B, et al. First report on a human percutaneous transluminal implantation of a self-expanding valve prosthesis for interventional treatment of aortic valve stenosis. Catheter Cardiovasc Interv. 2005;66:465–9. [DOI] [PubMed] [Google Scholar]
- 4.Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol. 2007;50:69–76. [DOI] [PubMed] [Google Scholar]
- 5.Webb JG, Pasupati S, Humphries K, Thompson C, Altwegg L, Moss R, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007;116:755–63. [DOI] [PubMed] [Google Scholar]
- 6.Grube E, Laborde JC, Gerckens U, Felderhoff T, Sauren B, Buellesfeld L, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation. 2006;114:1616–24. [DOI] [PubMed] [Google Scholar]
- 7.Bauer F, Eltchaninoff H, Tron C, Lesault PF, Agatiello C, Nercolini D, et al. Acute improvement in global and regional left ventricular systolic function after percutaneous heart valve implantation in patients with symptomatic aortic stenosis. Circulation. 2004;110:1473–6. [DOI] [PubMed] [Google Scholar]