Abstract
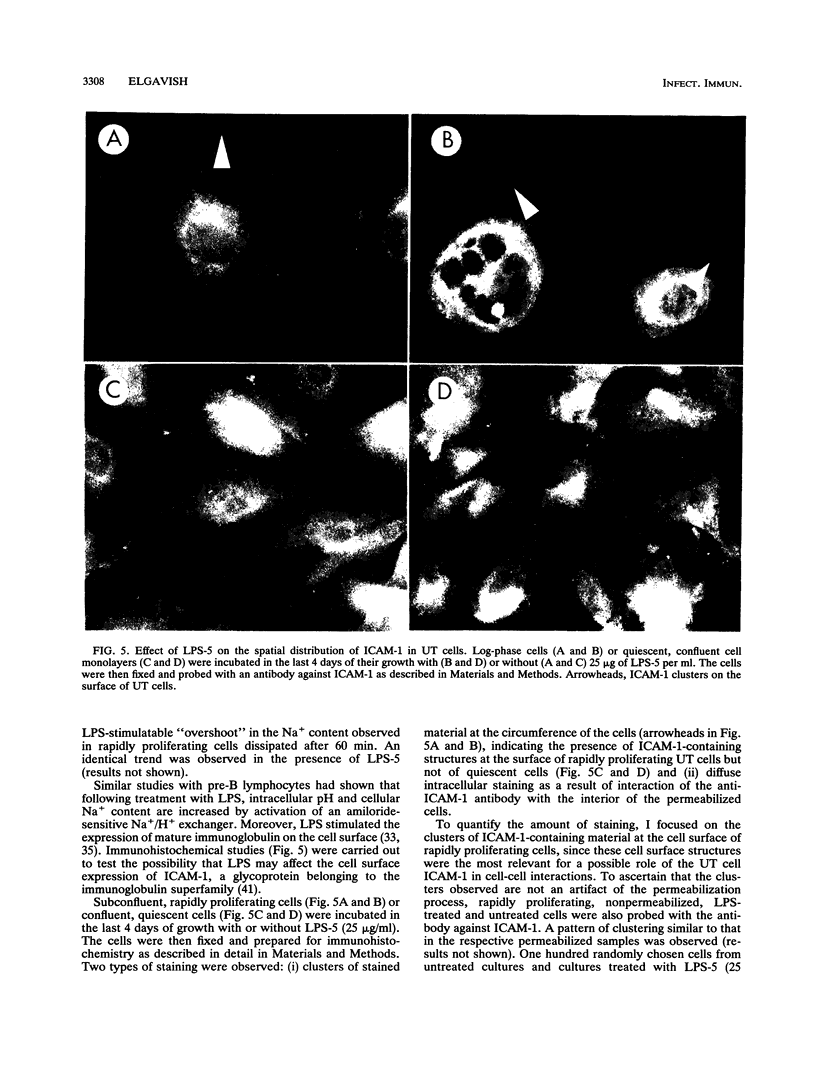
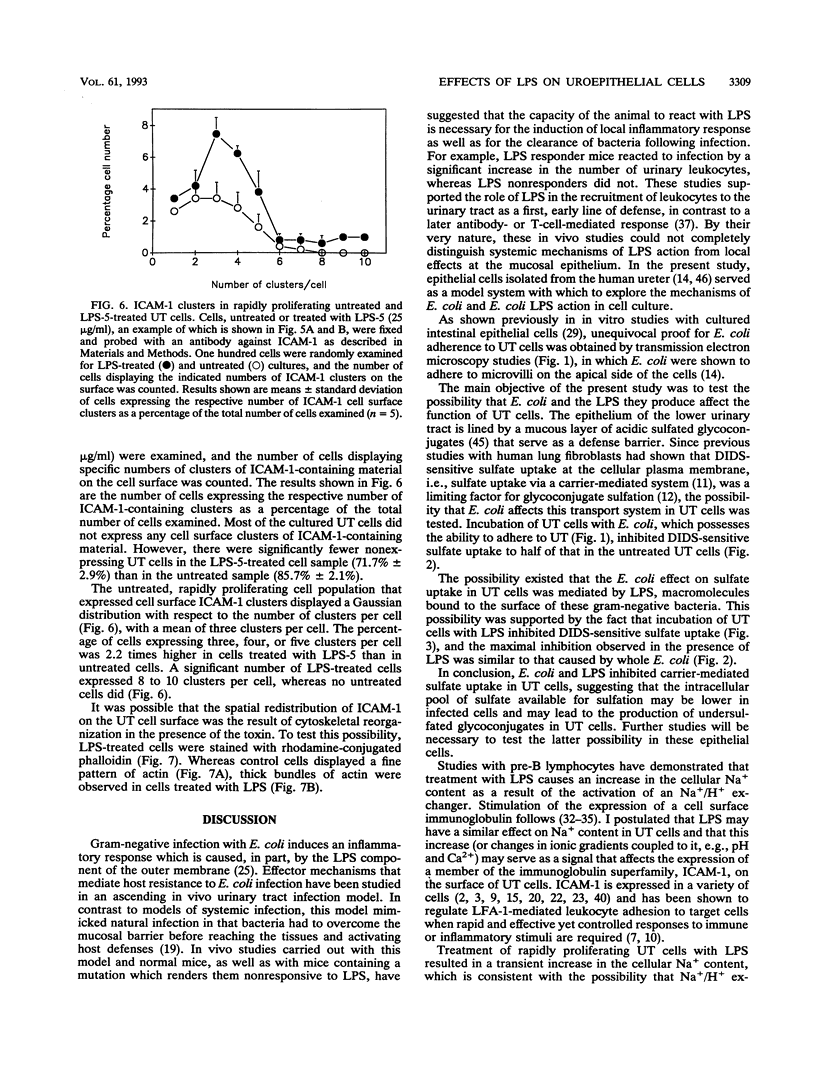
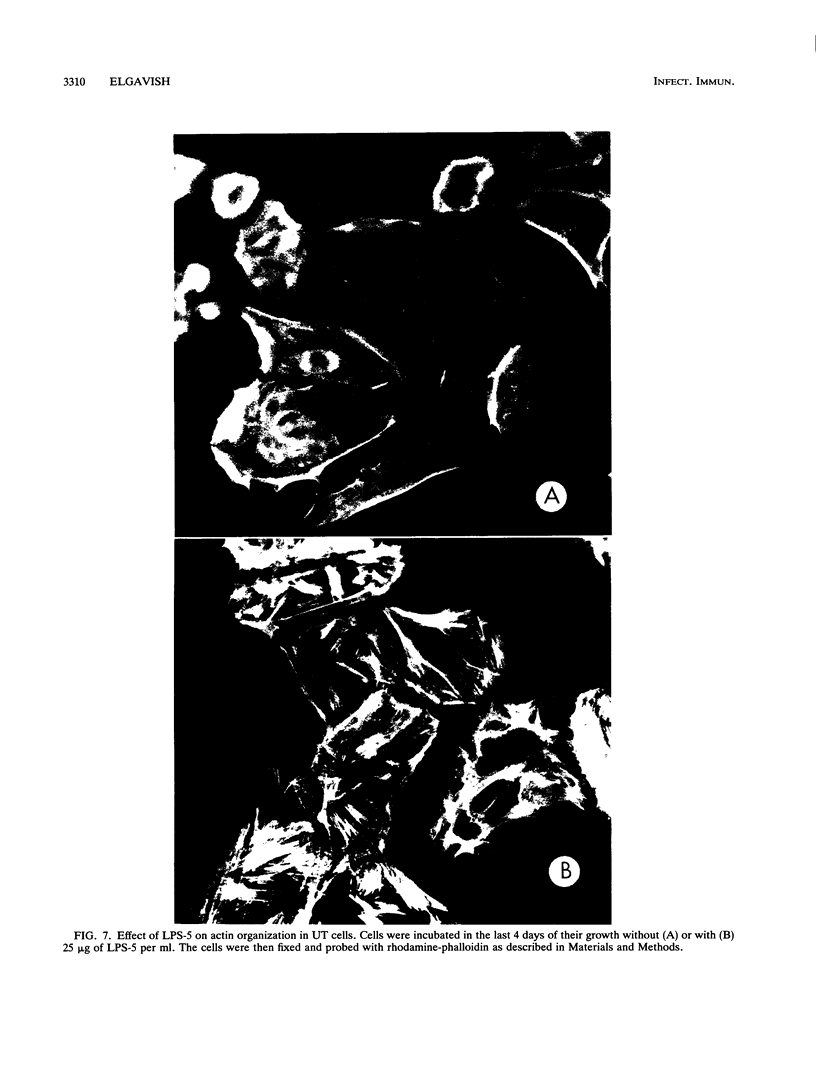
Escherichia coli is the microorganism most commonly isolated from human urinary tract infections. Earlier studies by others have shown that bacterial attachment and production of toxins (e.g., lipopolysaccharides [LPS]) enhance recruitment of leukocytes to the infection site and mucosal inflammation. The mechanisms by which these changes occur have not been completely defined. In the present study, epithelial cell cultures isolated from the human ureter (UT cells) (A. Elgavish, J. J. Wille, F. Rahemtulla, and L. Debro, Am. J. Physiol. 261:C916-C926, 1991; J. J. Wille, J. Park, and A. Elgavish, J. Cell. Physiol. 150:52-58, 1992) served as a model system with which to explore the mechanisms of action of Escherichia coli and E. coli LPS in UT cells. E. coli adhered to UT cells and inhibited carrier-mediated sulfate uptake to half of that in untreated UT cells, suggesting that the intracellular pool of sulfate available for sulfation may be lower in infected cells and may lead to the production of undersulfated glycoconjugates. Incubation of UT cells with E. coli LPS inhibited carrier-mediated sulfate uptake to an extent similar to that caused by whole E. coli, indicating that the effect of E. coli on sulfate uptake may be mediated by LPS. LPS caused an increase in Na+ content in rapidly proliferating UT cells but not in quiescent cells. We postulated that this change in the intracellular ionic environment or changes coupled to it (e.g., pH or Ca2+ levels) may serve as a transducing signal. This possibility was supported by the fact that LPS stimulated clustering of ICAM-1 on the cell surface of rapidly proliferating but not quiescent UT cells. This study suggests that, in vivo, LPS stimulation of ICAM-1 clustering on the surface of the urothelium may allow more effective binding of leukocytes. This may be the mechanism underlying earlier findings in vivo indicating a role for LPS in the recruitment of leukocytes to the urinary tract as a host defense mechanism following urinary tract infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur M., Campanelli C., Arbeit R. D., Kim C., Steinbach S., Johnson C. E., Rubin R. H., Goldstein R. Structure and copy number of gene clusters related to the pap P-adhesin operon of uropathogenic Escherichia coli. Infect Immun. 1989 Feb;57(2):314–321. doi: 10.1128/iai.57.2.314-321.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A. W., Dunn S. M., Fecondo J. V., Culvenor J. G., Dührsen U., Burns G. F., Wawryk S. O. Regulation of expression of a human intercellular adhesion molecule (ICAM-1) during lymphohematopoietic differentiation. Blood. 1989 May 15;73(7):1896–1903. [PubMed] [Google Scholar]
- Boyd A. W., Wawryk S. O., Burns G. F., Fecondo J. V. Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci U S A. 1988 May;85(9):3095–3099. doi: 10.1073/pnas.85.9.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpén O., Pallai P., Staunton D. E., Springer T. A. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J Cell Biol. 1992 Sep;118(5):1223–1234. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Arruda J. C., Williams T. J., Laux D. C. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect Immun. 1985 Apr;48(1):139–145. doi: 10.1128/iai.48.1.139-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean E. A., Kessler R. E. Quantitation of effects of subinhibitory concentrations of trimethoprim on P fimbria expression and in vitro adhesiveness of uropathogenic Escherichia coli. J Clin Microbiol. 1988 Jan;26(1):25–30. doi: 10.1128/jcm.26.1.25-30.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I., Buckle A. M., Hogg N. Early events of the immune response mediated by leukocyte integrins. Immunol Rev. 1990 Apr;114:29–44. doi: 10.1111/j.1600-065x.1990.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Eden C. S., Eriksson B., Hanson L. A. Adhesion of Escherichia coli to human uroepithelial cells in vitro. Infect Immun. 1977 Dec;18(3):767–774. doi: 10.1128/iai.18.3.767-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgavish A., Meezan E. Altered sulfate transport via anion exchange in CFPAC is corrected by retrovirus-mediated CFTR gene transfer. Am J Physiol. 1992 Jul;263(1 Pt 1):C176–C186. doi: 10.1152/ajpcell.1992.263.1.C176. [DOI] [PubMed] [Google Scholar]
- Elgavish A., Meezan E. Sulfate transport in human lung fibroblasts (IMR-90): effect of pH and anions. Am J Physiol. 1989 Mar;256(3 Pt 1):C486–C494. doi: 10.1152/ajpcell.1989.256.3.C486. [DOI] [PubMed] [Google Scholar]
- Elgavish A., Meezan E. Sulfation by human lung fibroblasts: SO4(2-) and sulfur-containing amino acids as sources for macromolecular sulfation. Am J Physiol. 1991 Jun;260(6 Pt 1):L450–L456. doi: 10.1152/ajplung.1991.260.6.L450. [DOI] [PubMed] [Google Scholar]
- Elgavish A., Wille J. J., Rahemtulla F., Debro L. Carrier-mediated sulfate transport in human ureteral epithelial cells cultured in serum-free medium. Am J Physiol. 1991 Nov;261(5 Pt 1):C916–C926. doi: 10.1152/ajpcell.1991.261.5.C916. [DOI] [PubMed] [Google Scholar]
- Elner S. G., Elner V. M., Pavilack M. A., Todd R. F., 3rd, Mayo-Bond L., Franklin W. A., Strieter R. M., Kunkel S. L., Huber A. R. Modulation and function of intercellular adhesion molecule-1 (CD54) on human retinal pigment epithelial cells. Lab Invest. 1992 Feb;66(2):200–211. [PubMed] [Google Scholar]
- Hagberg L., Engberg I., Freter R., Lam J., Olling S., Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983 Apr;40(1):273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E., Prusty D., Frangioni J. V., Cragoe E. J., Jr, Lechene C., Schwartz M. A. Control of intracellular pH and growth by fibronectin in capillary endothelial cells. J Cell Biol. 1990 May;110(5):1803–1811. doi: 10.1083/jcb.110.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevnikar A. M., Wuthrich R. P., Takei F., Xu H. W., Brennan D. C., Glimcher L. H., Rubin-Kelley V. E. Differing regulation and function of ICAM-1 and class II antigens on renal tubular cells. Kidney Int. 1990 Sep;38(3):417–425. doi: 10.1038/ki.1990.221. [DOI] [PubMed] [Google Scholar]
- Kaiserlian D., Rigal D., Abello J., Revillard J. P. Expression, function and regulation of the intercellular adhesion molecule-1 (ICAM-1) on human intestinal epithelial cell lines. Eur J Immunol. 1991 Oct;21(10):2415–2421. doi: 10.1002/eji.1830211018. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Linder H., Engberg I., Baltzer I. M., Jann K., Svanborg-Edén C. Induction of inflammation by Escherichia coli on the mucosal level: requirement for adherence and endotoxin. Infect Immun. 1988 May;56(5):1309–1313. doi: 10.1128/iai.56.5.1309-1313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Gugel E. A., Dustin M. L., Springer T. A., Shaw S. Functional evidence that intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1-dependent adhesion in T cell-mediated cytotoxicity. Eur J Immunol. 1988 Apr;18(4):637–640. doi: 10.1002/eji.1830180423. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Neeser J. R., Chambaz A., Golliard M., Link-Amster H., Fryder V., Kolodziejczyk E. Adhesion of colonization factor antigen II-positive enterotoxigenic Escherichia coli strains to human enterocytelike differentiated HT-29 cells: a basis for host-pathogen interactions in the gut. Infect Immun. 1989 Dec;57(12):3727–3734. doi: 10.1128/iai.57.12.3727-3734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Reznikoff C. A., Johnson M. D., Norback D. H., Bryan G. T. Growth and characterization of normal human urothelium in vitro. In Vitro. 1983 Apr;19(4):326–343. doi: 10.1007/BF02619511. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Cantley L. C. Increasing the intracellular Na+ concentration induces differentiation in a pre-B lymphocyte cell line. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7547–7550. doi: 10.1073/pnas.80.24.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff P. M., Cantley L. C. Lipopolysaccharide and phorbol esters induce differentiation but have opposite effects on phosphatidylinositol turnover and Ca2+ mobilization in 70Z/3 pre-B lymphocytes. J Biol Chem. 1985 Aug 5;260(16):9209–9215. [PubMed] [Google Scholar]
- Rosoff P. M., Stein L. F., Cantley L. C. Phorbol esters induce differentiation in a pre-B-lymphocyte cell line by enhancing Na+/H+ exchange. J Biol Chem. 1984 Jun 10;259(11):7056–7060. [PubMed] [Google Scholar]
- Schwartz M. A., Ingber D. E., Lawrence M., Springer T. A., Lechene C. Multiple integrins share the ability to induce elevation of intracellular pH. Exp Cell Res. 1991 Aug;195(2):533–535. doi: 10.1016/0014-4827(91)90407-l. [DOI] [PubMed] [Google Scholar]
- Shahin R. D., Engberg I., Hagberg L., Svanborg Edén C. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J Immunol. 1987 May 15;138(10):3475–3480. [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Roles of adhesion molecules in T-cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990 Apr;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Ham R. G. Improved medium and culture conditions for clonal growth with minimal serum protein and for enhanced serum-free survival of Swiss 3T3 cells. In Vitro. 1981 Aug;17(8):656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]
- Singer K. H., Denning S. M., Whichard L. P., Haynes B. F. Thymocyte LFA-1 and thymic epithelial cell ICAM-1 molecules mediate binding of activated human thymocytes to thymic epithelial cells. J Immunol. 1990 Apr 15;144(8):2931–2939. [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Svanborg Edén C., Hagberg L., Hanson L. A., Hull S., Hull R., Jodal U., Leffler H., Lomberg H., Straube E. Bacterial adherence--a pathogenetic mechanism in urinary tract infections caused by Escherichia coli. Prog Allergy. 1983;33:175–188. [PubMed] [Google Scholar]
- Tosi M. F., Stark J. M., Smith C. W., Hamedani A., Gruenert D. C., Infeld M. D. Induction of ICAM-1 expression on human airway epithelial cells by inflammatory cytokines: effects on neutrophil-epithelial cell adhesion. Am J Respir Cell Mol Biol. 1992 Aug;7(2):214–221. doi: 10.1165/ajrcmb/7.2.214. [DOI] [PubMed] [Google Scholar]
- Wille J. J., Park J., Elgavish A. Effects of growth factors, hormones, bacterial lipopolysaccharides, and lipotechoic acids on the clonal growth of normal ureteral epithelial cells in serum-free culture. J Cell Physiol. 1992 Jan;150(1):52–58. doi: 10.1002/jcp.1041500108. [DOI] [PubMed] [Google Scholar]
- Woods A., Hök M., Kjellén L., Smith C. G., Rees D. A. Relationship of heparan sulfate proteoglycans to the cytoskeleton and extracellular matrix of cultured fibroblasts. J Cell Biol. 1984 Nov;99(5):1743–1753. doi: 10.1083/jcb.99.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]