Abstract
Sickle cell disease (SCD) is characterized by intravascular hemolysis and inflammation coupled to a 400-fold greater incidence of invasive pneumococcal infection resulting in fulminant, lethal pneumococcal sepsis. Mechanistically, invasive infection is facilitated by a proinflammatory state that enhances receptor-mediated endocytosis of pneumococci into epithelial and endothelial cells. As statins reduce chronic inflammation, in addition to their serum cholesterol-lowering effects, we hypothesized that statin therapy might improve the outcome of pneumococcal infection in SCD. In this study, we tested this hypothesis in an experimental SCD mouse model and found that statin therapy prolonged survival following pneumococcal challenge. The protective effect resulted in part from decreased platelet-activating factor receptor expression on endothelia and epithelia, which led to reduced bacterial invasion. An additional protective effect resulted from inhibition of host cell lysis by pneumococcal cholesterol-dependent cytotoxins (CDCs), including pneumolysin. We conclude therefore that statins may be of prophylactic benefit against invasive pneumococcal disease in patients with SCD and, more broadly, in settings of bacterial pathogenesis driven by receptor-mediated endocytosis and the CDC class of toxins produced by Gram-positive invasive bacteria.
Introduction
Sickle cell disease (SCD) is the most common genetic disorder worldwide, with an estimated 300,000 affected individuals born each year (1). SCD causes a chronic hemolytic anemia characterized by distorted erythrocytes that aggregate and occlude blood flow in the microvasculature. Individuals with SCD experience a wide spectrum of vascular complications, including vaso-occlusive crisis, stroke, and hyposplenism. In addition, children with SCD have a 400-fold greater risk of fulminant, lethal pneumococcal sepsis than their healthy peers or patients with other hemolytic anemias (2). This invasive disease rate greatly exceeds the 2- to 3-fold increased risk of sepsis from other encapsulated bacteria in SCD, suggesting a unique vulnerability to the pathogenic mechanisms of pneumococci in particular.
The pneumococcus is the single most frequent cause of lethal pneumonia in children worldwide (3). The bacteria is carried in the nasopharynx by approximately 20% of children at any time, a rate that is unchanged in children with SCD (4). Invasive pneumococcal disease develops during pneumonia when inflammation promotes receptor-mediated translocation of bacteria from alveoli into the bloodstream. This invasive process involves 2 steps. First, pneumococci adhere to host cells by interactions mediated by adhesins such as bacterial CbpA (5). Subsequent to adherence, bacteria bind by surface phosphorylcholine to platelet-activating factor receptor (PAFr) and cross the host cell by receptor-mediated endocytosis (6). Preexisting inflammation accelerates both adherence and invasion as host receptor expression is positively regulated by inflammation (7), a setting recapitulated in the mouse model of SCD (8–10). To mitigate the very high risk of sepsis in SCD, young children are treated prophylactically with penicillin (11). However, the emergence of resistant strains underscores the importance of identifying additional preventive options (12). We hypothesized that the antiinflammatory activity of statins could be used prophylactically to decrease baseline cellular activation in the SCD lung and vasculature and potentially mitigate bacterial invasion.
Statins, 3-hydroxy-3-methylglutaryl CoA reductase inhibitors, are among the most widely prescribed drugs in the world and are used to treat elevated levels of cholesterol and heart disease. Statins inhibit the synthesis of cellular cholesterol, resulting in a compensatory increase in cholesterol uptake by cells and concomitant decrease in plasma cholesterol (13). Statins also have potent antiinflammatory properties that are independent of their lipid-lowering ability and are suggested to be of benefit in the setting of sepsis (14–18). Currently, it is believed that statins inhibit lipid raft formation and prenylation of signaling molecules, thereby disrupting cellular signaling networks (17). Furthermore, statins have been shown to reduce inflammation in response to lipopolysaccharide (19). The potential protective effect of statins during bacterial respiratory infections has been suggested (20, 21), a setting in which the inflammatory status of the host is an important aspect of pathogenesis and disease progression.
Using a mouse model of SCD, we tested whether the antiinflammatory properties of statins could be applied to SCD to confer protection against pneumococcal challenge. Herein, we demonstrate that statin therapy reduced bacterial adherence and invasion of host cells in association with decreased activation-induced expression of host receptors. Additionally, statins protected host cells from the cytotoxic effects of the cholesterol-dependent pneumococcal toxin pneumolysin.
Results
Simvastatin improves survival in SCD mice.
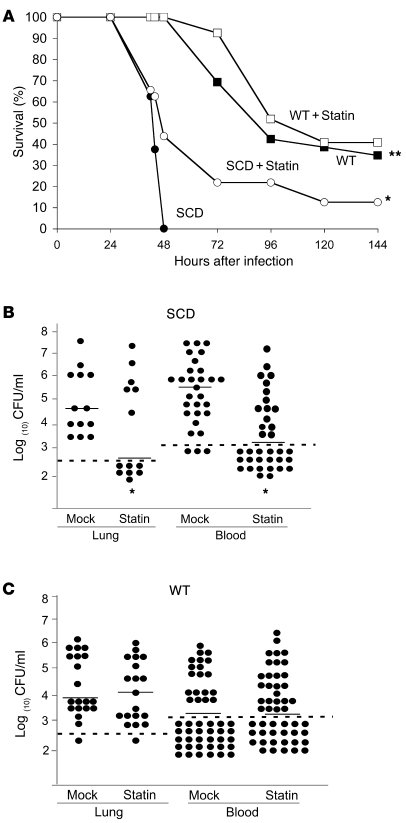
The development of transgenic knockout mice that exclusively express human sickle hemoglobin has greatly enhanced the understanding of SCD (22). Transplantation of bone marrow from sickle transgenic mice effectively recapitulates the manifestations of SCD, including erythrocytic sickling, multiorgan infarcts, anemia, vascular inflammation, and heightened white blood cell counts. Similar to children with SCD, the transplant mouse model of SCD showed heightened mortality following pneumococcal challenge (9). Consistent with these previous results, all SCD mice died within 48 hours of pneumococcal challenge of the respiratory tract, whereas there were no deaths in the WT group; 40% of WT animals showed long-term survival extending beyond the 6-day duration of the study (Figure 1A). To determine the effect of statins on disease severity, both WT and SCD mice were treated with simvastatin daily for 5 days prior to bacterial challenge. Treatment with simvastatin had no significant effect on the survival of infected WT mice (Figure 1A). In contrast, the SCD mice receiving simvastatin showed a significantly delayed time to death (P = 0.023) compared with SCD mice receiving carrier only (Figure 1A). These data indicated that prophylactic statin treatment prolonged survival in the face of invasive pneumococcal disease in SCD mice.
Figure 1. Impact of statin therapy on survival of pneumococcal infection in SCD mice.
(A) Survival of WT versus SCD mice receiving mock treatment (n = 26 and n = 24, respectively) or simvastatin (n = 27 and n = 32, respectively) following intranasal challenge with pneumococci; *P = 0.023 versus SCD, **P = 0.0001 versus SCD. (B) Blood and lung bacterial titers at 24 hours after challenge of SCD mice receiving mock or simvastatin treatment. (C) Blood and lung bacterial titers at 24 hours after challenge of WT mice receiving mock or simvastatin treatment. Each symbol represents an individual mouse; horizontal lines indicate the mean; dashed lines indicate limit of detection. *P < 0.01, mock versus simvastatin; Mann-Whitney U test.
The intranasal challenge model of infection mimics the course of human disease, including colonization of the nasal passages, aspiration of bacteria into the lungs, development of pneumonia, and translocation of bacteria from the lungs into the bloodstream, resulting in bacteremia, sepsis, and ultimately death. To further understand at what stage in the disease statin intervention was active, we determined bacterial burden in the lungs and blood following intranasal challenge. Statin treatment significantly decreased the amount of bacteria in the lungs and blood at 24 hours after infection (P = 0.0024 and P = 0.0001 respectively, Figure 1B). Moreover, in more than half of the animals, statin treatment completely blocked the appearance of bacteria in the bloodstream at 24 hours (Figure 1B). This protection was limited to SCD mice, as no significant difference in recoverable bacteria in the lungs or blood was observed in WT mice receiving simvastatin (Figure 1C). One possible explanation for the difference between WT and SCD mice is that the statins may have a more pronounced protective effect in models with significant preexisting airway or vascular inflammation such as SCD (23). This is in agreement with recent clinical data indicating that prior statin use leads to improved outcome in community-acquired pneumonia particularly in the setting of underlying chronic pulmonary inflammation (20).
Effect of statins on severity of pneumonia.
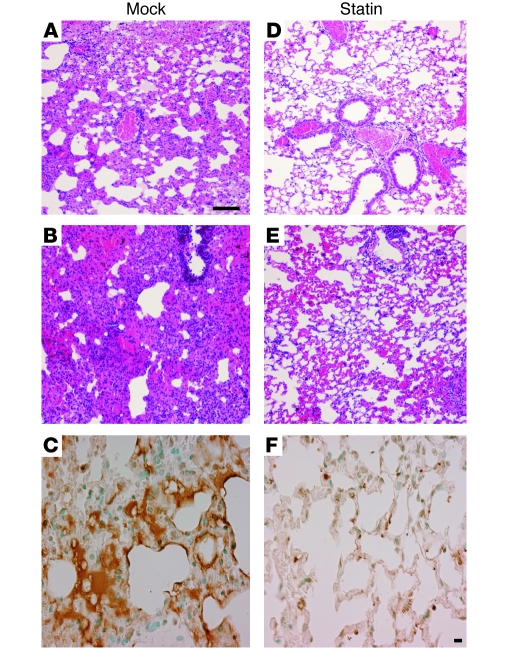
The effect of statin treatment on lung pathology was examined in greater detail at 18 hours after infection, the time point at which bacteria began to enter the bloodstream in mock-treated animals. Examination of lung sections revealed a marked reduction in lung consolidation in statin-treated SCD animals when compared with the mock-treated SCD group (Figure 2). Lungs of mock-treated animals exhibited a spectrum of moderate to severe pneumonia. For example, moderate cases showed widespread areas of leukocyte infiltration into alveoli and interstitial spaces accompanied by extensive thickening or obliteration of alveolar walls (Figure 2A), while severe cases showed dense consolidation with intense leukocyte infiltration and intra-alveolar hemorrhage resulting in red and gray hepatization (Figure 2B). In contrast, lungs of statin-treated mice demonstrated only mild pneumonitis, with minimal perivascular leukocyte infiltration and either normal alveolar architecture or patchy, mild thickening of the alveolar walls (Figure 2, D and E). To confirm these observations, lung pathology was blindly scored from 1 (mild) to 5 (severe), with at least 6 representative images examined from 6 mice per group. The statin-treated group had a mean score of 2.4 ± 0.2, whereas the untreated group had a significantly higher pathology index of 4.0 ± 0.5 (P = 0.0034). Lung sections were also examined for apoptosis using TUNEL staining. A high degree of apoptosis was observed in the mock-treated group (Figure 2C), whereas the statin-treated lungs showed only an occasional, more localized apoptotic response (Figure 2F). Thus, the degree of apoptosis paralleled the severity of histopathology, with the mice having the most severe pneumonia showing the highest degree of apoptosis.
Figure 2. Impact of statin therapy on histopathology of pneumonia in SCD mice.
SCD mice were pretreated daily for 5 days with vehicle control (A–C) or simvastatin (D–F) and then challenged intranasally with pneumococci. Lungs were harvested at 18 hours for histopathology (H&E staining: A, B, D, and E) or apoptosis (TUNEL: C and F). See text for full description; sections are representative of at least 6 mice per group. Scale bars: 100 μM.
Individuals with SCD have been shown to have increased levels of proinflammatory cytokines in the blood (10, 24, 25), and levels of proinflammatory cytokines are reduced in statin-treated patients with bacterial infections (26). In uninfected SCD mice, lung levels of IL-6 but not TNF-α, IL-1β, IL-10, IFN-γ, or IL-12 were elevated, and statin therapy decreased IL-6 from 34 ± 9.1 pg/ml to 7.0 ± 5.4 pg/ml (P = 0.046). Six hours after intratracheal challenge with nonreplicating bacteria, TNF-α increased in the lungs to 321 ± 215 pg/ml in untreated SCD mice, a change that was attenuated in statin-treated SCD mice to 154 ± 97 pg/ml (P = 0.03). No other cytokines (IL-1β, IL-10, IFN-γ, IL-12, IL-6, or IL-17) showed significant decrease with statin treatment in the infected SCD mice (data not shown). Collectively, these data suggest that significant amelioration of lung pathology in statin-treated SCD occurs with relatively modest changes in cytokines, suggesting that the statins may afford benefit through other non-antiinflammatory mechanisms.
Effect of statins on bacterial-host cell interactions.
The progression of disease from pneumonia to sepsis requires that Streptococcus pneumoniae interact with host cells, particularly those of the pulmonary epithelium and the vascular endothelium. Invasion into host cells occurs when bacteria bind to PAFr and are engulfed into vacuoles; upregulation of PAFr increases bacterial invasion (6, 27). Statin treatment is reported to lower the amount of Ptafr transcript in experimental aortic aneurysms, as well as improving various aspects of endothelial function (28–31). We first documented statin effects on PAFr on endothelial cells in vitro and then examined lung and vasculature in vivo.
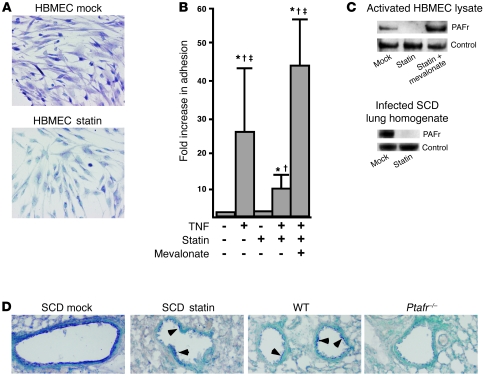
Using vascular endothelial cells activated with TNF-α to model the inflammation seen in SCD mice, we observed decreased expression of PAFr with simvastatin treatment as determined by immunohistochemistry and Western blot analysis (Figure 3, A and C). The decreased expression of PAFr was biologically relevant, as treatment with simvastatin also significantly lowered the number of bacteria associated with activated endothelial cells (Figure 3B). Importantly, the inhibitory effect of simvastatin on bacterial binding was reversed by the addition of mevalonate, the immediate downstream metabolic product of the acetyl-CoA/3-hydroxy-3-methylglutaryl CoA reductase reaction (Figure 3, B and C).
Figure 3. Effect of statin treatment on TNF-α–induced upregulation of PAFr on endothelial cells in vitro.
(A) Immunohistochemical detection (purple) of PAFr expression on HBMECs activated with TNF-α with or without simvastatin. Original magnification, ×200. (B) Relative bacterial adhesion to activated HBMECs with or without pretreatment with simvastatin, and reversal by mevalonate. *P < 0.01 versus no treatment; †P < 0.01 versus statin; ‡P < 0.01 versus TNF plus statin. Error bars represent SD. (C) Detection of PAFr by Western blot analysis in activated HBMEC lysates and infected lung homogenates of SCD mice with or without simvastatin treatment. β-Actin served as the loading control. (D) Uninfected lung sections of SCD (with or without simvastatin), WT, and SCD Ptafr–/– mice showing bronchial epithelium stained for PAFr (purple). Arrowheads indicate staining in sections showing patchy expression. Original magnification, ×100.
To correlate the in vitro findings with in vivo effects in SCD, we examined lungs from uninfected mice for PAFr expression with and without simvastatin treatment. WT mice showed patchy PAFr expression on airway epithelia (Figure 3D). SCD mice (mock-treated) exhibited strong, confluent staining for PAFr, a pattern that returned to WT levels upon treatment with statin; SCD Ptafr–/– mouse lung showed minimal staining (Figure 3D). Western blot analysis of cellular PAFr levels in the infected lung homogenates of SCD mice indicated that statin treatment reduced PAFr expression (Figure 3C). These results suggested that statins downmodulate PAFr expression in vivo in the lungs of a SCD model.
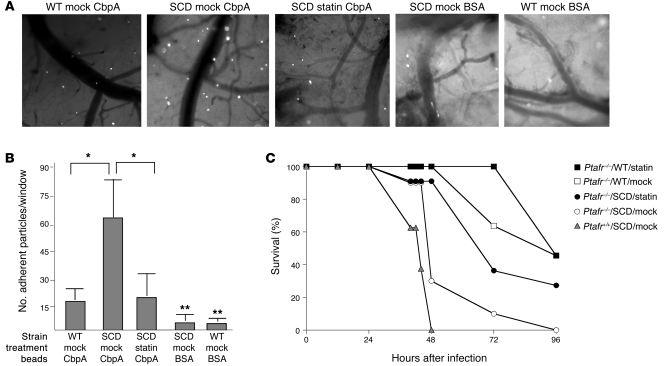
To investigate effects of statin against bacterial-vascular interactions in vivo, we fitted SCD mice with cranial windows and quantitated adherence of bacterial components to the vasculature. Fluorescent latex beads coated with pneumococcal CbpA only bind to activated cerebral endothelium (5). While WT mice showed minimal adherent beads, SCD mice showed significantly increased bead adherence (Figure 4, A and B), consistent with an activated endothelium. Treatment of the SCD mice with statins resulted in a significant decrease in the number of CbpA-coated beads adherent to the SCD vasculature (Figure 4A). Negative control beads coated with BSA showed virtually no binding (Figure 4A). These data indicated that statins attenuated the bacterial-host cell adherence interaction to lung and vasculature in vivo.
Figure 4. Effect of statin treatment on bacterial interactions with vasculature and survival of SCD mice.
(A) WT and SCD mice fitted with cranial windows were injected intravenously with fluorescent beads coated with the pneumococcal adhesin CbpA to assess activation-dependent adhesion to cerebral vessels (representative images at ×4 magnification). Negative control beads coated with BSA were also utilized in both WT and SCD mice as indicated. (B) Quantitation of bead adherence is presented as average ± SD of beads/×4 field for 10 fields per animal from at least 3 independent experiments. *P < 0.01 versus SCD mock; **P < 0.01 versus WT mock. (C) Ptafr–/– mice were transplanted with WT (squares; n = 11) or SCD (circles; n = 11) bone marrow. Survival following intranasal pneumococcal challenge was monitored after mice received mock (open symbols; n = 11) or simvastatin treatment (filled symbols; n = 11). Survival of Ptafr+/+ SCD mice (triangles; from Figure 1) is shown for comparison.
Statin activity in Ptafr–/– mice.
Pneumococci utilize PAFr for invasion into host cells, and mice lacking PAFr show a dramatic attenuation of invasive pneumococcal disease (6, 32). Since PAFr is upregulated in SCD mice (9) and statins downregulate PAFr in the lungs of SCD mice (Figure 3), we hypothesized that protection against mortality by statins occurred by downregulation of PAFr expression. If that were the only mechanism, statins should not add to the protection observed in SCD mice lacking PAFr. SCD mice WT and null for Ptafr were treated with and without statin. As expected from previous studies (9), the absence of PAFr improved the survival of SCD mice upon pneumococcal challenge (Figure 4C). Surprisingly, however, statin treatment of Ptafr–/– SCD mice further improved survival (Figure 4C, P = 0.02) compared with Ptafr–/– SCD mock controls. The protective effect of the statins resulted in the mean time to death being statistically indistinguishable from that of the Ptafr–/– WT mock group (P = 0.35) but still distinct from that of the WT Ptafr–/– statin group (P = 0.04). No significant differences were observed in time to death in the WT Ptafr–/– between the mock- and statin-treated groups (P = 0.31). Bacterial titers in the blood were consistent with these findings, in that statin lowered the level of bacteremia in Ptafr–/– SCD mice but not Ptafr–/– non-SCD mice (mean log titers: SCD, 4.2 ± 1.8 vs. SCD/statin, 3.0 ± 2.2; non-SCD, 3.2 ± 1.9 vs. non-SCD/statin, 3.2 ± 2.2; n = 11 per group). These data indicate that, although downregulation of PAFr by statin was operative in the SCD setting, it was not the only mechanism of protection.
Statins protect cells against cholesterol-dependent cytotoxins.
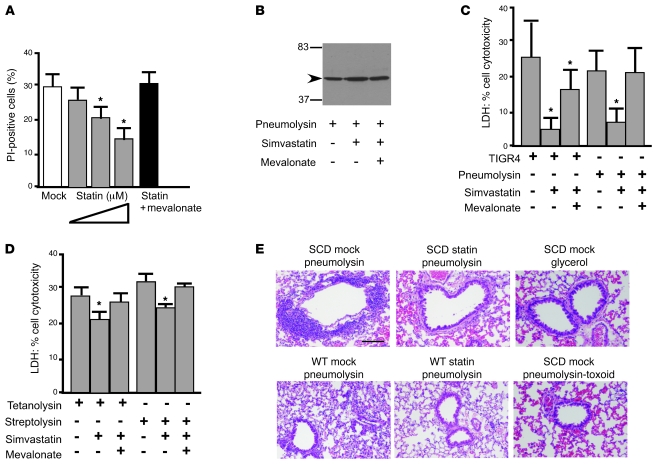
Pneumolysin is a pore-forming toxin that binds to cholesterol in lipid rafts on the host cell membrane (33). Since statins inhibit cholesterol synthesis and the formation of cholesterol-rich lipid rafts, we sought to determine whether statins modulated the ability of pneumolysin to cause cell death in vitro. Cytotoxicity of recombinant pneumolysin on endothelial cells was evidenced by rounding of spindle-shaped endothelial cells within 30 minutes, followed by detachment from the well. In contrast, simvastatin-treated cells exhibited some rounding at 30 minutes but regained spindle shape without detachment by 60 minutes. This protection was further measured by determination of the percentage of dead cells after pneumolysin treatment (Figure 5A) and by cytolysis-induced release of cytoplasmic lactate dehydrogenase (LDH) (Figure 5C). Western blot analysis of whole-cell lysates from endothelial cells exposed to pneumolysin demonstrated that statin had no effect on the amount of pneumolysin present in the cell membrane (Figure 5B), suggesting the mechanism of protection related to effects on activation of the toxin after binding to cholesterol in host cell membranes. Furthermore, toxin-induced red blood cell hemolysis was not inhibited by statins; addition of simvastatin (101%) and mevalonate (98%) alone or in combination (98%) did not alter the hemolytic capacity of pneumolysin, indicating that the drugs did not directly interfere with pneumolysin activity. Statin-mediated protection extended to challenge with live bacteria, and the addition of mevalonate counteracted the protective effect of the statin (Figure 5C). Further experiments indicated that the protective effects of the statins extended to other cholesterol-dependent cytolysins, including tetanolysin and streptolysin O (Figure 5D).
Figure 5. Effect of simvastatin on cytolysin-mediated endothelial cell death.
(A) PI staining of HBMECs following exposure to pneumolysin. Cells were treated with increasing concentrations of simvastatin overnight (0.1, 1.0, and 10 μM, respectively) or with simvastatin (1 μM) supplemented with mevalonate. For each condition, 20,000 cells were analyzed by FACS for PI-positive staining. Percentages of PI-positive cells in controls were: mock, 1.5%; simvastatin, 0.1%; mevalonate, 0.2%; simvastatin plus mevalonate, 0.1%. Data represent the mean ± SD from 2 independent experiments. *P < 0.01 versus mock. (B) Western blot of pneumolysin (arrowhead) in HBMEC membranes with or without simvastatin and/or mevalonate pretreatment (molecular weight markers indicated). (C and D) Release of LDH as a measure of HBMEC lysis after 1 hour of exposure to living bacteria (C) or cholesterol-dependent toxins pneumolysin (C), tetanolysin (D), or streptolysin O (D). Pretreatment with simvastatin and reversal by mevalonate as indicated. LDH released from HBMEC monolayer completely lysed by 1% Triton X-100 was assigned a value of 100%. *P < 0.01 versus mock. Error bars represent SD. (E) SCD and WT mice with or without simvastatin treatment were challenged intratracheally with 500 ng recombinant pneumolysin in 0.5% glycerol. Lung histology was assessed at 5 hours. Effects of pneumolysin toxoid and 0.5% glycerol alone are shown as negative controls. Data are representative images from at least 3 mice per group. Scale bar: 100 μM.
The protective effect observed in vitro was investigated in vivo in mice pretreated with statins and subsequently inoculated with purified pneumolysin into the lungs. Histological examination of lungs 5 hours after pneumolysin exposure showed a marked reduction in lung injury in the statin-treated SCD animals compared with the mock-treated control group (Figure 5E). Administration of either PBS or a nonhemolytic mutant pneumolysin protein showed no marked lung damage (Figure 5E), indicating that statin-mediated protection was directed to active toxin. Such a mechanism should be observable also in WT animals. Despite the fact that statins had no effect on survival of WT animals (Figure 1), a significant reduction in lung injury was observed upon pulmonary challenge of statin-treated WT mice with pneumolysin (Figure 5E). In summary, statins alter cellular susceptibility to cholesterol-dependent cytotoxins (CDCs) in WT and SCD settings, but attenuation of mortality is seen only in SCD.
Discussion
As a result of vascular occlusion and subsequent hypoxia, SCD is characterized by chronic activation of vascular endothelial cells, with marked increases in the expression of many inflammation-responsive cell surface receptors (10, 22). In the setting of pneumococcal challenge, chronic upregulation of PAFr promotes bacterial invasion and predisposes SCD individuals to invasive disease (7, 9, 27). Statins are potent inhibitors of systemic inflammation (16, 26, 29) and thus might serve as a prophylactic measure for SCD individuals, potentially reducing the incidence and severity of pneumococcal infection. Our data indicate that statin therapy prolonged survival in experimental pneumonia and sepsis. This protective effect arose by several mechanisms. Statins reduced PAFr expression on the chronically activated SCD vascular endothelium as well as in the lungs. This decrease was coupled to reduced bacterial adherence and invasion in vitro and in vivo. It is reasonable to hypothesize that the effects of statins in the context of SCD arise from a combination of effects on cholesterol-containing membrane rafts. These membrane microdomains organize and compartmentalize G protein–coupled receptors, such as PAFr and their signaling components. Our results indicate that statins also disrupt microdomain functions important for β-arrestin–mediated, PAFr-directed endocytosis of pneumococci. This activity would potentially also apply to infection by the many invasive bacteria that use activation of host receptors in membrane microdomains as a mechanism for endocytosis.
While the SCD mouse model clearly indicated that statins interfered with PAFr-dependent pneumococcal invasion, an additional protective mechanism was suggested by the beneficial activity of statins in infected SCD mice lacking Ptafr. As expected in this setting, bacteria failed to invade the bloodstream, indicating that the additional benefit to survival must have arisen within the alveolar compartment. Pneumococci and several other Gram-positive invasive bacteria produce a class of CDCs that bind to host cell lipid rafts and oligomerize to form pores (34). Direct testing of the effect of statins on cells treated with purified pneumolysin toxin or toxin-producing bacteria indicated that statins prevented cytolysis. The effect was not mediated by direct statin-toxin binding or decreased toxin binding to cells, suggesting that statin-treated lipid rafts contained cholesterol sufficient to ligate pneumolysin, but the interaction failed to support the pore formation required for cytotoxicity. Recent evidence indicating a role for cholesterol in toxin activation rather than cell binding (35) supports this contention. Furthermore, attenuation of CDC-induced lung damage by statins was also apparent in WT mice. This protection from injury, however, did not translate to increased survival, as only SCD mice experienced a survival benefit. The ability of statins to protect against cytolysis by tetanus toxin and streptolysin O indicate that statins might be of broader benefit in arresting tissue damage from fulminant Gram-positive toxemias.
Collectively, the multiple points of inhibition of pathogenesis afforded by statins in the context of pneumococcal challenge suggest that statin prophylaxis might not only decrease the heightened susceptibility of children with SCD to lethal pneumococcal disease but also attenuate progression of bacterial invasion in other settings of chronic inflammation, such as in individuals with chronic pulmonary or vascular inflammation that elevates the risk of pneumonia. These findings support clinical investigation of the protective efficacy of statins in children with SCD.
Methods
Preparation of bacteria and statins.
S. pneumoniae strains D39, R6, TIGR4, or T4R were grown on tryptic soy agar (EMD Chemicals) supplemented with 3% sheep blood or in Todd Hewitt broth (TH) or defined semisynthetic casein liquid medium (36, 37) supplemented with 0.5% yeast extract. Simvastatin or lovastatin (10 μM; Calbiochem) and mevalonate (100 μM; Sigma-Aldrich) did not affect bacterial growth. No major changes were observed in gene expression by array despite dosage of bacteria with simvastatin at levels 10-fold greater than that used in our infection models (Supplemental Table 1; supplemental material available online with this article; doi: 10.1172/JCI39843DS1).
Simvastatin was resuspended in ethanol and sodium hydroxide as an inactivated stock solution stored at –20°C for up to 3 weeks. Immediately prior to use, the stock solution was activated by addition of hydrogen chloride and subsequently diluted in sterile PBS (38). For animal experiments, simvastatin was administered at 1 μg/g body weight via intraperitoneal injection based on previous studies (38). Mock-treated controls were injected with PBS supplemented with ethanol as for the simvastatin group. Injections were performed daily for 5 days prior to pneumococcal challenge and each day subsequent to challenge. Serum cholesterol was measured 5 days after statin intervention and was found to decrease from 107 ± 8 mg/dl to 96 ± 7 mg/dl (P = 0.012, Mann-Whitney U test), indicating that the administration route was successful. Simvastatin had no effect on bacterial growth rate or pneumolysin secretion at the concentration used in these studies (Supplemental Figure 1).
Adhesion assay.
Assays were performed using human brain microvascular endothelial cells (HBMECs; ScienCell Research Laboratories). Cells were grown to 80%–90% confluence in 24-well plates (Costar Corning Inc.). Prior to the experiment, cells were treated overnight with fresh growth medium containing 1.0 μM simvastatin with or without 100 μM mevalonate (Sigma-Aldrich). This dose of simvastatin was selected based on previous investigations on the effect of simvastatin on endothelial cells (31, 39). The following day, cells were washed and incubated for 2 hours with serum-free F12 medium (Invitrogen) or F12 containing recombinant TNF-α (10 ng/ml; Sigma-Aldrich). Cells were washed and incubated with F12 containing 1 × 107 CFU/ml T4R for 1 hour at 37°C in 5% CO2. Cells were then washed 3 times with F12 to remove nonadherent bacteria, and the cells were lysed by 100 μl 0.1% Triton X-100. The number of bacteria in the cell lysate, representing adherent and intracellular bacteria, was determined by serial dilution, plating, and colony counting.
Western blot analysis.
HBMECs were pretreated with 1 μM simvastatin overnight and activated with TNF-α for 2 hours, followed by lysis in 0.1% Triton X-100. To ensure equal loading, protein concentration was determined for each lysate and loaded accordingly. Lysates were run on 4%–12% NuPAGE Bis-Tris gels (Invitrogen). Proteins were subsequently transferred to PVDF membranes by Western blot and probed with antibody to PAFr (1:500; Santa Cruz Biotechnology Inc.) and against β-actin (1:2,000; Santa Cruz Biotechnology Inc.). Various concentrations and durations of simvastatin treatment were analyzed (Supplemental Figure 2), with the overnight exposure at the low dose providing the most consistent results.
Cytokine analysis.
To model early events in inflammation, intratracheal challenge with 106 ethanol-fixed bacteria was utilized so as to eliminate differential bacterial outgrowth in the lung tissues. Mice were challenged after 5 days of statin treatment, and lungs were harvested at 6 hours and resuspended in 500 μl PBS supplemented with protease inhibitors. Lungs were then homogenized and debris was removed by centrifugation. Supernatants were frozen at –80°C, and cytokine levels were measured in duplicate for each sample using a Milliplex cytokine assay (Millipore). A standard curve was generated for each cytokine to ensure that values were within the linear range of detection for the assay. Two independent quality controls of cytokines of known concentration were also used to ensure validity of the data.
Immunohistochemistry.
Mice were perfused with 3% paraformaldehyde, after which lungs were excised and kept in 3% paraformaldehyde overnight. Lungs were then transferred to 30% sucrose for 3–5 days, frozen in OCT freezing medium (Tissue Tek, Electron Microscopy Sciences) in dry ice, and sectioned (20 μm). For PAFr staining, sections were submerged in 0.6% hydrogen peroxide for 30 minutes, washed, permeabilized with 0.4% Triton X-100 for 20 minutes, and washed. Sections were blocked with 1% BSA and 5% goat serum for 30 minutes and incubated with primary anti-PAFr antibody (Santa Cruz Biotechnology Inc.) at 1:100 overnight at 4°C. After washing, sections were incubated with goat anti-rabbit IgG-HRP (Bio-Rad) at 1:500 for 1 hour at room temperature. Sections were washed and visualized with VIP substrate kit and counterstained with methyl green (Vector Laboratories).
Generation of SCD mice.
Lethally irradiated 8-week-old female C57BL/J6 mice (The Jackson Laboratory) were transplanted as described previously with 2 × 106 bone marrow cells from either BERK SCD mice or WT mice (40). Baytril (enrofloxacin; 2.27% solution, diluted 1:100 in drinking water; Bayer) was administered as an antimicrobial prophylaxis for 3 weeks after transplantation. One hundred days after transplantation, the sickle phenotype was confirmed by hemoglobin cellulose acetate electrophoresis of red cell lysates (41). Complete blood counts were determined to ensure that the white blood cell number, hematocrit, hemoglobin, and red blood cell distribution were within expected values for both WT and SCD mice.
Intravital fluorescence microscopy.
All experiments using animals were performed with the prior approval of and in accordance with guidelines of the St. Jude Institutional Animal Care and Use Committee. Lack of availability of intravital microscopy in BL2 animal facilities precluded the use of fluorescently labeled bacteria; hence, one of the major proteins required for pneumococcal adherence to the brain endothelium, CbpA, was selected for these studies. Cranial windows were installed overlying the cerebral cortex as described previously (5, 42). WT and SCD mice were immediately imaged after surgery due to challenges in the long-term survival of the SCD mice after the procedure. Mice with cranial windows were anesthetized (2.5% isoflurane; Baxter Healthcare), immobilized on a stereotaxic frame, and placed under an industrial-scale microscope (model MM-11, Nikon) with a camera assembly and bright-field and fluorescent light sources. Yellow-green fluorescent 2-μm microspheres (Polysciences) were coated with recombinant CbpA according to the supplier’s instructions and injected intravenously (2.5 μl/g body weight of a 108 sphere/ml suspension). Video images and still pictures were captured during injection and at 3 and 6 minutes after injection at an excitation wavelength of 490 nm using MetaMorph software (Molecular Devices Analytical Technologies). To ensure that beads were adherent and not merely passing through the vasculature, imaging was done at multiple time points, and only beads remaining stationary from the previous frames were included in the enumeration of adherence.
Mouse challenge.
Bacterial challenge studies were performed as previously described (43). All mice were maintained in BSL2 facilities, and all experiments were done under inhaled isoflurane (2.5%). Bacteria (strain D39) were introduced by intranasal administration of 106 CFU in 25 μl PBS. Mice were monitored daily for signs of infection, and differences in time to death were compared via Kaplan-Meier survival estimates. Bacterial density in blood was quantified at 24 hours after infection and compared by Mann-Whitney U test. Bacterial density in lungs was measured by harvesting lungs at 24 hours after infection followed by homogenization in 500 μl PBS and subsequent serial dilution and enumeration of CFU. For histopathology, lungs were collected at 18 hours after infection and immediately perfused with 10% formaldehyde and embedded in paraffin. Sections were stained with H&E for tissue morphology and with TUNEL for indications of cell death (ApopTag Plus Peroxidase in Situ Apoptosis Detection Kit, Chemicon International).
Effect of simvastatin on cytotoxin activity.
The ability of pneumolysin to kill statin-treated cells in vitro was determined using fluorescence microscopy to detect acridine orange (AO) and ethidium bromide (EtBr) staining (44). HBMEC monolayers in 24-well plates at 20%–40% confluence were treated overnight with 1 μg/ml simvastatin, washed, and incubated with serum-free medium containing 0.5 μg/ml pneumolysin, AO (100 mg/ml), and EtBr (100 mg/ml). At the designated times, images of the cells were taken using a Leica DMIM fluorescence microscope. The percentage of dying cells was determined by dividing the number of necrotic and apoptotic cells (orange) by the total number of cells present per field of vision (orange plus green). At least 8 fields were counted from 3 independent experiments.
The ability of pneumolysin, streptolysin O, or tetanolysin (Sigma-Aldrich) to form lytic pores was determined by the release of intracellular LDH into the cell culture medium (45). His-tagged pneumolysin, nonhemolytic pneumolysin toxoid (34), and streptolysin O (46) were expressed in E. coli BL21(DE3) induced by 1 mM IPTG for 4 hours and purified by metal affinity chromatography. Streptolysin O expression plasmid was provided by R. Tweten (University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA). Endotoxin was removed by gentle mixing with Endotoxin Decontamination Beads (Thermo Scientific) for 2 hours, and its removal was confirmed using Lonza’s colorimetric LAL assay. Endotoxin levels were similar for all constructs, approximately 5 EU/ml of purified protein. This corresponds to approximately 0.001 EU for the in vivo lung administration. HBMEC monolayers at 80% confluence were treated with simvastatin overnight, washed, and incubated at 37°C in 5% CO2 with serum-free medium containing 1 μg/ml recombinant cytolysin for 90 minutes. The supernatant was removed and centrifuged to remove cellular debris, and LDH in the supernatant was measured using the LDH-Cytotoxicity Assay Kit II (BioVision). Pneumolysin cytotoxicity was quantified as a percentage of the total LDH in the entire monolayer determined by complete lysis of control monolayers with 1.0% Triton X-100. Both cell viability and LDH experiments were performed in triplicate, with a minimum of 8 wells quantified for each lysin per experiment. Values for each experiment were based on the average of a minimum of 3 separate wells per experimental condition tested. Binding of toxin to cells was determined by Western blot analysis using rabbit anti-pneumolysin antibody (1:20,000).
Hemolysis assay.
The hemolytic activity of pneumolysin and streptolysin O was determined using washed, defibrinated sheep red blood cells (Hemostat Laboratories) suspended in PBS to a final concentration of 3%. In a 96-well plate, 100 μl of the 3% blood solution was added to 100 μl of serially diluted toxin and incubated at 37°C. The unlysed blood cells were centrifuged at 200 g for 5 minutes. The supernatant (180 μl) was then transferred to a new plate, and the absorbance at 540 nm was measured. Controls included PBS only (0% lysis), simvastatin alone at 1–10 μM (0% lysis), mevalonate alone (0% lysis), and distilled water (100% lysis). Hemolytic activity was determined by the amount of protein required to lyse 50% of the cells within the allotted time.
Live/dead staining.
HBMECs were seeded in 6-well plates and treated overnight with 0, 0.1, 1, or 10 μM simvastatin. The next day, cells were treated with serum-free medium containing hemolytic units lysing 50% of cells (HU50) of 3 μg/ml of pneumolysin for 4 hours at 37°C in 5% CO2. After 4 hours, the cells and cell culture supernatants were collected. Washed cells were suspended in 500 μl HEPES buffer and 5 μl propidium iodide (PI) and assessed for the percentage of live/dead cells on a BD FACSCalibur. Control staining of cells treated with simvastatin, mevalonate, or simvastatin and mevalonate in combination did not exhibit levels of PI-positive staining above background of the untreated controls (data not shown).
Statistics.
Kaplan-Meier analysis and generalized Wilcoxon tests were performed to determine statistical significance of mortality data. Mann-Whitney U tests were performed to determine statistical significance of bacterial lung and blood titers, and adherent bead counts for cranial windows. Student’s 2-tailed t test was used to determine significance of cytokine levels. A P value less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank the Animal Imaging Facility at St. Jude Children’s Hospital for excellent technical assistance with the cranial windows, Heather Forrest for histological scoring of lung pathology, and Karla Gorena of the UTHSCSA Flow Cytometry Core Facility for data processing and analysis. This work was supported by National Institute of Allergy and Infectious Diseases grant 27913 and National Heart, Lung, and Blood Institute grant U54HL070590 to E.I. Tuomanen, National Institute on Aging grant AG29313 to C.J. Orihuela, and the American Lebanese Syrian Associated Charities. Funding agencies had no role in conduct of the study or interpretation of the data.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(2):627–635. doi:10.1172/JCI39843.
References
- 1.WHO. Sickle-cell disease and other haemoglobin disorders [fact sheet]. WHO Media Centre; August 2006. http://www.who.int/mediacentre/factsheets/fs308/en/. Accessed December 3, 2009. [Google Scholar]
- 2.Overturf GD. Infections and immunizations of children with sickle cell disease. Adv Pediatr Infect Dis. 1999;14:191–218. [PubMed] [Google Scholar]
- 3. Wardlaw T, Johansson EW, Hodge M.Pneumonia: The Forgotten Killer of Children . UNICEF/WHO; 2006. http://www.unicef.org/publications/index_35626.html. Accessed December 3, 2009. [Google Scholar]
- 4.Overturf G, Field R, Lam C, Lee S, Powars DR. Nasopharyngeal carriage of pneumococci in children with sickle cell disease. Infect Immun. 1980;28(3):1048–1050. doi: 10.1128/iai.28.3.1048-1050.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orihuela CJ, et al. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest. 2009;119(6):1638–1646. doi: 10.1172/JCI36759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radin JN, et al. beta-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. . Infect Immun. 2005;73(12):7827–7835. doi: 10.1128/IAI.73.12.7827-7835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinojosa E, Boyd AR, Oriheula CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. . J Infect Dis. 2009;200(4):546–554. doi: 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belcher JD, et al. Transgenic sickle mice have vascular inflammation. Blood. 2003;101(10):3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- 9.Miller ML, Gao G, Pestina T, Persons D, Tuomanen E. Hypersusceptibility to invasive pneumococcal infection in experimental sickle cell disease involves platelet-activating factor receptor. J Infect Dis. 2007;195(4):581–584. doi: 10.1086/510626. [DOI] [PubMed] [Google Scholar]
- 10.Lanaro C, Franco-Penteado CF, Albuqueque DM, Saad STO, Conran N, Costa FF. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. J Leukoc Biol. 2009;85(2):235–242. doi: 10.1189/jlb.0708445. [DOI] [PubMed] [Google Scholar]
- 11.Gatson MH, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314(25):1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 12.Butler JC, Hofmann J, Cetron MS, Elliott JA, Facklam RR, Breiman RF. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention’s Pneumococcal Sentinel Surveillance System. . J Infect Dis. 1996;174(5):986–993. doi: 10.1093/infdis/174.5.986. [DOI] [PubMed] [Google Scholar]
- 13.Collina R, Armitage J, Parish S, Sleight P, Peto R. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: A randomized placebo controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 14.Merx MW, et al. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2004;109(21):2560–2565. doi: 10.1161/01.CIR.0000129774.09737.5B. [DOI] [PubMed] [Google Scholar]
- 15.Winkler F, Angele B, Pfister H, Koedel U. Simvastatin attenuates leukocyte recruitment in experimental bacterial meningitis. Int Immunopharmacol. 2009;9(3):371–374. doi: 10.1016/j.intimp.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis. 2007;7(5):358–368. doi: 10.1016/S1473-3099(07)70111-1. [DOI] [PubMed] [Google Scholar]
- 17.Hothersall E, McSharry C, Thomson NC. Potential therapeutic role for statins in respiratory disease. Thorax. 2006;61(8):729–734. doi: 10.1136/thx.2005.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almog Y, et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110(7):880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288(6):L1026–L1032. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- 20.Chalmers JD, Singanayagam A, Murray MP, Hill AT. Prior statin use is associated with improved outcomes in commuity-acquired pneumonia. . Am J Med. 2008;121(11):1002–1007. doi: 10.1016/j.amjmed.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen RW, et al. Preadmission use of statins and outcomes after hospitalization with pneumonia: population-based cohort study of 29,000 patients. Arch Intern Med. 2008;168(19):2081–2087. doi: 10.1001/archinte.168.19.2081. [DOI] [PubMed] [Google Scholar]
- 22.Paszty C, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278(5339):876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 23.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med. 2009;180(8):731–740. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtzclaw JD, Jack D, Aguayo SM, Eckman JR, Roman J, Hsu LL. Enhanced pulmonary and systemic response to endotoxin in transgenic sickle mice. Am J Respir Crit Care Med. 2004;169(6):687–695. doi: 10.1164/rccm.200302-224OC. [DOI] [PubMed] [Google Scholar]
- 25.Brittain JE, Parise LV. Cytokines and plasma factors in sickle cell disease. Curr Opin Hematol. 2007;14(5):438–443. doi: 10.1097/MOH.0b013e3282a4a673. [DOI] [PubMed] [Google Scholar]
- 26.Novack V, et al. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: a randomized double-blind placebo controlled clinical trials. Intensive Care Med. 2009;35(7):1255–1260. doi: 10.1007/s00134-009-1429-0. [DOI] [PubMed] [Google Scholar]
- 27.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. . Nature. 1995;377(6548):435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 28.Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol. 2003;23(5):729–736. doi: 10.1161/01.ATV.0000063385.12476.A7. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood J, Mason JC. Statins and the vascular endothelial inflammatory response. Trends Immunol. 2007;28(2):88–98. doi: 10.1016/j.it.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalyanasundaram A, et al. Simvastatin suppresses experimental aortic aneurysm expansion. J Vasc Surg. 2006;43(1):117–124. doi: 10.1016/j.jvs.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Dichtl W, et al. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. . Arterioscler Thromb Vasc Biol. 2003;23(1):58–63. doi: 10.1161/01.ATV.0000043456.48735.20. [DOI] [PubMed] [Google Scholar]
- 32.Rijneveld AW, et al. Improved host defense against pneumococcal pneumonia in platelet-activating factor receptor-deficient mice. J Infect Dis. 2004;189(4):711–716. doi: 10.1086/381392. [DOI] [PubMed] [Google Scholar]
- 33.Marriott HM, Mitchell TJ, Dockrell DH. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr Mol Med. 2008;8(6):497–509. doi: 10.2174/156652408785747924. [DOI] [PubMed] [Google Scholar]
- 34.Rosado CJ, et al. The MACPF/CDC family of pore-forming toxins. Cell Microbiol. 2008;10(9):1765–1774. doi: 10.1111/j.1462-5822.2008.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soltani CE, Hotze EM, Johnson AE, Tweten RK. Structural elements of the cholesterol-dependent cytolysins that are responsible for their cholesterol-sensitive membrane interactions. Proc Natl Acad Sci U S A. 2007;104(51):20226–20231. doi: 10.1073/pnas.0708104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosch JW, Sublett J, Gao G, Wang YD, Tuomanen EI. Calcium efflux is essential for bacterial survival in the eukaryotic host. Mol Microbiol. 2008;72(1):12–25. doi: 10.1111/j.1365-2958.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacks S, Hotchkiss RD. A study of the genetic material determining enzyme in the pneumococcus. Biochim Biophys Acta. 1960;39:508–517. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- 38.Solovey A, et al. Endothelial cell expression of tissue factor in sickle mice is augmented by hypoxia/reoxygenation and inhibited by lovastatin. Blood. 2004;104(3):840–846. doi: 10.1182/blood-2003-10-3719. [DOI] [PubMed] [Google Scholar]
- 39.Kureishi Y, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6(9):1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pestina TI, Hargrove PW, Jay D, Gray JT, Boyd KM, Persons DA. Correction of murine sickle cell disease using gamma-globin lentiviral vectors to mediate high-level expression of fetal hemoglobin. Mol Ther. 2009;17(2):245–252. doi: 10.1038/mt.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Persons D, Hargrove W, Allay E, Hanawa H, Neinhuis A. The degree of phenotypic correction of murine beta thalassemia intermedia following lentiviral-mediated transfer of human gamma-globulin gene is influenced by chromosomal position effects and vector copy number. Blood. 2003;101(6):2175–2183. doi: 10.1182/blood-2002-07-2211. [DOI] [PubMed] [Google Scholar]
- 42.Fillon S, et al. Platelet-activating factor receptor and innate immunity: uptake of gram-positive bacterial cell wall into host cells and cell-specific pathophysiology. J Immunol. 2006;177(9):6182–6191. doi: 10.4049/jimmunol.177.9.6182. [DOI] [PubMed] [Google Scholar]
- 43.Orihuela CJ, Radin JN, Sublett JE, Gao G, Kaushal D, Tuomanen EI. Microarray analysis of pneumococcal gene expression during invasive disease. Infect Immun. 2004;72(10):5582–5596. doi: 10.1128/IAI.72.10.5582-5596.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kasibhatla S, Amarante-Mendes GP, Finucane D, Brunner T, Bossy-Wetzel E, Green DR. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis [published online 2006].Cold Spring Harb Protoc . doi:10.1101/pdb.prot4493. [DOI] [PubMed] [Google Scholar]
- 45.Braun JS, Novak R, Gao G, Murray PJ, Shenep JL. Pneumolysin, a protein toxin of Streptococcus pnuemoniae, induces nitric oxide production from macrophages. . Infect Immun. 1999;67(8):3750–3756. doi: 10.1128/iai.67.8.3750-3756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinkney M, Beachy E, Kehoe M. The thiol-activated toxin streptolysin O does not require a thiol group for cytolytic activity. Infect Immun. 1989;57(8):2553–2558. doi: 10.1128/iai.57.8.2553-2558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.