Abstract
Background:
The mammalian target of rapamycin (mTOR) has emerged as an attractive cancer therapeutic target. Accordingly, several mTOR inhibitors (e.g., rapamycin and its analogs; rapalogs) are currently being tested in many cancer clinical trials. Despite the encouraging results that some rapalogs improved overall survival among patients with metastatic renal-cell carcinoma, the single agent activity of rapalogs in most other tumor-types has been modest, at best.
Objective:
To review the current understanding of the mTOR axis and discuss potential strategies to enhance mTOR-targeted cancer therapy.
Methods:
Preclinical and clinical data in peer-reviewed reports on the novel biological and therapeutic parts of the mTOR axis are discussed.
Conclusion:
The mTOR axis involves complex regulatory networks. Inhibition of the mTOR axis with a rapalog induces feedback activation of several survival signaling pathways such as Akt activation, which in turn blunt rapalogs' anticancer efficacy. Thus, blockage or prevention of the activation of these survival signaling pathways may enhance mTOR-targeted cancer therapy.
Keywords: Cancer, mTOR, rapalogs, RAD001, rapamycin
1.Introduction
The mammalian target of rapamycin (mTOR) belongs to the phosphatidylinositol kinase-related kinase family (1). It plays a central role in regulating cell growth, proliferation and survival, in part by regulation of translation initiation, primarily through interactions with other proteins such as raptor (forming mTOR complex 1, mTORC1) and rictor (forming mTOR complex 2, mTORC2) (2-4). mTORC1 is composed of mTOR, proline-rich Akt substrate 40 kDa (PRAS40), raptor, and mLST8 (also known as GβL), whereas mTORC2 consists of mTOR, rictor, Sin1 (SAPK interacting protein), and mLST8 (4). Recent data have revealed that the formation or stabilization of both complexes requires phosphatidic acid (PA), a product generated by phospholipase (PLD) (5).
The best characterized downstream effectors of mTORC1 are the 70 kDa ribosomal S6 kinase (p70S6K) and the eukaryotic translation initiation factor 4E (eIF4E) binding protein 1 (4E-BP1) (2). In response to mitogenic stimuli or nutrient availability, mTORC1 is activated, leading to phosphorylation of p70S6K and 4E-BP1. Phosphorylated p70S6K further phosphorylates the 40S ribosomal protein S6, leading to enhancement of the translation of mRNAs. Moreover phosphorylated 4E-BP1 promotes dissociation of 4E-BP1 from eIF4E, thereby increasing the cap-dependent translation of mRNAs, such as cyclin D1 and c-Myc (2, 4). Therefore, phospho-p70S6K (or phospho-S6) and phospho-4E-BP1 are common readouts of the mTORC1 signaling. In contrast to the mTORC1 that is regulated by Akt, mTORC2 is a Ser473 Akt kinase and thus functions upstream of Akt (6). Moreover, mTORC2 regulates protein kinase Cα (PKCα) phosphorylation, which is critical for controlling cytoskeletal organization (7). More recently, serum- and glucocorticoid-induced protein kinase 1 (SGK1), which may regulate cancer cell growth and survival (8-12), has been identified as a specific substrate of mTORC2 (13).
It was originally suggested that the mTORC1, but not the mTORC2, is sensitive to rapamycin (7). However, prolonged treatment with rapamycin does inhibit the mTORC2 by disrupting its assembly (14, 15). One recent study using S2481 phosphorylation as a marker for mTORC2 has shown that mTORC2 formation is in fact rapamycin sensitive in several cancer cell lines in which it had been previously reported that mTORC2 assembly and function were rapamycin insensitive (16).
It is known that the mTOR axis is activated by phosphoinositide 3-kinase (PI3K)/Akt survival signaling. Recent studies have also linked mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling to the activation of the mTOR axis. Thus, mTOR serves as a convergence point of the PI3K/Akt and MAPK/ERK signaling pathways (see below for detailed description), which are often hyper-activated in many types of cancers. Moreover, the tumor suppressor LKB1 negatively regulates the mTOR axis. In certain types of cancer such as lung cancer, high frequency of LKB1 mutations occurs, leading to activation of the mTOR signaling (see below description). Thus, mTOR signaling has emerged as an attractive cancer therapeutic target (2, 17, 18).
Accordingly, the potential applications of mTOR inhibitors (e.g., rapamycin and its analogs; rapalogs) for treating various types of cancer have been actively studied both pre-clinically and clinically. While recent studies have shown encouraging results that the rapalog CCI-779 or RAD001 improved overall survival among patients with metastatic renal-cell carcinoma (19-21), in most other tumor-types, the single agent activity of rapalogs has been modest, at best (22-24).
While there are many recent elegant review articles describing mTOR signaling (4, 25-28), the current review will focus on discussing major obstacles to mTOR-targeted cancer therapy and possible strategies that may potentially enhance mTOR-targeted cancer therapy based on our understanding of the complexity of the mTOR signaling network.
2. mTOR as a cancer therapeutic target
mTORC1 signaling is well documented in regulation of translation of several oncogenic proteins such as cyclin D1, c-Myc, ornithine decarboxylase (ODC), hypoxia-Inducible Factor 1α (HIF1α), vascular endothelial growth factor (VEGF), fibroblast growth factors (FGF) and myeloid cell leukemia sequence 1 (Mcl-1) because of its critical roles in activating p70S6K and 4E-BP1. Since the PI3K/Akt and MAPK/ERK signaling pathways, which positively regulate the mTOR axis, are often activated in many types of cancer, and LKB1, which suppresses mTOR signaling, is frequently mutated in certain types of cancers, mTOR signaling is consequently hyper-activated in many types of cancers. Although the upstream regulation of mTORC2 has not been well documented, mTORC2 functions as a direct Ser473 kinase for Akt, which is critical for cell survival. Thus, it is rational to target the mTOR axis for cancer therapy.
2.1. mTORC1 plays a critical role in the regulation of translation of oncogenic proteins
Protein translational control is an important strategy by which eukaryotic cells regulate gene expression. A prime target of translational control is eIF4E, which recognizes and binds to the 7-methylguanosine (m7GppN) cap structure present at the 5′ untranslated regions (5′UTRs) of cellular mRNAs and delivers these mRNAs to the eIF4F translation initiation complex. Assembly of the eIF4F complex, which involves eIF4E, eukaryotic translation initiation factor 4A (eIF4A) and eukaryotic translation initiation factor 4G (eIF4G), is dependent upon eIF4E availability. Given that eIF4E is the least abundant among the initiator factors involved in the eIF4F complex, eIF4E is the rate limiting factor for cap-dependent translation initiation (26, 29, 30). Consequently, changes in eIF4E levels profoundly affect translation rates of certain proteins, particularly those related to cell growth and survival involved in oncogenesis (e.g., c-Myc, VEGF, ODC, cyclin D1, HIF-1 and Mcl-1), which, under normal cellular conditions, are translationally repressed. Although enhanced eIF4F complex formation increases the translation of all cap-dependent mRNAs and thereby increases global protein synthesis rates, the variability of mRNAs in the length and structure of their 5′-UTRs determines their inherent “translatability”. The short, unstructured 5′-UTRs of most cellular mRNAs (e.g. “housekeeping” genes) enable the eIF4F complex to scan readily for the translation initiation codon. Therefore, these strong mRNAs are efficiently translated, even when the active eIF4F complex is limiting. In contrast, the lengthy, G/C-rich, highly structured 5′-UTRs typical of growth and survival factor mRNAs (i.e. weak mRNAs), encumber efficient scanning and start codon recognition. Consequently, these mRNAs are inefficiently translated, especially when the active eIF4F complex is limiting. Whereas strong mRNAs [such as β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] are only minimally affected by alterations in eIF4F complex formation, weak mRNAs, which typically encode growth and survival factors (e.g., c-Myc, VEGF, ODC, cyclin D1, HIF1α, and Mcl-1), are preferentially and disproportionately affected by eIF4E availability (30-32).
The eIF4E inhibitor 4E-BP1 competes with eIF4G for an overlapping binding site on eIF4E, thus preventing eIF4G from interacting with eIF4E. mTORC1 directly phosphorylates 4E-BP1 that causes 4E-BP1 to dissociate from eIF4E, thereby increasing the availability of functional eIF4E (26). The increase in free eIF4E levels thus leads to more efficient cap-dependent translation initiation, particularly increasing the translation of weak mRNAs important for oncogenesis. Therefore, mTORC1 is critical for regulation of translation of many oncogenic proteins.
2.2. PI3K/Akt regulates mTORC1 signaling
It has been well documented that mTORC1 functions downstream of the PI3K/Akt pathway and is activated in response to stimuli that activate the PI3K/Akt pathway. However, the mechanism through which mTORC1 is regulated by PI3K/Akt has not been fully understood. The tumor-suppressor proteins tuberous sclerosis 1 (TSC1; also called hamartin) and tuberous sclerosis 2 (TSC2; also named tuberin) form a heterodimer that acts as a functional unit in the suppression of mTORC1 activity. TSC2 has GTPase-activating protein (GAP) activity towards the Ras family small GTPase Rheb (ras homolog enriched in brain) and thereby can enhance the conversion of Rheb into its GDP-bound inactive state, leading to inactivation of the mTORC1 (26, 27). In response to growth factors, Akt can directly phosphorylate on several residues including Ser939 and Thr1462 of TSC2 (human TSC2), which may impair the ability of TSC2 to inhibit Rheb and mTORC1 (26, 27). It is known that PLD and its product PA mediate mitogenic activation of mTORC1 signaling (33). Recent data have demonstrated that Rheb activates the mTORC1 signaling through a PLD1/PA-dependent manner (34).
Several recent studies have revealed that PRAS40 is a novel mTORC1-binding partner that mediates Akt signals to mTOR1 (35-37). Upon activation (i.e., phosphorylation) by Akt, PRAS40 will dissociate from mTORC1 through 14-3-3 binding of the phosphorylated PRAS40, leading to activation of mTORC1 signaling (35). Moreover, PRAS40 is also a substrate of mTORC1 and mTORC1-mediated phosphorylation of PRAS40 facilitates the removal of its inhibition on downstream signaling of mTORC1 (38-40), suggesting a positive-feedback mechanism for AKT-induced mTORC1 signaling events.
Therefore, PI3K/Akt signaling might positively regulate mTORC1 by activating Rheb through inhibition of TSC1/TSC2 and/or by directly inhibiting PRAS40 independent of TSC1/TSC2.
2.3. MAPK/ERK regulates mTORC1 signaling
In addition to positive regulation of mTORC1 by PI3k/Akt, recent studies have demonstrated that MAPK/ERK signaling activates the mTORC1 axis. ERK-dependent phosphorylation of TSC2, particularly at Ser664, leads to TSC1-TSC2 dissociation and markedly impairs the ability of TSC2 to inhibit mTOR signaling (41, 42). Moreover, the MAPK-activated kinase, p90 ribosomal S6 kinase (RSK) 1, also interacts with and phosphorylates TSC2 at a regulatory site, Ser1798, located at the evolutionarily conserved C terminus of TSC2, leading to inhibition of the tumor suppressor function of the TSC1/TSC2 complex and subsequent activation of mTORC1 signaling (43). Intriguingly, a recent study has shown that MAPK/ERK signaling can stimulate mTORC1 activity by promoting RSK-mediated raptor phosphorylation (44). In this study, it was shown that RSK (both RSK1 and RSK2) directly targets the mTORC1 complex by phosphorylating raptor, leading to an increase in mTORC1 kinase activity (44). Collectively, it appears that mitogen-activated MAPK/ERK/RSK signaling, in parallel to the PI3K/Akt pathway, functions upstream of the mTORC1 and activates mTORC1 signaling.
2.4. LKB1/AMP-activated protein kinase (AMPK) regulates mTORC1 signaling
LKB1 is a serine/threonine kinase with tumor suppression activity (45) and plays an important role in negative regulation of the mTORC1 axis (46). AMPK is the primary regulator of the cellular response to lowered ATP levels in eukaryotic cells (47). It is activated by stimuli that include pathological stresses, such as oxidative damage, hypoxia, and glucose deprivation, as well as physiological stimuli, such as exercise, muscle contraction, and hormones including leptin and adiponectin (47). LKB1 can activate TSC2 through AMPK, whereas LKB1 is required for repression of the mTOR pathway under low ATP conditions in cell culture in an AMPK- and TSC2-dependent manner. Therefore, it has been proposed that, in response to cellular energy stress, AMPK is activated through LKB1-mediated phosphorylation and then phosphorylates TSC2 to enhance TSC2 function (46). TSC2 subsequently inhibits mTORC1 function via TSC2's GAP activity toward the Rheb small GTPase (48). Under normal conditions, LKB1/AMPK activation will override the mitogenic signal from Akt and tightly control mTOR signaling. However, in the absence of LKB1, AMPK cannot be activated, nor can mTOR be inactivated, in response to cellular energy stress (46).
Although Peutz-Jeghers syndrome patients frequently present with neoplasm of the colon, stomach, small intestine, pancreas, breast, ovaries, and cervix, somatic LKB1 mutations are rare in most sporadic tumor types including the above tumor types (49). Exceptionally, there is a high frequency of LKB1 mutations in human non-small cell lung cancers, particularly adenocarcinomas. It was reported that LKB1 gene alterations were present in 54% of lung adenocarcinoma cell lines and in about 30% of primary lung adenocarcinomas (50, 51). Thus, LKB1 inactivation is also a critical event in the development of sporadic lung adenocarcinomas (52).
3. Biological obstacles in targeting the mTOR axis for cancer therapy
Because of the frequent activation of the mTOR axis in cancer due to the aforementioned mechanisms, mTOR inhibitors [e.g., rapalogs including temsirolimus (CCI-779), everolimus (RAD001) and deforolimus (AP23573)] that inhibit mTOR signaling have been widely tested in clinical trials against various types of cancers. Encouraging results came from studies in renal cancer in which the rapalog CCI-779 or RAD001 improved overall survival among patients with metastatic renal-cell carcinoma (19-21). However, rapalogs have failed to show any appreciable single agent activity in most other tumor types (22-24). Thus, it is urgently needed to know why rapalogs as a single agent do not work well in these cancer types. In this regard, it is crucial to understand the complexity of the regulation of mTOR network including mTORC1 inhibition-induced feedback activation of multiple survival signaling pathways (e.g., PI3K/Akt and MAPK/ERK), which might compromise the anticancer efficacy of rapalogs.
3.1. mTORC1 inhibition induces activation of PI3K/Akt signaling
Although mTORC1 functions downstream of the PI3K/Akt signaling, we (53) and others (54-56) have shown that rapalogs (e.g., rapamycin) increase Akt phosphorylation (both at Ser473 and at Thr308) while inhibiting mTORC1 signaling in various types of cancer cells. Subsequently, rapalog-induced Akt phosphorylation was observed in xenograft tissues (15) and in human cancer tissues (54, 57, 58).
Rapalog-induced increase in phosphorylated Akt (p-Akt) levels occurs very rapidly (i.e., within 10-20 min post treatment) and almost parallels the inhibition of p-p70S6K (15, 53, 54). Moreover, the increase in Akt phosphorylation is a sustained process, which lasts for up to 72 h when cancer cells are exposed to low doses of a rapalog (e.g., 1 or 10 nM) (15, 53, 54). Although prolonged treatment with a rapalog at high doses (100 nM or higher) decreases Akt phosphorylation in certain types of cancer cells (approximately 20%), presumably due to disruption of mTORC2 assembly (14, 59), we found that rapalogs at a low dose (e.g., 1 nM) strongly increased Akt phosphorylation even after a sustained treatment (e.g., 24 and 48 h) in the same cell systems, in which high dose of a rapalog indeed decreased p-Akt levels (15). Interestingly, we found that rapamycin at both 1 nM and 100 nM was equally potent in disrupting the assembly of mTORC2 as in inhibiting mTORC1 signaling (15). Thus, rapalogs' effects on Akt phosphorylation in certain types of cancer cell lines are dose-dependent.
In several cancer cell lines, we found that prolonged treatment with a rapalog potently inhibited mTORC2 assembly, as it did mTORC1 assembly, but clearly increased p-Akt levels (15). In a rapamycin-resistant cell line (i.e., A549-RR), which was routinely cultured in the presence of 1 μM rapamcyin for over 6 months, we found that p-Akt levels were much higher than those in its parental cell lines (i.e., A549). Under such conditions, the assembly of mTORC2 was apparently inhibited (15). Moreover, silencing of rictor expression failed to prevent rapamycin from increasing Akt phosphorylation (15). Collectively, these data suggest that rapalogs are unlikely to induce Akt activation through an mTORC2-dependent mechanism.
By disrupting mTORC1 through silencing the expression of raptor, we could detect a substantial increase in Akt phosphorylation in cancer cells, thus mimicking the effects of rapalogs on Akt phosphorylation (15). Similar findings were also reported by others (6). Therefore, it appears that rapalog-induced Akt activation is a consequence of mTORC1 inhibition. Insulin receptor substrate-1 (IRS-1) is an important mediator of insulin receptor-dependent activation of PI3K. Chronic insulin stimulation causes the phosphorylation and degradation of IRS-1 protein in a rapamycin-sensitive manner (60, 61). Thus, studies on insulin signaling in mammalian skeletal muscle cells, adipocytes, and fibroblasts have demonstrated that mTOR activation by insulin initiates a feedback inhibition of PI3K/Akt, likely through p70S6K activation and its subsequent phosphorylation of IRS-1. The phosphorylation of IRS-1 promotes IRS-1 degradation and reduces IRS-1 expression, leading to decreased activity of PI3K/Akt. Thus, it has been proposed that rapamycin suppresses p70S6K and thus relieves this negative feedback inhibition of Akt, leading to activation of the PI3K/Akt survival pathway (60, 61). Indeed, rapalogs were reported to increase IRS-1 levels in certain cancer cell types (54, 55). Thus, one potential model that may explain rapalog-induced PI3K-dependent Akt activation is the relief of mTORC1/p70S6K-mediated feedback inhibition of IRS-1/PI3K as suggested (54, 62). However, another study showed that suppression of IRS-1 with siRNA failed to abrogate rapalog-induced Akt phosphorylation, suggesting an IRS-1-independent mechanism, even though this process is dependent on insulin-like growth factor-1 receptor (56).
DEPTOR is a newly identified mTOR-interacting protein (63). DEPTOR binds directly to mTOR near the FRB domain and is associated with both mTORC and mTORC2 and functions as an mTOR inhibitor (63). Although downregulation of DEPTOR activates both mTORC1 and mTORC2, leading to an increase in phosphorylation of p70S6K (Thr389) and Akt (Ser473), DEPTOR overexpression suppress mTORC1-dependent p70S6K phosphorylation, but activates Akt (i.e., Akt Ser473 phsohporylation), likely by relieving feedback inhibition from mTORC1 to PI3K signaling (63).
3.2. mTORC1 inhibition induces MAPK/ERK activation
Although MAPK/ERK positively regulates mTORC1 activity as described above, recent studies have shown that mTORC1 inhibition can also induce feedback activation of MAPK/ERK signaling (64, 65), thus adding another layer of complexity to the already complicated mTOR signaling network. Rapalog-induced ERK phosphorylation was observed not only in cell cultures (64, 65), but also in mouse tumor models and most importantly in cancer patients (64). Similar to feedback activation of PI3K/Akt signaling, MAPK/ERK activation by mTORC1 inhibition is also suggested to be a consequence of p70S6K inhibition and subsequent PI3K activation, which in turn activates Ras, leading to the activation of MAPK/ERK signaling (64).
It has been suggested that RSKs can directly activate mTORC1 by phosphorylating raptor (44). In addition to ERK phosphorylation, we found that rapamycin also increased RSK2 phosphorylation (65), suggesting that mTORC1 inhibition activates RSK as well. In contrast to Akt activation, which is very rapid and common in many types of cancer cells (15, 53, 54), we found that rapalogs increased ERK phosphorylation only after a prolonged treatment and only in a few lung cancer cells lines among those tested in which p-Akt can be increased by rapalogs (65). Thus, the activation of the MAPK/ERK signaling does not seem to occur in parallel to Akt activation. The question is why rapalog-induced MAPK/ERK activation does not couple to Akt activation if both of the events are secondary to p70S6K inhibition and subsequent PI3K activation. Thus, further studies are needed in this regard to fully demonstrate the mechanism by which rapalogs activate MAPK/ERK signaling.
3.3. mTORC1 inhibition increases eIF4E phosphorylation
eIF4E plays a critical role in initiating translation of mRNAs including those encoding oncogenic proteins. Therefore, eIF4E is considered to be a survival protein involved in cell cycle progression, cell transformation and apoptotic resistance (66-68). mTORC1 signaling positively regulates the synthesis of many oncogenic proteins through activating eIF4E function in cap-dependent translation initiation by phosphorylating 4E-BP1 (4, 26). Additionally, eIF4E is phosphorylated (usually at Ser209) in many systems in response to extracellular stimuli including growth factors, hormones and mitogens (67, 69, 70). The biological role of phosphorylated eIF4E in regulation of cap-dependent translation initiation is still controversial although it has been suggested that phosphorylation of eIF4E may increase its affinity for the cap of mRNA, and may also favor its entry into initiation complexes (67, 69, 70). However, phosphorylation of eIF4E may play a critical role in regulation of cell transformation and oncogenesis. A genetic study in Drosophila melanogaster suggested that eIF4E phosphorylation is biologically significant and is essential for normal growth and development (71). Overexpression of a mutant of eIF4E in which Ser209 has been altered to alanine is much less efficient than wild-type eIF4E in transforming NIH3T3 cells. In addition, the overexpression of wild-type, but not mutant eIF4E, increases cyclin D1 levels (72). Most importantly, a recent study using a mouse lymphoma model has convincingly demonstrated that eIF4E phosphorylation at Ser209 is absolutely required for eIF4E's ability to inhibit apoptosis and promote tumorigenesis (73).
The best candidate for eIF4E phosphorylation is the MAPK-activated protein kinase called MAP kinase-interacting kinase 1 (Mnk1), which physically associates with eIF4F and directly phosphorylates eIF4E at Ser209. In addition to Mnk1, Mnk2 also phosphorylates eIF4E, albeit to a lesser extent. Both Mnk1 and Mnk2, particularly Mnk1, are directly phosphorylated by ERK and p38 MAPKs (69, 70).
In addition to activation of Akt and ERK survival signaling pathways, we also reported that rapalogs paradoxically increase eIF4E phosphorylation (Ser209) in various types of cancer cells while inhibiting mTORC1 signaling. Like Akt phosphorylation, eIF4E phosphorylation by rapalogs occurs very rapidly and is sustained for a long time (up to 72 h) (53, 74). Rapalogs increase eIF4E phosphorylation at Ser209 through a Mnk-dependent mechanism since both Mnk inhibition with the Mnk inhibitor CGP57380 and Mnk deficiency abolished the ability of rapalogs to increase p-eIF4E levels (74). However, the MEK inhibitors UO126 and PD98059 and the p38 MAPK inhibitor SB203580 failed to block rapamycin-induced eIF4E phosphorylation, suggesting that mTOR inhibitors induce Mnk-mediated eIF4E phosphorylation independently of MAPK signaling pathways (53). Importantly, we found that inhibition of PI3K with small molecule PI3K inhibitors (i.e., LY294002 and wortmannin) or PI3K deficiency (e.g., p85 knockout) blocked rapalog-induced eIF4E phosphorylation, suggesting that rapalogs induce a PI3K-dependent, Mnk-mediated eIF4E phosphorylation (53, 74). Thus, our findings, for the first time, link PI3K to the activation of the Mnk/eIF4E survival signaling pathway (74).
4. Strategies to enhance mTOR-targeted cancer therapy
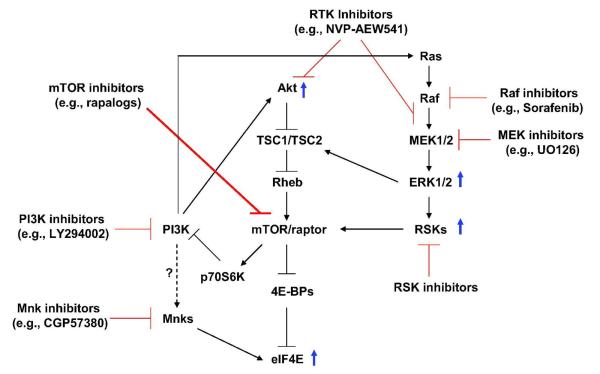
As discussed above, inhibition of mTORC1 paradoxically initiates feedback activation of several survival signaling pathways including Akt, MAPK/ERK and Mnk/eIF4E (Fig. 1), which may counteract or blunt the anticancer efficacy of rapalogs. Indeed, several studies have shown that inhibition of the feedback activation of these survival signaling pathways enhances the anticancer efficacy of rapalogs both in vitro and in vivo. Thus, these findings at least in part explain why rapalogs show very limited single agent activity in the majority of tested cancer types. Thus, combining a rapalog with other agents that can prevent or inhibit the feedback activation of these survival signaling pathways should be an important direction to achieve our goal of enhancing mTOR-targeted cancer therapy (Fig. 1). Moreover, we have shown that sustained Akt activation during mTORC inhibition is tightly associated with development of cell resistance (i.e., acquired rapamycin resistance) to rapalogs (15), a critical issue in mTOR-based cancer therapy. Thus, inhibition of sustained Akt activation may avoid or prevent development of rapamycin resistance as well.
Fig. 1. A schematic model for feedback activation of PI3K/Akt, ERK/RSK and Mnk/eIF4E survival signaling pathways by inhibition of mTORC1 with rapalogs.
Based on the current literature, it appears that PI3K activation plays a central role in mediating activation of these survival signaling pathways, although the underlying mechanisms need further study. The combination of a rapalog and one of the targeting agents (e.g., a PI3K inhibitor) as indicated may achieve enhanced anticancer activity.
It is worthy mentioning that the combination strategy may also result in enhanced side effects and toxicities in addition to augmenting therapeutic efficacies. Thus safety concerns on rapalog-based combinations should be considered and carefully investigated before we can move these potential combinations to the clinic.
4.1. Rapalogs combined with PI3K inhibitors
Inhibition of mTORC1 with a rapalog triggers PI3K-dependent Akt activation (53-55). Moreover, rapalog-induced activation of MAPK/ERK and Mnk/eIF4E signaling pathways is also PI3K-dependent (64, 74). Thus, it is plausible to speculate that blockage of PI3K-dependent activation of these survival signaling pathways will augment rapalogs' anticancer efficacy. As a proof-of-principle study, we examined the effect of rapamycin or RAD001 in combination with the PI3K inhibitor LY294002 on the growth of lung cancer cells in vitro and in vivo. As expected, the combination of rapamycin or RAD001 with LY294002 exhibited enhanced inhibitory effects on the growth of human lung cancer cells in cell cultures as evaluated by a short-term monolayer culture assay (i.e., sulforhodamine B assay) and a long-term colony formation assay (15, 53). Importantly, the RAD001 and LY294002 combination worked better than each single agent alone in significantly inhibiting the growth of human lung cancer xenografts in nude mice (15), indicating an enhanced anticancer activity in vivo. In agreement with in vitro findings, treatment of xenografts with RAD001 for a prolonged time (14 consecutive days) increased p-Akt levels, which could be abrogated by co-treatment with LY294002. Besides, we found that RAD001 plus LY294002 also exerted an enhanced effect on reduction of p-S6 levels, indicating that inhibition of PI3K/Akt enhances the rapalog's effect on inhibition of mTORC1 signaling (15). A similar result was also reported in adult T-cell leukemia cells. When rapamycin was combined with LY294002, rapamycin-induced phosphorylation of Akt was blocked, and the ability of rapamycin to induce growth arrest of HTLV-1-infected T-cells and suppress the p-p70S6K and p-4E-BP1 proteins was potentiated (75). Collectively, these results validate a strategy for cancer therapy of co-targeting mTOR and PI3K/Akt signaling.
4.2. Rapalogs combined with MAKP/ERK/RSK inhibitors
Recent studies have suggested that MAPK/ERK/RSK signaling functions upstream of and activates mTORC1 signaling (42, 44). Moreover, inhibition of mTORC1 induces feedback activation of MAPK/ERK/RSK signaling, which also attenuates the efficacy of rapalogs (64, 65). We (65) and others (64) reported that the rapamycin or RAD001 combined with a MEK (the direct upstream activator of ERK) inhibitor (e.g., UO126 or PD0325901) was more potent than each single agent in inhibiting the growth of cancer cells; this effect was associated with the abrogation of the feedback ERK activation. Moreover, the combination of RAD001 and PD0325901 exhibited additive antitumor effect in a mouse xenograft model in line with abrogation of RAD001-induced ERK activation (64). Similarly, the synergistic anticancer activity of rapamycin combined with PD0325901 was also reported in lung cancer xenograft models (76). Thus, it appears that pharmacological inhibition of the MAPK pathway enhances the antitumor effect of mTORC1 inhibition by a rapalog, suggesting that co-targeting mTORC1 and the MAPK/ERK signaling pathways should be an effective cancer therapeutic strategy.
Correspondingly, we can speculate that the combination of a rapalog with a Raf (direct upstream of MEK) or RSK (downstream of ERK) inhibitor may achieve enhanced anticancer efficacy as well. One report shows that the combination of rapamycin and sorafenib (a Raf inhibitor) increases survival and decreases tumor volume as compared to rapamycin or sorafenib treatment alone in a Tsc2−/− subcutaneous tumor model (77).
4.3. Rapalogs combined with Mnk inhibitors
Although the role of eIF4E phosphorylation in regulation of cap-dependent translation initiation is still controversial, some studies have suggested that phosphorylation of eIF4E may increase its affinity for the cap of mRNA, and may also favor its entry into initiation complexes (67, 69, 70). Moreover, Mnk-mediated eIF4E phosphorylation is critical for conferring apoptotic resistance and facilitating oncogenesis (73). Therefore, it is important to prevent feedback activation of Mnk1/eIF4E signaling during mTOR-targeted cancer therapy. In this way, we may achieve the goal of enhancing the anticancer activity of rapalogs as well. In our study, we found that the presence of the Mnk inhibitor CGP57380 not only abrogated rapamycin-induced increase in eIF4E phosphorylation, but also enhanced rapamycin's effects on inhibiting the growth of several human lung cancer cells (74). Our findings warrant further study in the direction of co-targeting mTORC1 signaling and Mnk/eIF4E phosphorylation as a potential cancer therapeutic strategy.
4.4. Rapalogs combined with receptor tyrosine-kinase inhibitors (RTKIs)
The findings demonstrating the involvement of insulin-like growth factor-1 receptor (IGF-1R) in mediating rapalog-induced Akt phosphorylation (54-56) led to the hypothesis that combining a rapalog with an IGF-1R inhibitor may be an appropriate strategy to enhance mTOR-targeted anticancer therapy. Indeed, rapamycin combined with the IGF-1R inhibitor NVPAEW541 was reported to exert additive antiproliferative effects as compared with either agent alone in prostate and breast cancer cells (54). Similar results were also observed in myeloma cells when RAD001 was combined with NVP-AEW541 (78).
In addition to IGF-1R, platelet-derived growth factor receptors (PDGFRs) have also been demonstrated to mediate mTORC1 inhibition-induced PI3K/Akt activation (79). Thus, it is possible to generate enhanced anticancer efficacy when a rapalog is combined with another RTKI, particularly a multikinase inhibitor. In renal cell carcinoma cells, the combination of RAD001 and the multikinase inhibitor AEE788 resulted in more pronounced growth inhibition, greater rates of G0/G1 cells and lower rates of S-phase cells than either agent alone (80). In glioma cells, combined RAD001 and AEE788 resulted in increased rates of cell cycle arrest and apoptosis and reduced proliferation compared with either agent alone. Moreover the combination given orally to athymic mice bearing established human malignant glioma tumor xenografts resulted in greater tumor growth inhibition and greater increases in median survival than monotherapy (81). Similarly, the combination of rapamycin and another RTKI, ABT-869, was significantly better than each single agent in reducing the growth of hepatocellular carcinoma xenograft in mice (82). In prostate cancer cells, rapamycin combined with imatinib mesylate (Gleevec), a PDGFR, c-kit and bcr/abl antagonist, showed enhanced growth-inhibitory effects as well (83).
Epidermal growth factor receptor (EGFR) inhibitors represent another group of RTKIs that show clinical efficacy, particularly in lung cancer patients. Several studies including ours have shown that a rapalog in combination with an EGFR inhibitor (e.g., erlotinib or gefitinib) exerted enhanced anticancer activity both in cell cultures and in mouse tumor xenograft models of several types of cancers such as lung cancer (65, 84-88). We found that the presence of erlotinib suppressed rapamycin-induced phosphorylation of Akt, ERK and eIF4E (65), implying that erlotinib can suppress mTORC1 inhibition-induced feedback activation of several survival signaling pathways including Akt, ERK and eIF4E.
5. Conclusion
Targeting the mTOR signaling network appears to be an attractive cancer therapeutic strategy. The success of using conventional mTOR inhibitors (i.e., rapalogs) as a monotherapy is limited in the clinic, largely due to the not-yet fully understood complexity of the mTOR signaling network. Thus, further studies are needed to fully understand the biology of the mTOR signaling network to guide the development of more efficacious therapeutic regimens and/or novel mTOR inhibitors. Currently, one strategy that may enhance mTOR-targeted cancer therapy is to combine a rapalog with a targeting drug that may inhibit rapalog-induced feedback activation of one or multiple survival signaling pathways (Fig. 1).
6. Expert opinion
Although the mTOR axis represents an attractive cancer therapeutic target, the single agent activities of rapalogs have not been encouraging in clinical trials against the majority of tumor types, with the exception of advanced renal cell carcinomas. This is likely due to the complexity of the mTOR signaling networks as we discussed above. Inhibition of the mTORC1 signaling with a rapalog induces feedback activation of several survival signaling pathways including PI3K/Akt, MAPK/ERK and Mnk/eIF4E, which will blunt the anticancer efficacy of rapalogs or lead to cell resistance to rapalogs. Thus, it is crucial to fully understand the biology of the mTOR signaling network. Then, we may develop efficacious therapeutic regimens (e.g., rational combinations) or identify other novel targets in the mTOR signaling for developing novel mTOR inhibitors.
Considering the critical role of PI3K activation in mTORC1 inhibition-induced feedback activation of these survival signaling pathways, future drug direction toward targeting the mTOR axis should focus on the development of novel mTOR and PI3K dual inhibitors. These dual inhibitors may maintain their potent mTOR inhibitor activity, but without activating Akt, ERK and eIF4E survival mechanisms. Theoretically, these agents may have better single agent anticancer activity than the conventional rapalogs. Efforts toward this direction have developed some mTOR/PI3K dual inhibitors such as PI-103 (89) and NVP-BEZ235 (90), which exhibit promising preclinical anticancer activity. NVP-BEZ235 is currently being tested in clinical trials. Thus, time will tell us whether this group of agents have better anticancer efficacy than the conventional rapalogs.
Akt is an essential cancer survival kinase and is frequently activated in many cancer types (91). The findings that mTORC2 acts as an Akt ser473 kinase (6) and is critical for the survival of prostate cancer cells (92) have made mTORC2 an attractive cancer therapeutic target. Thus, efforts have been made to develop mTORC2-specific inhibitors or mTORC1 and mTORC2 dual inhibitors including mTOR catalytic inhibitors (18). Such inhibitors are not only potential cancer therapeutic agents (93, 94), but also useful tools for studying the complexity of the mTOR signaling (95). In this regard, Akt Ser 473 phosphorylation is generally used as a readout of mTORC2 activity. We recently have shown that rapamycin treatment increases Akt Ser473 phosphorylation under conditions in which mTORC2 assembly is disrupted (15). A similar finding was also reported in TSC2−/− MEF cells (96). Thus it is questionable whether Akt Ser473 phosphorylation can necessarily reflect mTOR2 activity. In addition to Akt Ser473 phosphorylation, mTORC2 has also been implicated in phosphorylation of other proteins including Akt (at Ser450), SGK1 (at Ser422), and PKCa (at Ser 638 and Ser657) (7, 13, 25, 97-99). Thus, additional protein phosphorylation (e.g., SGK1 or PKCa) should be monitored as well when evaluating mTORC2 activity.
Another important issue that may improve the clinical outcomes of mTOR-targeted cancer therapy is patient selection. We need to identify those patient populations who are most likely to respond to mTOR-targeted cancer therapy. In this regard, identification of reliable biomarkers that may predict tumor response to rapalogs or other mTOR inhibitors is both crucial and challenging. While PTEN deficiency has been suggested to predict cancer cell sensitivity to rapalogs (100), a recent study has shown that PTEN loss does not predict for response to RAD001 in a glioblastoma orthotopic xenograft test panel (101). The E3 ligase F-box and WD repeat domain containing 7 (FBXW7) has recently been demonstrated to mediate ubiquitination and proteasome degradation of mTOR protein (102). Accordingly, breast cancer cell lines harboring deletions or mutations in FBXW7 were particularly sensitive to rapamycin treatment, thus suggesting that loss of FBXW7 may be a biomarker for human cancers that are susceptible to treatment with rapalogs (102). The overall frequency of FBXW7 mutation or deletion is approximately 6% in human primary cancers (103). Thus, further work is needed to validate the predictive value of FBXW7 mutation or deletion in certain types of cancers harboring high mutation frequency of FBXW7. Fully understanding the biology of mTOR signaling will facilitate our research in this regard.
Many human cancers exhibit elevated PLD activity (28). Because rapamycin is suggested to inhibit mTOR activity by preventing the interaction between mTOR and PA (28), cellular PA levels affects cell responses to rapamycin. Thus we need to consider the potential negative impact of PLD activity or PA levels on rapalog-based cancer therapy. Whether PLD activity or PA levels can be used to predict cancer cell sensitivity to rapalogs or to select patients who may better respond to rapalogs clinically needs investigation. Accordingly, a potential strategy foe enhancing mTOR-targeted cancer therapy by reducing the level of PA needs to be explored as well.
Acknowledgements
Studies in the author's laboratory were supported by the Georgia Cancer Coalition Distinguished Cancer Scholar award, the Department of Defense IMPACT award W81XWH-05-0027 (Project 5 to F.R. K. and S-Y. S.) and BATTLE award W81XWH-06-1-0303 (Project 4 to F.R. K. and S-Y. S.), and the National Institute of Health R01 CA118450-01 (to S-Y. S.) and P01 CA116676-01 (Project 1 to F. Khuri and S-Y. Sun).
S-Y. Sun is a Georgia Cancer Coalition Distinguished Cancer Scholar.
References
- 1.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 2.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–48. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 3.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 4*.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. A comprehensive review on the role of mTOR signaling in cancer. [DOI] [PubMed] [Google Scholar]
- 5.Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol Cell Biol. 2009;29:1411–20. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. First study showing that mTORC2 functions as a Ser473 Akt kinase. [DOI] [PubMed] [Google Scholar]
- 7.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. 2004 doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 8.Amato R, Menniti M, Agosti V, et al. IL-2 signals through Sgk1 and inhibits proliferation and apoptosis in kidney cancer cells. J Mol Med. 2007;85:707–21. doi: 10.1007/s00109-007-0205-2. [DOI] [PubMed] [Google Scholar]
- 9.Tangir J, Bonafe N, Gilmore-Hebert M, Henegariu O, Chambers SK. SGK1, a potential regulator of c-fms related breast cancer aggressiveness. Clin Exp Metastasis. 2004;21:477–83. doi: 10.1007/s10585-004-4226-8. [DOI] [PubMed] [Google Scholar]
- 10.Sahoo S, Brickley DR, Kocherginsky M, Conzen SD. Coordinate expression of the PI3-kinase downstream effectors serum and glucocorticoid-induced kinase (SGK-1) and Akt-1 in human breast cancer. Eur J Cancer. 2005;41:2754–9. doi: 10.1016/j.ejca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Dehner M, Hadjihannas M, Weiske J, Huber O, Behrens J. Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through up-regulation of serum- and glucocorticoid-inducible kinase 1. J Biol Chem. 2008;283:19201–10. doi: 10.1074/jbc.M710366200. [DOI] [PubMed] [Google Scholar]
- 12.Shanmugam I, Cheng G, Terranova PF, Thrasher JB, Thomas CP, Li B. Serum/glucocorticoid-induced protein kinase-1 facilitates androgen receptor-dependent cell survival. Cell Death Differ. 2007;14:2085–94. doi: 10.1038/sj.cdd.4402227. [DOI] [PubMed] [Google Scholar]
- 13*.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–85. doi: 10.1042/BJ20081668. First study to show that SGK1 is a mTORC2-specific substrate. [DOI] [PubMed] [Google Scholar]
- 14.Sarbassov dos D, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun SY. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008;68:7409–18. doi: 10.1158/0008-5472.CAN-08-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–7. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choo AY, Blenis J. TORgeting oncogene addiction for cancer therapy. Cancer Cell. 2006;9:77–9. doi: 10.1016/j.ccr.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 18**.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. An important review on development of mTOR inhibitors. [DOI] [PubMed] [Google Scholar]
- 19.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England journal of medicine. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 20.Amato RJ, Jac J, Giessinger S, Saxena S, Willis JP. A phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic clear cell renal cell cancer. Cancer. 2009;115:2438–46. doi: 10.1002/cncr.24280. [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 22.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007;13:433–42. doi: 10.1016/j.molmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res. 2007;13:3109–14. doi: 10.1158/1078-0432.CCR-06-2798. [DOI] [PubMed] [Google Scholar]
- 25**.Alessi DR, Pearce LR, Garcia-Martinez JM. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal. 2009;2:pe27. doi: 10.1126/scisignal.267pe27. A detailed review on mTOR complexes and their potential substrates. [DOI] [PubMed] [Google Scholar]
- 26*.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–18. doi: 10.1038/nrm2672. A comprehensive review on mTOR signaling and translational control. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–22. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster DA. Phosphatidic acid signaling to mTOR: Signals for the survival of human cancer cells. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbalip.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodfellow IG, Roberts LO. Eukaryotic initiation factor 4E. Int J Biochem Cell Biol. 2007 doi: 10.1016/j.biocel.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–99. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 31.Clemens MJ. Targets and mechanisms for the regulation of translation in malignant transformation. Oncogene. 2004;23:3180–8. doi: 10.1038/sj.onc.1207544. [DOI] [PubMed] [Google Scholar]
- 32.Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 2008;68:631–4. doi: 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]
- 33*.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–5. doi: 10.1126/science.1066015. First study that links phosphatidic acid to the mTOR signaling. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Fang Y, Yoon MS, et al. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci U S A. 2008;105:8286–91. doi: 10.1073/pnas.0712268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 36.Sancak Y, Thoreen CC, Peterson TR, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–15. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Harris TE, Roth RA, Lawrence JC., Jr. PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–44. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Harris TE, Lawrence JC., Jr. Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation. J Biol Chem. 2008;283:15619–27. doi: 10.1074/jbc.M800723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fonseca BD, Smith EM, Lee VH, MacKintosh C, Proud CG. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007;282:24514–24. doi: 10.1074/jbc.M704406200. [DOI] [PubMed] [Google Scholar]
- 40.Oshiro N, Takahashi R, Yoshino K, et al. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem. 2007;282:20329–39. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma L, Teruya-Feldstein J, Bonner P, et al. Identification of S664 TSC2 phosphorylation as a marker for extracellular signal-regulated kinase mediated mTOR activation in tuberous sclerosis and human cancer. Cancer Res. 2007;67:7106–12. doi: 10.1158/0008-5472.CAN-06-4798. [DOI] [PubMed] [Google Scholar]
- 42*.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–93. doi: 10.1016/j.cell.2005.02.031. First study to show that ERK activates mTORC1 signaling. [DOI] [PubMed] [Google Scholar]
- 43.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A. 2004;101:13489–94. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carriere A, Cargnello M, Julien LA, et al. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol. 2008;18:1269–77. doi: 10.1016/j.cub.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 45.Yoo LI, Chung DC, Yuan J. LKB1--a master tumour suppressor of the small intestine and beyond. Nat Rev Cancer. 2002;2:529–35. doi: 10.1038/nrc843. [DOI] [PubMed] [Google Scholar]
- 46.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–9. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene. 2007;26:7825–32. doi: 10.1038/sj.onc.1210594. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Cespedes M, Parrella P, Esteller M, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–62. [PubMed] [Google Scholar]
- 51.Carretero J, Medina PP, Pio R, Montuenga LM, Sanchez-Cespedes M. Novel and natural knockout lung cancer cell lines for the LKB1/STK11 tumor suppressor gene. Oncogene. 2004;23:4037–40. doi: 10.1038/sj.onc.1207502. [DOI] [PubMed] [Google Scholar]
- 52.Makowski L, Hayes DN. Role of LKB1 in lung cancer development. Br J Cancer. 2008;99:683–8. doi: 10.1038/sj.bjc.6604515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53**.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E Survival Pathways by Rapamycin-Mediated Mammalian Target of Rapamycin Inhibition. Cancer Res. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. First report on Akt and eIF4E phosphorylation by rapalogs in cancer cells. [DOI] [PubMed] [Google Scholar]
- 54.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–40. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 56.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 57.Duran I, Kortmansky J, Singh D, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95:1148–54. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–10. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 59.Zeng Z, Sarbassov dos D, Samudio IJ, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–12. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Easton JB, Kurmasheva RT, Houghton PJ. IRS-1: auditing the effectiveness of mTOR inhibitors. Cancer Cell. 2006;9:153–5. doi: 10.1016/j.ccr.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 63.Peterson TR, Laplante M, Thoreen CC, et al. DEPTOR Is an mTOR Inhibitor Frequently Overexpressed in Multiple Myeloma Cells and Required for Their Survival. Cell. 2009;137:873–86. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008 doi: 10.1172/JCI34739. First study to show that mTORC1 inhibition activates ERK signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Hawk N, Yue P, et al. Overcoming mTOR inhibition-induced paradoxical activation of survival signaling pathways enhances mTOR inhibitors' anticancer efficacy. Cancer Biol Ther. 2008;7:1952–8. doi: 10.4161/cbt.7.12.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E--from translation to transformation. Oncogene. 2004;23:3172–9. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 67.Raught B, Gingras AC. eIF4E activity is regulated at multiple levels. Int J Biochem Cell Biol. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- 68.Bjornsti MA, Houghton PJ. Lost in translation: dysregulation of cap-dependent translation and cancer. Cancer Cell. 2004;5:519–23. doi: 10.1016/j.ccr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 69.Mahalingam M, Cooper JA. Phosphorylation of mammalian eIF4E by Mnk1 and Mnk2: tantalizing prospects for a role in translation. Prog Mol Subcell Biol. 2001;27:132–42. [PubMed] [Google Scholar]
- 70.Pyronnet S. Phosphorylation of the cap-binding protein eIF4E by the MAPK-activated protein kinase Mnk1. Biochem Pharmacol. 2000;60:1237–43. doi: 10.1016/s0006-2952(00)00429-9. [DOI] [PubMed] [Google Scholar]
- 71.Lachance PE, Miron M, Raught B, Sonenberg N, Lasko P. Phosphorylation of eukaryotic translation initiation factor 4E is critical for growth. Mol Cell Biol. 2002;22:1656–63. doi: 10.1128/MCB.22.6.1656-1663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Topisirovic I, Ruiz-Gutierrez M, Borden KL. Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res. 2004;64:8639–42. doi: 10.1158/0008-5472.CAN-04-2677. [DOI] [PubMed] [Google Scholar]
- 73*.Wendel HG, Silva RL, Malina A, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–7. doi: 10.1101/gad.1604407. First mouse genetic study on the critical role of eIF4E phosphorylation in oncogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74*.Wang X, Yue P, Chan CB, et al. Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3 kinase-dependent and Mnk-mediated eIF4E phosphorylation. Mol Cell Biol. 2007 doi: 10.1128/MCB.00760-07. First study showing that rapalogs induces PI3K-dependent, Mnk-mediated eIF4E phosphorylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ikezoe T, Nishioka C, Bandobashi K, et al. Longitudinal inhibition of PI3K/Akt/mTOR signaling by LY294002 and rapamycin induces growth arrest of adult T-cell leukemia cells. Leukemia research. 2007;31:673–82. doi: 10.1016/j.leukres.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 76.Legrier ME, Yang CP, Yan HG, et al. Targeting protein translation in human non small cell lung cancer via combined MEK and mammalian target of rapamycin suppression. Cancer Res. 2007;67:11300–8. doi: 10.1158/0008-5472.CAN-07-0702. [DOI] [PubMed] [Google Scholar]
- 77.Lee N, Woodrum CL, Nobil AM, Rauktys AE, Messina MP, Dabora SL. Rapamycin weekly maintenance dosing and the potential efficacy of combination sorafenib plus rapamycin but not atorvastatin or doxycycline in tuberous sclerosis preclinical models. BMC Pharmacol. 2009;9:8. doi: 10.1186/1471-2210-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baumann P, Hagemeier H, Mandl-Weber S, Franke D, Schmidmaier R. Myeloma cell growth inhibition is augmented by synchronous inhibition of the insulin-like growth factor-1 receptor by NVP-AEW541 and inhibition of mammalian target of rapamycin by Rad001. Anticancer Drugs. 2009;20:259–66. doi: 10.1097/CAD.0b013e328328d18b. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H, Bajraszewski N, Wu E, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–8. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Juengel E, Engler J, Natsheh I, et al. Combining the receptor tyrosine kinase inhibitor AEE788 and the mammalian target of rapamycin (mTOR) inhibitor RAD001 strongly inhibits adhesion and growth of renal cell carcinoma cells. BMC Cancer. 2009;9:161. doi: 10.1186/1471-2407-9-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goudar RK, Shi Q, Hjelmeland MD, et al. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther. 2005;4:101–12. [PubMed] [Google Scholar]
- 82.Jasinghe VJ, Xie Z, Zhou J, et al. ABT-869, a multi-targeted tyrosine kinase inhibitor, in combination with rapamycin is effective for subcutaneous hepatocellular carcinoma xenograft. J Hepatol. 2008;49:985–97. doi: 10.1016/j.jhep.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 83.Masiello D, Mohi MG, McKnight NC, et al. Combining an mTOR antagonist and receptor tyrosine kinase inhibitors for the treatment of prostate cancer. Cancer Biol Ther. 2007;6:195–201. doi: 10.4161/cbt.6.2.3588. [DOI] [PubMed] [Google Scholar]
- 84.Rao RD, Mladek AC, Lamont JD, et al. Disruption of parallel and converging signaling pathways contributes to the synergistic antitumor effects of simultaneous mTOR and EGFR inhibition in GBM cells. Neoplasia. 2005;7:921–9. doi: 10.1593/neo.05361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bianco R, Garofalo S, Rosa R, et al. Inhibition of mTOR pathway by everolimus cooperates with EGFR inhibitors in human tumours sensitive and resistant to anti-EGFR drugs. Br J Cancer. 2008;98:923–30. doi: 10.1038/sj.bjc.6604269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buck E, Eyzaguirre A, Brown E, et al. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Mol Cancer Ther. 2006;5:2676–84. doi: 10.1158/1535-7163.MCT-06-0166. [DOI] [PubMed] [Google Scholar]
- 87.Birle DC, Hedley DW. Signaling interactions of rapamycin combined with erlotinib in cervical carcinoma xenografts. Mol Cancer Ther. 2006;5:2494–502. doi: 10.1158/1535-7163.MCT-05-0504. [DOI] [PubMed] [Google Scholar]
- 88.Azzariti A, Porcelli L, Gatti G, Nicolin A, Paradiso A. Synergic antiproliferative and antiangiogenic effects of EGFR and mTor inhibitors on pancreatic cancer cells. Biochem Pharmacol. 2008;75:1035–44. doi: 10.1016/j.bcp.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 89.Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–9. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–63. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 91.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guertin DA, Stevens DM, Saitoh M, et al. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009;15:148–59. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thoreen CC, Kang SA, Chang JW, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–32. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu K, Toral-Barza L, Shi C, et al. Biochemical, Cellular, and In vivo Activity of Novel ATP-Competitive and Selective Inhibitors of the Mammalian Target of Rapamycin. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- 95.Feldman ME, Apsel B, Uotila A, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–15. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. Embo J. 2008;27:1919–31. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Facchinetti V, Ouyang W, Wei H, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. Embo J. 2008;27:1932–43. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guertin DA, Stevens DM, Thoreen CC, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 100.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98:10314–9. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang L, Clarke MJ, Carlson BL, et al. PTEN loss does not predict for response to RAD001 (Everolimus) in a glioblastoma orthotopic xenograft test panel. Clin Cancer Res. 2008;14:3993–4001. doi: 10.1158/1078-0432.CCR-07-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mao JH, Kim IJ, Wu D, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321:1499–502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Akhoondi S, Sun D, von der Lehr N, et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–12. doi: 10.1158/0008-5472.CAN-07-1320. [DOI] [PubMed] [Google Scholar]