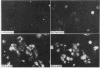
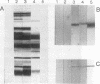
Abstract
Leishmania is a genus of parasitic protozoa capable of causing a spectrum of human diseases. The GP46/M-2 membrane glycoprotein has been demonstrated in a murine model system to elicit a protective immune response against infection with Leishmania amazonensis; in highly susceptible BALB/c mice, immunization leads to significant protection against infection. In the present study, for induction of long-term immunological effects, two recombinant vaccinia viruses, derived from the wild type and attenuated variant 48-7 and expressing the GP46/M-2 protein, were constructed; to ensure safety, we used the attenuated vaccinia virus mutant (48-7) as a live vector. Susceptible BALB/c mice immunized with either GP46/M-2-recombinant vaccinia virus were significantly protected against infection with L. amazonensis; 45 to 76% of the animals were completely protected (sterile) against a challenge inoculum of 10(3) infective organisms. The protectively immunized animals demonstrated T- and B-cell-dependent immunological responses; both lymphokine responses as well as antibody responses and long-term memory are indicative of T-cell activation. This first report of the use of a recombinant vaccinia virus to induce protection against a Leishmania infection indicates that recombinant vaccinia viruses should be of value in the design of a safe and effective vaccine against this parasitic disease.
Full text
PDF
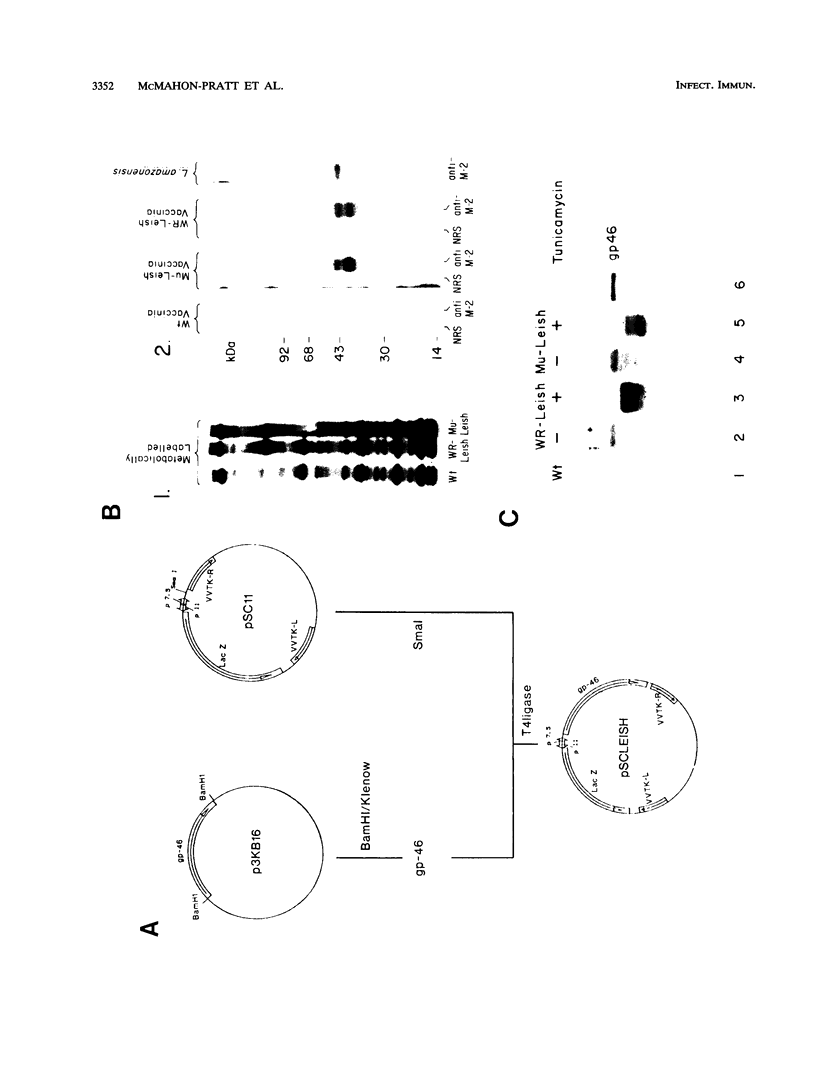


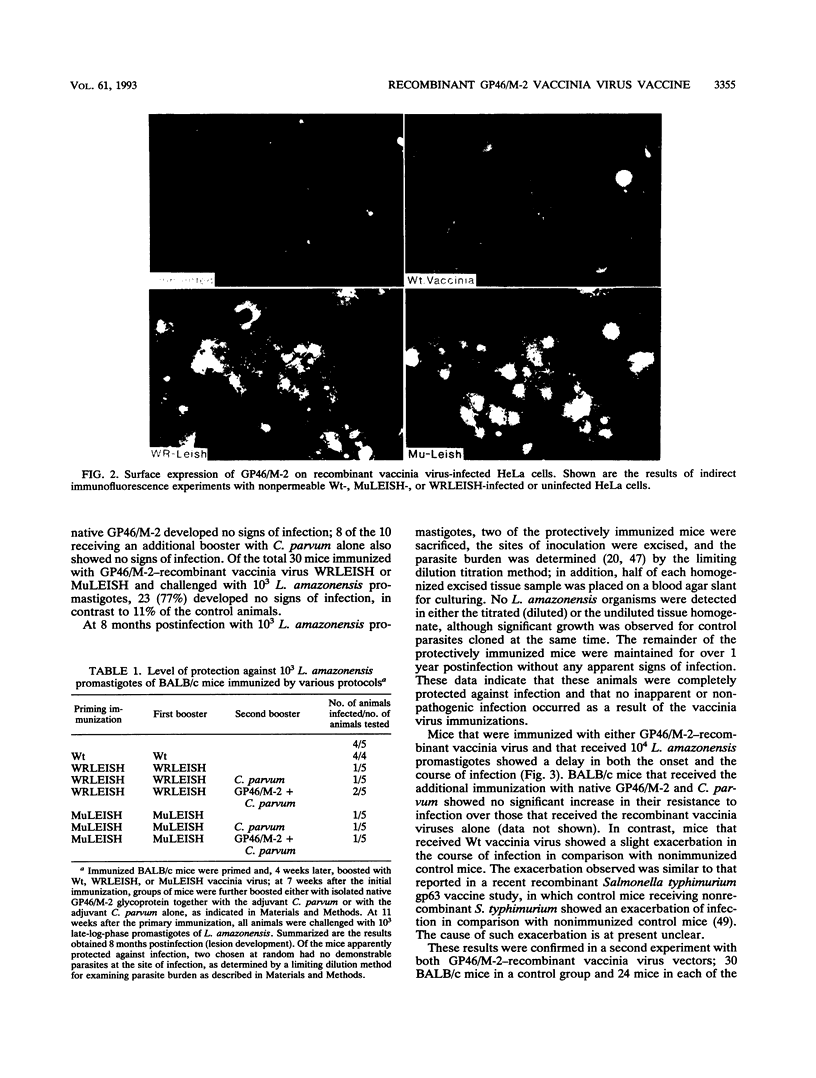


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennink J. R., Yewdell J. W. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153–184. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Smith G. L., Moller C., Moss B. Recombinant vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. Nature. 1984 Oct 11;311(5986):578–579. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- Burns J. M., Jr, Scott J. M., Carvalho E. M., Russo D. M., March C. J., Van Ness K. P., Reed S. G. Characterization of a membrane antigen of Leishmania amazonensis that stimulates human immune responses. J Immunol. 1991 Jan 15;146(2):742–748. [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champsi J., McMahon-Pratt D. Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect Immun. 1988 Dec;56(12):3272–3279. doi: 10.1128/iai.56.12.3272-3279.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallo S., Esteban M. Isolation and characterization of attenuated mutants of vaccinia virus. Virology. 1987 Aug;159(2):408–422. doi: 10.1016/0042-6822(87)90480-6. [DOI] [PubMed] [Google Scholar]
- Farrell J. P., Muller I., Louis J. A. A role for Lyt-2+ T cells in resistance to cutaneous leishmaniasis in immunized mice. J Immunol. 1989 Mar 15;142(6):2052–2056. [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Gong S. C., Lai C. F., Dallo S., Esteban M. A single point mutation of Ala-25 to Asp in the 14,000-Mr envelope protein of vaccinia virus induces a size change that leads to the small plaque size phenotype of the virus. J Virol. 1989 Nov;63(11):4507–4514. doi: 10.1128/jvi.63.11.4507-4514.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. M., Stern M. L., Haviland D. L., Mills B. J., Ware C. F. Cytotoxic lymphokines produced by cloned human cytotoxic T lymphocytes. I. Cytotoxins produced by antigen-specific and natural killer-like CTL are dissimilar to classical lymphotoxins. J Immunol. 1985 Dec;135(6):4034–4043. [PubMed] [Google Scholar]
- Greenblatt C. L. The present and future of vaccination for cutaneous leishmaniasis. Prog Clin Biol Res. 1980;47:259–285. [PubMed] [Google Scholar]
- Grimaldi G., Jr, David J. R., McMahon-Pratt D. Identification and distribution of New World Leishmania species characterized by serodeme analysis using monoclonal antibodies. Am J Trop Med Hyg. 1987 Mar;36(2):270–287. doi: 10.4269/ajtmh.1987.36.270. [DOI] [PubMed] [Google Scholar]
- Handman E., Button L. L., McMaster R. W. Leishmania major: production of recombinant gp63, its antigenicity and immunogenicity in mice. Exp Parasitol. 1990 May;70(4):427–435. doi: 10.1016/0014-4894(90)90127-x. [DOI] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks L. D., Wood D. E., Hajduk M. E. Haemoflagellates: commercially available liquid media for rapid cultivation. Parasitology. 1978 Jun;76(3):309–316. doi: 10.1017/s0031182000048186. [DOI] [PubMed] [Google Scholar]
- Hill J. O., Awwad M., North R. J. Elimination of CD4+ suppressor T cells from susceptible BALB/c mice releases CD8+ T lymphocytes to mediate protective immunity against Leishmania. J Exp Med. 1989 May 1;169(5):1819–1827. doi: 10.1084/jem.169.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. O., Fahey J. R. Leishmania spp.: agar plating as an alternative to limiting dilution and impression smears for the enumeration of viable parasites in tissue. Exp Parasitol. 1987 Feb;63(1):108–111. doi: 10.1016/0014-4894(87)90083-x. [DOI] [PubMed] [Google Scholar]
- Jardim A., Alexander J., Teh H. S., Ou D., Olafson R. W. Immunoprotective Leishmania major synthetic T cell epitopes. J Exp Med. 1990 Aug 1;172(2):645–648. doi: 10.1084/jem.172.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl L. P., Scott C. A., Lelchuk R., Gregoriadis G., Liew F. Y. Vaccination against murine cutaneous leishmaniasis by using Leishmania major antigen/liposomes. Optimization and assessment of the requirement for intravenous immunization. J Immunol. 1989 Jun 15;142(12):4441–4449. [PubMed] [Google Scholar]
- Klavinskis L. S., Whitton J. L., Joly E., Oldstone M. B. Vaccination and protection from a lethal viral infection: identification, incorporation, and use of a cytotoxic T lymphocyte glycoprotein epitope. Virology. 1990 Oct;178(2):393–400. doi: 10.1016/0042-6822(90)90336-p. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lohman K. L., Langer P. J., McMahon-Pratt D. Molecular cloning and characterization of the immunologically protective surface glycoprotein GP46/M-2 of Leishmania amazonensis. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8393–8397. doi: 10.1073/pnas.87.21.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath M. J., Murray H. W., Cohn Z. A. The dynamics of granuloma formation in experimental visceral leishmaniasis. J Exp Med. 1988 Jun 1;167(6):1927–1937. doi: 10.1084/jem.167.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Taylor E., Willey D. E., Cantin E. M., Eberle R., Moss B., Openshaw H. A recombinant vaccinia virus expressing herpes simplex virus type 1 glycoprotein B induces cytotoxic T lymphocytes in mice. J Gen Virol. 1988 Jul;69(Pt 7):1731–1734. doi: 10.1099/0022-1317-69-7-1731. [DOI] [PubMed] [Google Scholar]
- McMahon-Pratt D., Traub-Cseko Y., Lohman K. L., Rogers D. D., Beverley S. M. Loss of the GP46/M-2 surface membrane glycoprotein gene family in the Leishmania braziliensis complex. Mol Biochem Parasitol. 1992 Jan;50(1):151–160. doi: 10.1016/0166-6851(92)90252-f. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Moss B., Flexner C. Vaccinia virus expression vectors. Annu Rev Immunol. 1987;5:305–324. doi: 10.1146/annurev.iy.05.040187.001513. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies recognizing determinants specific for the promastigote state of Leishmania mexicana. Mol Biochem Parasitol. 1982 Nov;6(5):317–327. doi: 10.1016/0166-6851(82)90064-0. [DOI] [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies that distinguish between New World species of Leishmania. Nature. 1981 Jun 18;291(5816):581–583. doi: 10.1038/291581a0. [DOI] [PubMed] [Google Scholar]
- Rivas L., Kahl L., Manson K., McMahon-Pratt D. Biochemical characterization of the protective membrane glycoprotein GP46/M-2 of Leishmania amazonensis. Mol Biochem Parasitol. 1991 Aug;47(2):235–243. doi: 10.1016/0166-6851(91)90183-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez D., Rodriguez J. R., Rodriguez J. F., Trauber D., Esteban M. Highly attenuated vaccinia virus mutants for the generation of safe recombinant viruses. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1287–1291. doi: 10.1073/pnas.86.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. R., Rodriguez D., Esteban M. Structural properties of HIV-1 Env fused with the 14-kDa vaccinia virus envelope protein. Virology. 1991 Apr;181(2):742–748. doi: 10.1016/0042-6822(91)90910-4. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Bergman C. M., McGrath K. M., Lingenheld E. G., Grunnet M. L., Padula S. J., Clark R. B. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990 Oct 1;172(4):1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Pearce E., Natovitz P., Sher A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987 Jul 1;139(1):221–227. [PubMed] [Google Scholar]
- Stern J. J., Oca M. J., Rubin B. Y., Anderson S. L., Murray H. W. Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol. 1988 Jun 1;140(11):3971–3977. [PubMed] [Google Scholar]
- Titus R. G., Marchand M., Boon T., Louis J. A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985 Sep;7(5):545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D. M., Fairweather N., Button L. L., McMaster W. R., Kahl L. P., Liew F. Y. Oral Salmonella typhimurium (AroA-) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990 Oct 1;145(7):2281–2285. [PubMed] [Google Scholar]
- Zubler R. H., Lowenthal J. W., Erard F., Hashimoto N., Devos R., MacDonald H. R. Activated B cells express receptors for, and proliferate in response to, pure interleukin 2. J Exp Med. 1984 Oct 1;160(4):1170–1183. doi: 10.1084/jem.160.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]