Abstract
Common γ chain (γc)-receptor dependent cytokines are required for regulatory T cell (Treg) development as γc−/− mice lack Tregs. However, it is unclear which γc-dependent cytokines are involved in this process. Furthermore, TSLP has also been suggested to play a role in Treg development. Herein, we demonstrate that developing CD4+Foxp3+ Tregs in the thymus express the IL2Rβ, IL4Rα, IL7Rα, IL15Rα and IL21Rα chains, but not the IL9Rα or TSLPRα chains. Moreover, only IL2, and to a much lesser degree IL7 and IL15, were capable of transducing signals in CD4+Foxp3+ Tregs as determined by monitoring STAT5 phosphorylation. Likewise, IL2, IL7 and IL15, but not TSLP, were capable of inducing the conversion of CD4+CD25+Foxp3− thymic Treg progenitors into CD4+Foxp3+ mature Tregs in vitro. To examine this issue in more detail we generated IL2Rβ−/− x IL7Rα−/− and IL2Rβ−/− x IL4Rα−/− mice. We found that IL2Rβ−/− x IL7Rα−/− mice were devoid of Tregs thereby recapitulating the phenotype observed in γc−/− mice; in contrast, the phenotype observed in IL2Rβ−/− x IL4Rα−/− mice was comparable to that seen in IL2Rβ−/− mice. Finally, we observed that Tregs from both IL2−/− and IL2Rβ−/− mice show elevated expression of IL7Rα and IL15Rα chains. Addition of IL2 to Tregs from IL2−/− mice led to rapid downregulation of these receptors. Taken together, our results demonstrate that IL2 plays the predominant role in Treg development, but that in its absence the IL7Rα and IL15Rα chains are upregulated and allow for IL7 and IL15 to partially compensate for loss of IL2.
Keywords: Regulatory T cells, Cytokines, T cell Development
Introduction
CD4+Foxp3+ regulatory T cells (Tregs)4 that develop in the thymus play a key role in preventing autoimmune disease (1). Multiple signals are required to induce the development of Tregs. These include signals emanating from the TCR and the costimulatory molecule CD28 (2–5). In addition, several studies have suggested that cytokines play a key role in this process (6–11). However, which cytokines are involved has remained controversial. For example, it has been known for many years that mice lacking IL2, or the IL2Rα or IL2Rβ chains, develop lethal autoimmune disease (12–14). This was initially attributed to defective Treg development as these mice lacked CD4+CD25+ T cells. More recent studies utilizing the transcription factor Foxp3 as an identifier of Tregs found that young IL2−/− and IL2Rα−/− mice have relatively normal numbers of CD4+Foxp3+ Tregs (8, 15, 16). In contrast, both IL2Rβ−/− and IL2−/− x IL15−/− mice exhibited significant decreases in Treg numbers suggesting that IL2 and IL15 play a redundant role in Treg development (8, 9).
Importantly, although Treg differentiation is inhibited in IL2Rβ−/− mice, Tregs are not completely absent (8, 9). This raises the possibility that other cytokines can also drive Treg differentiation. Along these lines, Watanabe et al. have suggested a role for the cytokine TSLP. Specifically, they suggested that TSLP production by Hassall’s corpuscles plays an important role in human Treg development (17). Likewise, the common gamma chain (γc), which is closely related to the TSLPR, has also been shown to be involved in Treg development. For example, we and others have demonstrated that mice lacking the common gamma chain are devoid of Tregs (8, 15). The common gamma chain forms a component of multiple cytokine receptors including those for IL2, IL4, IL7, IL9, IL15, and IL21 (18). Thus, two key questions in Treg development are (i) which γc-dependent cytokines can induce Treg development and (ii) whether TSLP signals are also involved in this process. Herein, we demonstrate that developing Tregs express IL2Rβ, IL7Rα and IL15Rα and respond to IL2, IL7, and to a much lesser degree IL15, by inducing STAT5 activation in CD4+Foxp3+ thymocytes. Similarly, IL2 induced conversion of CD4+CD25+Foxp3− thymic Treg progenitors into CD4+Foxp3+ mature Tregs. IL7 and IL15 also induced conversion of thymic Treg progenitors into mature CD4+Foxp3+ Tregs, albeit much less effectively; in contrast, TSLP showed no activity in this conversion assay. IL4 signaling also does not appear to play a role in Treg development as IL4Rα−/− x IL2Rβ−/− mice show comparable numbers of Tregs as that seen in IL2Rβ−/− mice. In contrast, IL2Rβ−/− x IL7Rα−/− mice exhibit a developmental block which mimics that seen in γc−/− mice. Finally, the expression of IL7Rα and IL15Rα chains is suppressed in mature Tregs; this suppression does not occur in IL2−/− or IL2Rβ−/− mice demonstrating that IL7Rα and IL15Rα downregulation occurs via an IL2/IL2Rβ-dependent signaling pathway. Our findings demonstrate that IL2, IL7, and IL15, are the critical γc-dependent cytokines that are responsible for promoting Treg development. In contrast, developing Tregs do not express the TSLPRα chain, nor respond to TSLP, by inducing phospho-STAT5. Thus, at least in the mouse, TSLP does not appear to play a direct role in Treg development.
Materials and Methods
Mice
IL2−/−, IL2Rβ−/−, IL7Rα−/−, and IL4Rα−/− mice were obtained from Jackson Laboratories. IL2Rβ−/−, IL7Rα−/−, and IL4Rα−/− mice were crossed in our lab to obtain IL2Rβ−/− x IL7Rα−/− and IL4Rα−/− x IL2Rβ−/− mice. Mice used were on a C57Bl/6 background with the exception of the IL4Rα−/− and IL4Rα−/− x IL2Rβ−/− mice, which were on a mixed Balb/c × C57Bl/6 background, and Foxp3-GFP reporter mice, which were on a mixed C57Bl/6 × 129 background. Foxp3-GFP reporter mice were kindly provided by Dr. Sasha Rudensky (University of Washington School of Medicine, Department of Immunology).
Flow Cytometry and FACS analysis
Mice were sacrificed and lymph node, spleen and thymus were isolated. Five million cells were used per staining condition. Cells were first pretreated with an antibody which blocks Fc receptor binding (Clone 24G2). Cells were subsequently stained with the following antibodies from eBioscience (San Diego, CA): CD4-Alexa 700, CD8-allophycocyanin-alexa fluor 750 or CD8-FITC, CD3ε-FITC, CD25-Phycoerythrin-Cy7 (PE-Cy 7) or CD25-allophycocyanin. In addition, biotinylated antibodies for CD122 (IL2Rβ), CD124 (IL4Rα), CD127 (IL7Rα), and IL21Rα, were obtained from eBioscience. Antibodies for IL9Rα, TSLPRα, and IL15Rα were obtained from R&D Systems (Minneapolis, MN). Isotype control antibodies for TSLPRα and IL15Rα were obtained from R&D Systems, while isotype controls for IL9Rα were obtained from eBioscience. Both the IL9Rα and TSLPRα were biotinylated according to manufacturer’s instructions (Sigma, Cat. # BTAG-1KT), while the IL15Rα antibody was purchased in a biotinylated form. Streptavidin allophycocyanin from eBioscience was used as a secondary reagent to reveal staining with biotinylated antibodies. Intracellular Foxp3 staining was done after fixation, permeabilization and overnight incubation at 4°C as described previously (8).
To examine whether IL2 alters IL7Rα and IL15Rα expression on Tregs in IL2−/− mice, we purified CD4+ splenocytes from IL2−/− mice by MACS beads enrichment (Miltenyi Biotech, Auburn, CA). Purified cells were then stimulated with IL2 (100 units/mL) for 4, 8, 12 and 24 hours. Cells were then harvested and stained for IL7Rα, IL15Rα and Foxp3 expression and analyzed on an LSR II flow cytometer (BD Biosciences, San Jose, CA).
Flow cytometry for phospho-STAT5
Single cell suspensions were generated from isolated spleens and thymii from Foxp3-GFP reporter mice. These cell suspensions were pretreated with an antibody that blocks Fc receptor binding. Cells were then stained for the surface markers CD4, CD8, and CD25. Five million cells were then serum starved in 500 µl of 1× DMEM for 30 minutes at 37°C, then stimulated with either 100 units/mL of IL2 (PeproTech; Rocky Hill, NJ), or 50 ng/mL of IL4 (PeproTech), IL7(R&D Systems), IL9 (R&D Systems), TSLP (R&D Systems), IL15 (R&D Systems) or IL21 (PeproTech) for 20 minutes. After stimulation, the cells were washed with 1× DMEM to remove all traces of the supernatant; they were then resuspended in 100 µl of fixation medium from the Caltag Fix and Perm® kit and incubated at 37° C for 15 minutes. Afterwards, 1 mL of 4 °C 100% methanol was added and the cells were incubated overnight at 4° C in the dark. Intracellular phospho-STAT5 (p-STAT5) staining was done using the Caltag Fix and Perm® kit and PE-conjugated anti-phospho-STAT5 (BD Biosciences). Non-stimulated cells were used as negative controls.
Treg Conversion assay
The conversion assay of Treg progenitors into CD4+Foxp3+ Tregs was carried out as previously described (10). In brief, CD4+CD25+Foxp3− Treg progenitors were sorted from Foxp3-GFP reporter mice (19) and placed in culture in the presence of the indicated amounts of cytokine. Twenty-four hours later cells were stained for CD4 and CD25 and analyzed for expression of these markers plus Foxp3-GFP using an LSR II flow cytometer.
Results
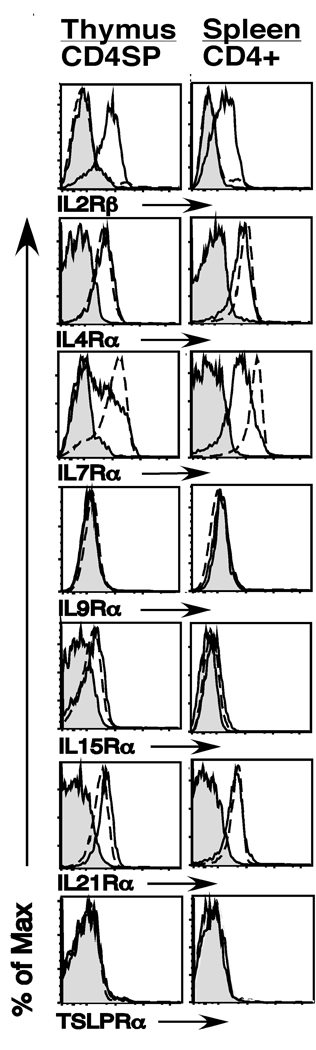
To examine the role that different cytokines play in Treg development in the thymus, we first analyzed the expression of IL2Rβ, IL4Rα, IL7Rα, IL9Rα, IL15Rα, IL21Rα and TSLPRα on CD4+Foxp3+ thymocytes and CD4+Foxp3+ splenocytes. We found four basic patterns of cytokine receptor expression. First, the IL2Rβ chain was selectively expressed on CD4+Foxp3+ thymocytes (Fig. 1). This pattern of expression was maintained in splenic Foxp3+ versus Foxp3− T cells. Second, IL9Rα and TSLPRα were not observed on either Foxp3+ or Foxp3− thymocytes. To confirm that our staining for these receptors was working, we also stained peritoneal B1 B cells and CD19+ Pre-B cells which have previously been reported to express the IL9Rα and TSLPRα chains respectively (17,18). We detected IL9Rα expression on peritoneal B1 B cells; as expected, we also observed TSLPRα expression on pre-B cells in the bone marrow, thereby indicating that our antibodies to IL9Rα and TSLPRα are capable of detecting expression of these receptors (data not shown). Third, IL4Rα and IL21Rα were expressed equally on Foxp3+ versus Foxp3− thymocytes and splenic T cells (Fig. 1). Fourth and last, we observed a dynamic expression pattern for the IL7Rα and IL15Rα chains. Expression of both of these receptors was observed in CD4+Foxp3+ thymocytes, but was significantly reduced in splenic Tregs (Fig. 1). Thus multiple γc-dependent cytokine receptors, but not the TSLPRα chain, are expressed on developing Tregs in the thymus.
Fig. 1. γc-cytokine-family receptor expression on CD4+Foxp3+ SP thymocytes and CD4+Foxp3+ splenocytes.
Thymus and spleen cells from 5–9 week old C57Bl/6 mice were harvested and stained with antibodies to CD4, CD8 and Foxp3, to identify distinct thymic and splenic T cell subsets, as well as with antibodies to IL2Rβ, IL4Rα, IL7Rα, IL9Rα, IL15Rα, IL21Rα and TSLPRα. Shown are flow cytometry histograms of CD4 single positive (SP) thymocytes (left column) and CD4+ splenocytes (right column). Gray filled in histograms represent staining of the corresponding CD4+Foxp3+ population with isotype control antibody. Solid lines and broken lines represent staining of CD4+Foxp3+ and CD4+Foxp3− cells, respectively. A representative example of 4 independent experiments is depicted (n=17).
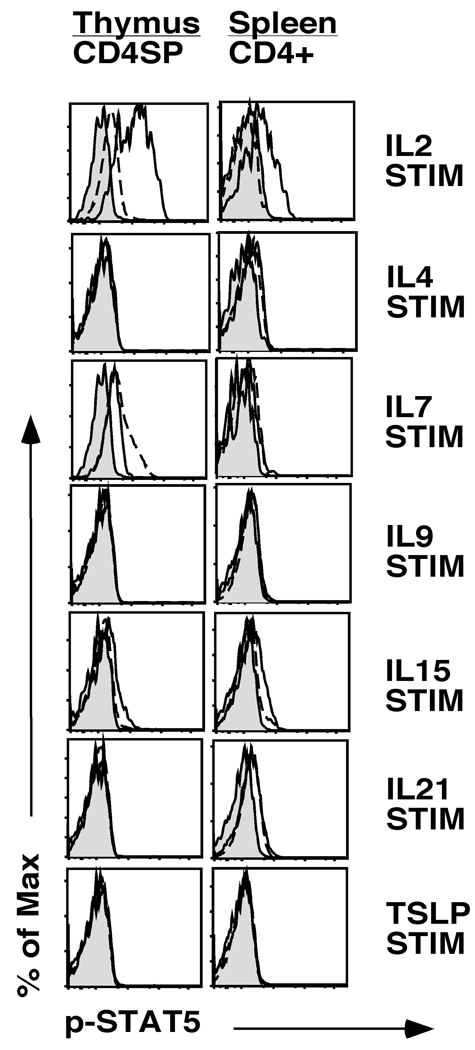
To assess whether receptor expression on Tregs correlated with function, we stimulated cells with IL2, IL4, IL7, IL9, IL15, IL21 and TSLP and examined STAT5 activation by intracellular staining for phospho-STAT5. We focused on STAT5 as previous reports have indicated that STAT5 plays a critical role in Treg development (8, 10, 20). For these studies we identified Tregs using Foxp3-GFP reporter mice; similar studies were also carried out using CD25 as a marker of Tregs. We identified three distinct response patterns. First, IL2 induced robust STAT5 activation in CD4+Foxp3+ thymocytes; splenic Tregs remained highly responsive to IL2 stimulation (Fig. 2). Second, IL7 and IL15 induced modest STAT5 activation in CD4+Foxp3+ thymocytes. However, these responses were almost completely eliminated in CD4+Foxp3+ splenocytes (Fig. 2). Third, IL4, IL9, IL21 and TSLP did not induce detectable STAT5 activation on CD4+Foxp3+ thymocytes (Fig. 2). Similar results were obtained when gating on CD4+CD25+ T cells, with the exception that under those staining conditions IL4 led to very weak STAT5 phosphorylation in CD4+Foxp3+ thymocytes and splenocytes (data not shown). A potential caveat with the IL15 studies is that IL15 could be presented by the IL15Rα chain via trans presentation in vivo (21). It is possible, therefore, that our ex vivo stimulation studies may not have allowed for optimal transpresentation of IL15 to CD4+Foxp3+ Tregs. Thus, developing thymic Tregs respond to IL2 and IL7, and to a lesser degree IL15.
Fig. 2. Cytokine stimulation and Phospho-STAT5 expression on CD4+Foxp3+ thymocytes and splenocytes.
Single cell suspensions of thymocytes or splenocytes from Foxp3-GFP mice were serum starved for 30 minutes and then stimulated with IL2, IL4, IL7, IL9, IL15, IL21 or TSLP for 20 minutes. Cells were then stained with antibodies to CD4, CD8, CD25 and phospho-STAT5 as described in the methods section. Shown are histograms of phospho-STAT5 expression in CD4SP thymocytes (left column) and CD4+ splenocytes (right column). Gray filled in histograms represent staining of unstimulated Foxp3-GFP+ cells. Solid lines and broken lines represent staining of stimulated Foxp3-GFP+ and Foxp3-GFP− cells, respectively. A representative example of 2 independent experiments is depicted (n=3 mice). Similar results were obtained when using CD25 to identify Tregs in C57Bl/6 mice (n=13 mice, data not shown).
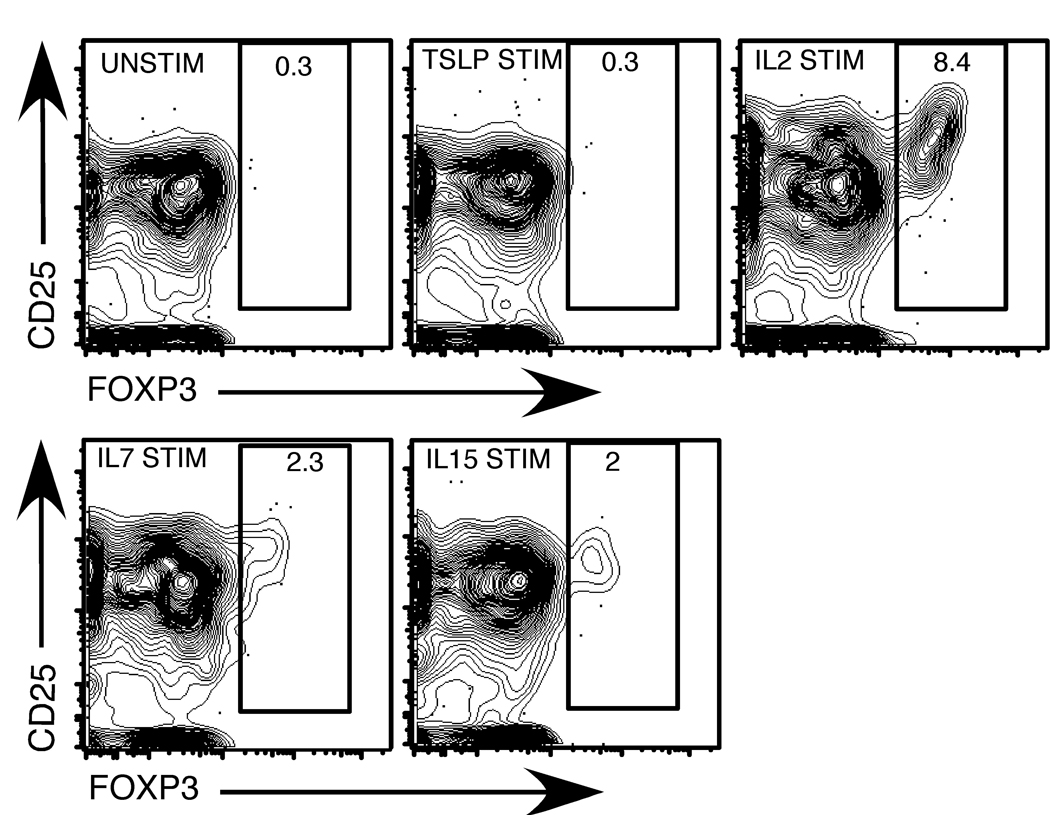
Previous reports have suggested that TSLP plays a role in Treg development (22). However, TSLPR−/− mice have no reported defects in Treg develoment or function (23). This latter observation is consistent with our failure to observe TSLPR expression on developing Tregs. A potential caveat with our studies is that TSLPR expression levels may be below the limits of detection by flow cytometery. Likewise, the amount of STAT5 phosphorylation induced by TSLP might be below the level that we can detect by flow cytometry. To explore this in more detail, we examined whether TSLPR mRNA was detectable by RT-PCR in CD4+Foxp3-GFP+ thymcoytes. These studies indicated that TSLPR mRNA could be detected at some level in developing Tregs (data not shown). A key question then is whether this results in expression of a receptor capable of inducing biological responses. To address this question we made use of the recent identification of CD4+CD25+Foxp3− thymocytes as penultimate Treg progenitors that can be converted into CD4+Foxp3+ mature Tregs following stimulation with IL2 (10, 11). For these studies we isolated CD4+CD25+Foxp3-GFP− Treg progenitors and stimulated them overnight with either IL2, IL7, IL15 or TSLP. The cultured cells were then examined for Foxp3-GFP expression. As shown in figure 3, IL2 induced clear conversion of Treg progenitors into CD4+Foxp3-GFP+ Tregs. IL7 and IL15 were also capable of inducing the conversion of Treg progenitors into Foxp3+ Tregs, although they were much less effective than IL2. In contrast, TSLP stimulation failed to induce conversion of any Treg progenitors into Foxp3+ Tregs. Thus, even if the TSLPR is expressed at very low levels on developing Tregs, it is incapable of inducing Treg differentiation following stimulation with TSLP.
Fig. 3. IL2, IL7 and IL15, but not TSLP, induce the conversion of CD4+CD25+Foxp3− thymic Treg progenitors into CD4+Foxp3+ Tregs.
Sorted CD4+CD25+ Foxp3− thymic Treg progenitors from Foxp3-GFP reporter mice were stimulated with 10 U/mL IL2, 5 ng/mL IL7, 100 ng/mL IL15 and 50 ng/mL TSLP in culture overnight. After 24 hours of stimulation, cells were stained with antibodies to CD4 and CD25. Shown are contour plots of Foxp3 versus CD25 for CD4-gated cells (95% of cells were CD4+) after stimulation with medium alone (top left), TSLP (top middle), IL2 (top right), IL7 (bottom left) or IL15 (bottom right). A representative example of 3 independent experiments is depicted.
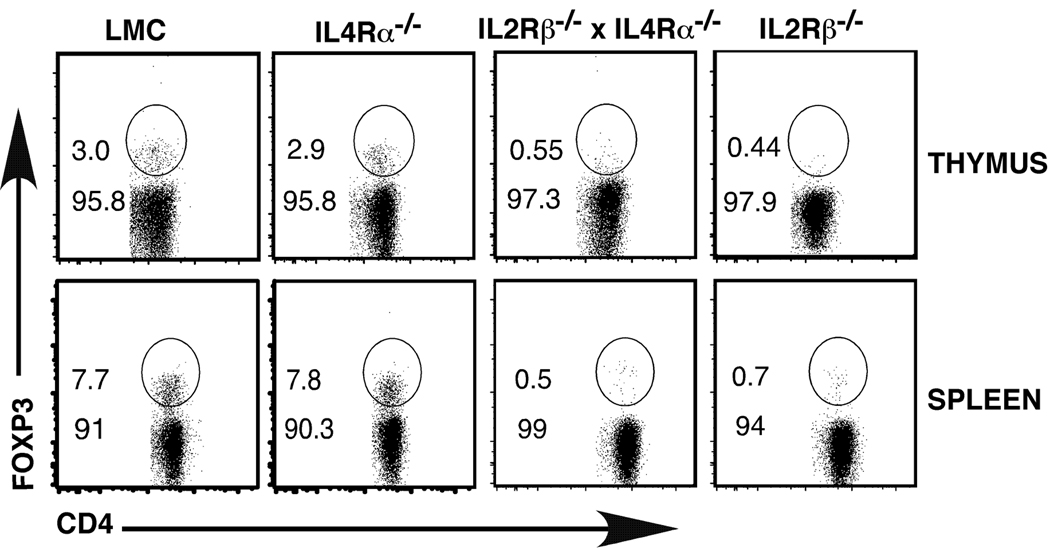
Our observation that IL2Rβ and IL7Rα were the predominant receptors expressed on developing thymocytes suggested that IL2, IL7 and IL15 were most likely the key γc-dependent cytokines that drive Treg development. However, given the expression of the IL4Rα chain on developing Tregs, we examined whether IL4-dependent signals also played a role in this process. IL4Rα−/− mice show no decrease in the percentage of Tregs in the thymus relative to littermate control (LMC) mice (Fig. 4). Furthermore, splenic Tregs were also not reduced in IL4Rα−/− mice (Fig. 4). To examine whether IL2Rβ and IL4Rα-dependent signals played a redundant role in Treg development, we generated IL2Rβ−/− x IL4Rα−/− mice. As previously reported, IL2Rβ−/− mice exhibited reduced numbers of Tregs in both the thymus and spleen; a further reduction was not observed in IL2Rβ−/− x IL4Rα−/− mice (Fig. 4). These findings strongly suggest that IL4Rα-dependent signals are not required for Treg development. It is important to note here that unlike our previous studies, which used IL2Rβ−/− mice on the C57Bl/6 background, the IL4Rα−/− and IL2Rβ−/− x IL4Rα−/− mice in these experiments are on a mixed C57Bl/6×Balb/c background. We have consistently noticed that the IL2Rβ−/− mice on the C57Bl/6 × Balb/c background mice have a more severe phenotype (i.e., fewer Tregs at an earlier age) than IL2Rβ−/− on the C57Bl/6 background. This results in IL2Rβ−/− mice on the mixed background having a reduced percentage of Tregs relative to that seen in IL2Rβ−/− mice on the C57Bl/6 background.
Fig. 4. Treg development in IL4Rα−/− and IL2Rβ−/− x IL4Rα−/− mice.
Thymus and spleen were harvested from 4–5 week old LMC, IL4Rα−/−, IL2Rβ−/− and IL2Rβ−/− x IL4Rα−/− mice. Cells were stained with antibodies to CD4, CD8 and Foxp3 to identify Tregs. Shown are flow cytometry plots of thymus (top panel) or spleen cells (bottom panel) gated on CD4+ T cells. A representative example of 6 independent experiments is depicted.
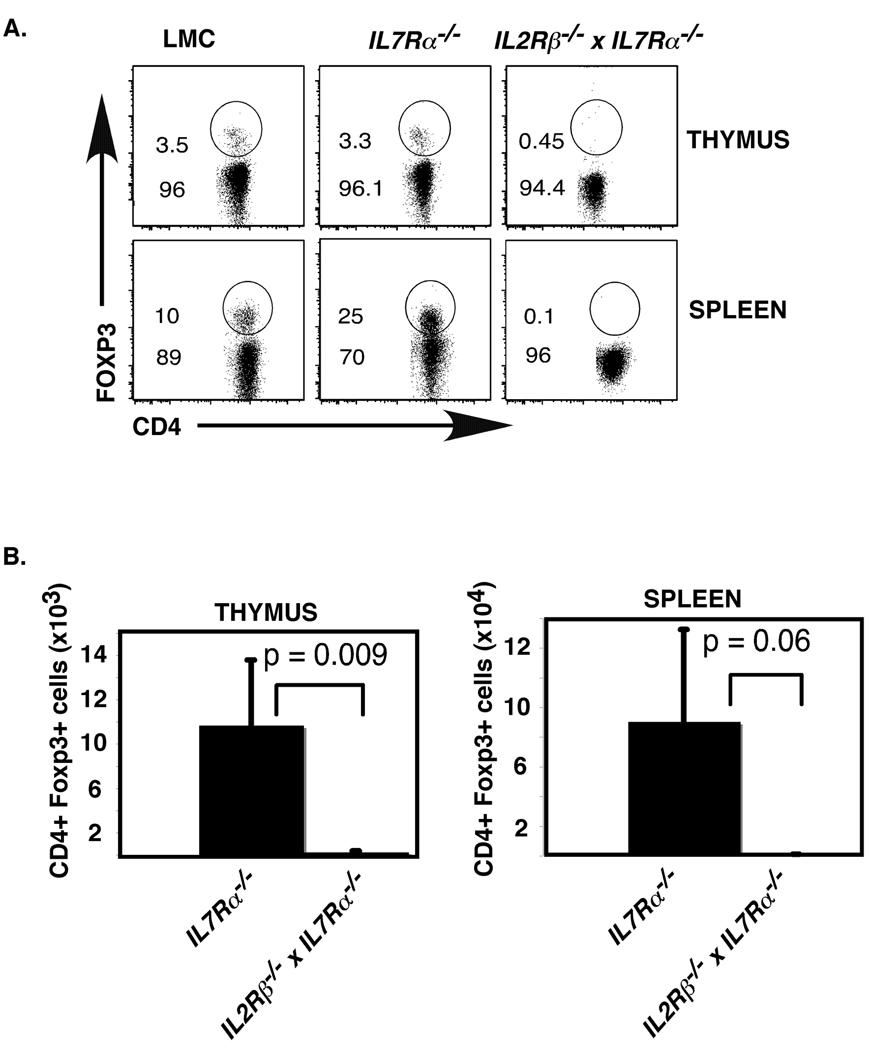
Given the expression of both functional IL7Rα and IL2Rβ on developing Tregs, we predicted that these two cytokine receptors might both be capable of driving Treg development. Consistent with our previous report, we found that although total numbers of T cells are greatly reduced in IL7Rα−/− mice, the percentage of Tregs relative to other T cell subsets was not affected (Fig. 5A)(8). Thus, IL7Rα signaling is not required for Treg development. However, it remains possible that IL2Rβ and IL7Rα can act redundantly to drive Treg development. To test this possibility, we compared Treg differentiation in IL7Rα−/− versus IL2Rβ−/− x IL7Rα−/− mice. We found that IL2Rβ−/− x IL7Rα−/− mice showed a significant decrease in Treg numbers when compared with IL7Rα−/− mice (p=0.009, students t-test) (Fig. 5A,B). Importantly, the numbers of Tregs found in IL2Rβ−/− x IL7Rα−/− mice (thymus=195 ± 75; spleen= 686 ± 136) were comparable to that which we observed in age matched γc−/− mice (thymus= 80 ± 22; spleen= 1500 ± 651) in our previous studies (Fig. 5B)(8). These experiments demonstrate that IL2Rβ and IL7Rα dependent cytokines are the only γc-dependent cytokines required for Treg development.
Fig. 5. Treg development in IL2Rβ−/− and IL2Rβ−/− x IL7Rα−/− mice.
A. Thymus and spleen were harvested from 4–5 week old LMC, IL7Rα−/−, and IL2Rβ−/− x IL7Rα−/− mice. Cells were stained with antibodies to CD4, CD8 and Foxp3 to identify Tregs. Shown are flow cytometry plots of thymus (top panel) or spleen cells (bottom panel) gated on CD4+ T cells. A representative example of 6 independent experiments is depicted. B. Shown are bar graphs representing total numbers of CD4+Foxp3+ Tregs in the thymus (left panel) and spleen (right panel). Error bars represent standard error of the mean; n= 6 IL7Rα−/− and 6 IL2Rβ−/− x IL7Rα−/− mice; p-values were calculated using two-tailed students t-test.
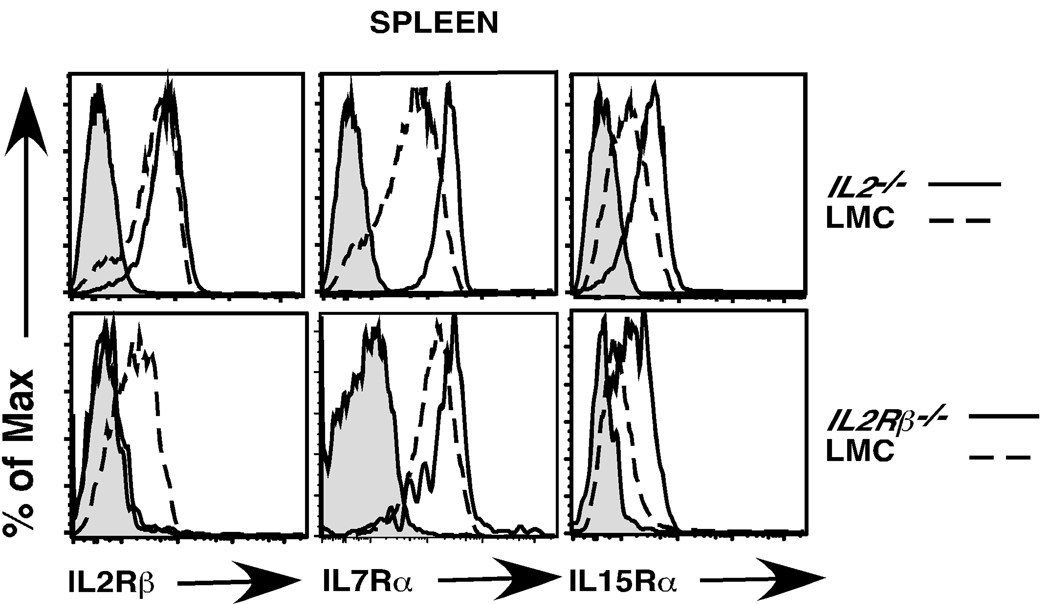
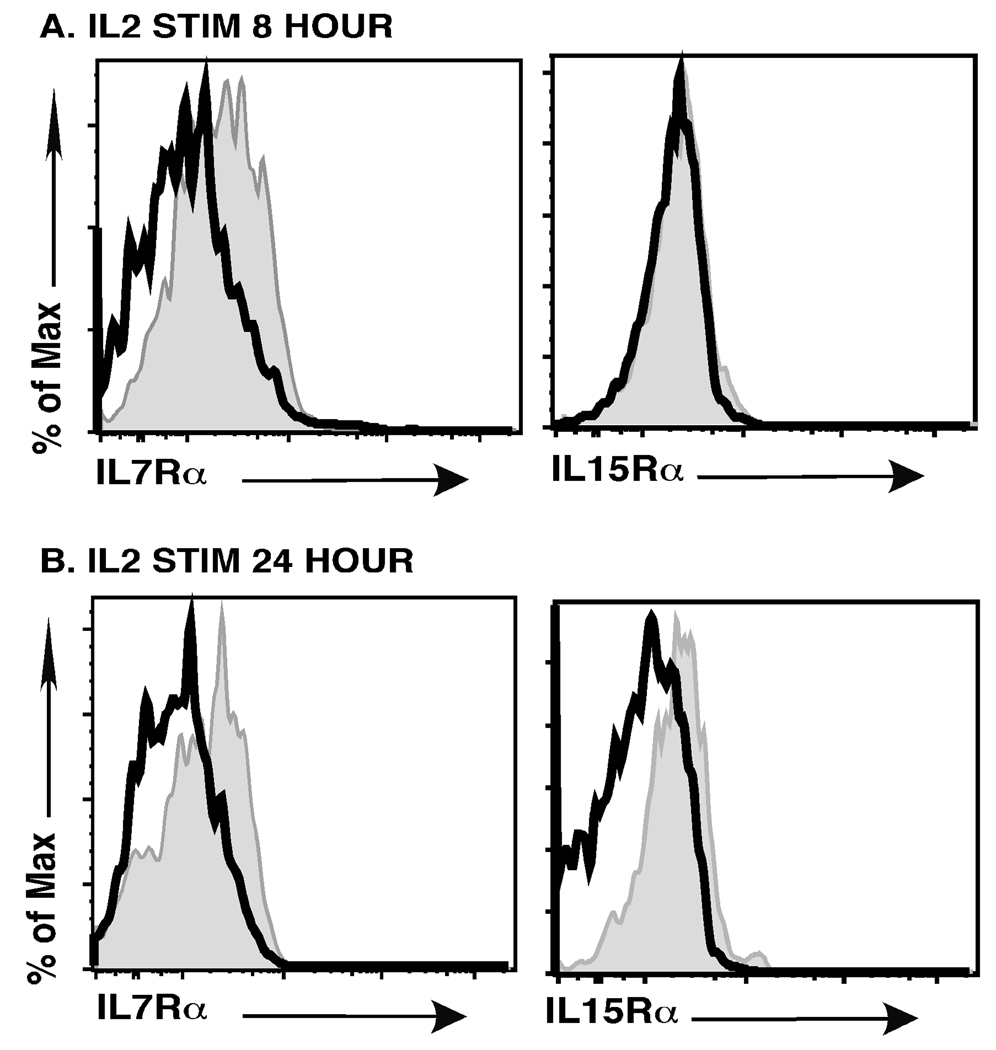
IL7Rα and IL15Rα are expressed at quite low levels on mature splenic Tregs. Thus, it is rather surprising that splenic Tregs are maintained in young IL2−/− mice. To examine this further, we stained CD4+Foxp3+ Tregs from littermate control (LMC) and IL2−/− mice for the expression of IL7Rα and IL15Rα. We found that CD4+Foxp3+ Tregs in IL2−/− mice expressed significantly higher levels of both the IL7Rα and IL15Rα chains (Fig. 6). We considered two explanations for these findings. First, it is possible that in the absence of IL2 any splenic Tregs that express higher levels of IL7Rα or IL15Rα have a competitive advantage and are selectively expanded. Alternatively, it is possible that IL2/ IL2Rβ-dependent signals actively downregulate IL7Rα or IL15Rα expression. To distinguish between these two possibilities, we stained the few CD4+Foxp3+ Tregs in IL2Rβ−/− mice for IL7Rα and IL15Rα expression. Once again, we observed increased expression of both IL7Rα and IL15Rα on Tregs from IL2Rβ−/− versus LMC mice (Fig. 6). In IL2Rβ−/− mice, IL15Rα expression provides no competitive advantage. Thus, this latter finding strongly suggests that IL15Rα downregulation, and likely IL7Rα as well, is due to IL2Rβ-dependent signals. To investigate this further, we took CD4+ splenocytes from IL2−/− mice and stimulated those cells with IL2. We then examined expression of IL7Rα and IL15Rα chains on CD4+Foxp3+ cells (Fig. 7). We observed downregulation of the IL7Rα chain as early as 8 hours after IL2 stimulation; both the IL7Rα and IL15Rα chains were clearly downregulated after 24 hours of IL2 stimulation. Taken together, these studies indicate that IL2 dependent signals can negatively regulate IL7Rα and IL15Rα expression on CD4+Foxp3+ Tregs.
Fig. 6. Expression of IL2Rβ, IL7Rα and IL15Rα on CD4+Foxp3+ Tregs from IL2−/− and IL2Rβ−/− mice compared to wild type littermate controls.
Splenocytes were isolated from 4–5 week old LMC, IL2−/− and IL2Rβ−/− mice and stained with antibodies for CD4, CD8, and Foxp3 to identify splenic Tregs. Shown are CD4+Foxp3+ gated cells stained for IL2Rβ (left panels), IL7Rα (middle panels) and IL15Rα (right panels). Gray histograms represent staining of CD4+Foxp3+ cells with isotype control antibody. Solid lines represent histograms of CD4+Foxp3+ T cells from IL2−/− (top panel) or IL2Rβ−/− (bottom panel) mice; broken lines represent staining of CD4+Foxp3+ Tregs from WT LMC mice. A representative example of 3 independent experiments is depicted (n= 6 IL2−/− and 3 IL2Rβ−/− mice).
Fig. 7. Expression of IL7Rα and IL15Rα on CD4+Foxp3+ Tregs in IL2−/− mice after ex vivo IL2 stimulation.
CD4+ T cells were isolated as described in the methods section and cells were stimulated with 100 units/mL of IL2 for 8 or 24 hours. Cells were then stained for Foxp3, IL7Rα and IL15Rα and analyzed by flow cytometry. Dark unshaded histogram represents IL7Rα and IL15Rα expression after IL2 stimulation for the times indicated. Shaded histograms represent non-stimulated controls. Shown is a representative example of six independent experiments for IL7Rα expression and two independent experiments for IL15Rα expression.
Discussion
These studies identify IL2, IL7 and IL15 as the sole γc-dependent cytokines required for Treg development in the thymus. Four pieces of data support this conclusion. First, receptors for these three cytokines are expressed on developing Tregs in the thymus. Second, these cells can respond to IL2 and IL7 by inducing robust STAT5 activation. The only other γc-dependent cytokine receptors expressed on thymic Tregs are IL4Rα and IL21Rα. However, IL4 and IL21 did not induce STAT5 activation in CD4+Foxp3+ thymocytes and only induced minimal STAT5 activation in mature CD4+Foxp3+ splenocytes. The role of IL15 is somewhat more complicated. We observed only weak STAT5 induction following ex vivo stimulation of thymic CD4+Foxp3+ Tregs with IL15. This may reflect the absence of accessory cells in our ex vivo stimulation cultures that would allow for effective trans presentation of IL15 to developing Tregs. Third, IL2 and to a lesser degree IL7 and IL15 were capable of inducing the conversion of CD4+CD25+Foxp3− Treg progenitors into mature Foxp3+ Tregs. In contrast, TSLP was completely ineffective in this assay. Finally, we found that IL2Rβ−/− x IL7Rα−/− mice recapitulated the phenotype reported in γc−/− mice which are essentially devoid of Tregs. In contrast, the reduction in thymic Tregs in IL2Rβ−/− x IL4Rα−/− mice was no more severe than that seen in IL2Rβ−/− mice. Taken together with our previous observation that IL2−/− x IL15−/− mice have significantly fewer Tregs that IL2−/− mice, our findings strongly support the conclusion that IL2, IL7 and IL15, but not other γc-dependent cytokines, can contribute to Treg differentiation in the thymus.
We also examined the role of TSLP on Treg differentiation as previous studies have suggested that TSLP plays an important role in human and murine Treg development (17, 22). Our studies rule out a direct role for TSLP in murine Treg differentiation. First, consistent with our earlier observation, IL7Rα−/− mice show no reduction in Tregs relative to non-Tregs. Second, we could not detect expression of TSLPRα on thymic Tregs nor induce STAT5 activation following stimulation of these cells with TSLP. Third TSLP was incapable of inducing the conversion of thymic Treg progenitors into CD4+Foxp3+ Tregs. These findings demonstrate that TSLP cannot play a direct role in Treg development. It remains possible that TSLP plays an indirect role by acting on other cell types that may be involved in promoting Treg differentiation. However, this function is either not unique to TSLP, or not critical, as Tregs clearly develop in mice lacking the IL7Rα chain which is a critical component of the TSLPR.
Although our studies demonstrate that IL2, IL7 and IL15 can redundantly contribute to Treg development and homeostasis, it seems likely that IL2 is the relevant cytokine in wild-type mice. Specifically, we found that expression of IL7Rα and IL15Rα were significantly increased on Tregs in both IL2−/− and IL2Rβ−/− mice. Ex vivo stimulation of CD4+Foxp3+ Tregs from IL2−/− mice with IL2 led to rapid downregulation of both of these receptor subunits. Thus, IL2 plays an important role in rendering CD4+Foxp3+ Tregs uniquely responsive to IL2-dependent signals in wild type mice. This most likely serves to link Treg homeostasis directly to effector T cell activation and IL2 secretion. Effector T cell IL2 production appears to be critical for Tregs to expand in step with activated effector T cells and thereby mediate effective suppression. Supporting this conclusion, IL2−/− mice, but not IL7−/− or IL15−/− mice, show signs of T cell activation and ultimately succumb to lethal multi-organ autoimmune disease. Thus, while IL7 and IL15 are capable of sustaining Treg populations in young mice, they are not effective at expanding these cells sufficiently during ongoing immune responses. Finally, these findings also have implications for the use of low-level IL7Rα expression to identify Tregs (24, 25) as this receptor may be upregulated under conditions of limited IL2 availability.
Acknowledgements
We thank Rachel Agneberg and Jared Liebelt for assistance with animal husbandry, Paul Champoux for assistance with flow cytometry, and Dr. Alexander Khoruts for review of the manuscript.
Footnotes
This work was supported in part by a Pew Scholar Award, a Cancer Investigator Award, a Leukemia and Lymphoma Society Scholar Award, and by NIH grant AI061165 to MAF. KBV was supported by a Supplement to Promote Diversity in Health-related Research.
Abbreviations used in this paper: regulatory T cell, Treg; littermate control, LMC; γc, common gamma chain; wild type, WT; single positive, SP.
References
- 1.Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 2.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. The Journal of experimental medicine. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nature immunology. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 4.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nature immunology. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 5.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 6.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 7.Malek TR, Porter BO, Codias EK, Scibelli P, Yu A. Normal lymphoid homeostasis and lack of lethal autoimmunity in mice containing mature T cells with severely impaired IL-2 receptors. J Immunol. 2000;164:2905–2914. doi: 10.4049/jimmunol.164.6.2905. [DOI] [PubMed] [Google Scholar]
- 8.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 9.Soper DM, Kasprowicz DJ, Ziegler SF. IL-2Rbeta links IL-2R signaling with Foxp3 expression. Eur J Immunol. 2007;37:1817–1826. doi: 10.1002/eji.200737101. [DOI] [PubMed] [Google Scholar]
- 10.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadlack B, Lohler J, Schorle H, Klebb G, Haber H, Sickel E, Noelle RJ, Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 13.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science (New York, N.Y. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 16.D'Cruz LM, Klein L. Development and function of agonist-induced CD25(+)Foxp3(+) regulatory T cells in the absence of interleukin 2 signaling. Nature immunology. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 18.Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 19.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, J. O'Shea J. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Q, Su H, Knudsen G, Helms W, Su L. Delayed functional maturation of natural regulatory T cells in the medulla of postnatal thymus: role of TSLP. BMC Immunol. 2006;7:6. doi: 10.1186/1471-2172-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpino N, Thierfelder WE, Chang MS, Saris C, Turner SJ, Ziegler SF, Ihle JN. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Molecular and cellular biology. 2004;24:2584–2592. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. The Journal of experimental medicine. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. The Journal of experimental medicine. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]