Abstract
We recently demonstrated a contributing role of spinal cord infiltrating CD4+ T lymphocytes in the maintenance of mechanical hypersensitivity in a rodent model of neuropathic pain, spinal nerve L5 transection (L5Tx). It has been demonstrated that microglia play a role in the etiology of pain states. We hypothesized that infiltrating CD4+ T lymphocytes communicate with microglia via a CD40-CD154 interaction. Here, we investigated the role of CD40 in the development of mechanical hypersensitivity post-L5Tx. CD40 KO mice displayed significantly decreased mechanical sensitivity compared with WT mice starting from day 5 post-L5Tx. Using bone marrow chimeric mice, we further identified a pro-nociceptive role of CNS microglial CD40 rather than the peripheral leukocytic CD40. Flow cytometric analysis determined a significant increase of CD40+ microglia in the ipsilateral side of lumbar spinal cord post-L5Tx. Further, spinal cord proinflammatory cytokine (IL-1β, IL-6, IL-12, and TNF-α) profiling demonstrated an induction of IL-6 in both WT and CD40 KO mice post-L5Tx prior to the increase of microglial CD40 expression, indicating a CD40-independent induction of IL-6 following L5Tx. These data establish a novel role of microglial CD40 in the maintenance of nerve injury-induced behavioral hypersensitivity, a behavioral sign of neuropathic pain.
Keywords: CD40 knockout mice, Bone marrow chimeras, Microglia, IL-6
Introduction
Chronic pain is a serious health problem. According to a report released by the Centers for Disease Control and Prevention (CDC) in 2006, one in ten adults in the US suffers from pain that lasts 1 year or more. Neuropathic pain, defined as pain initiated or caused by a primary lesion or dysfunction in the nervous system [1], is one of the most devastating kinds of chronic pain. The concept that microglial activation is crucial in the development of neuropathic pain has been extensively studied and reviewed [2, 3]. A phenotype of activated microglia, represented by elevated CD11b, TLR4, B7.2, and MHC II surface expression, has been detected during neuropathic pain development [3, 4]. However, despite the emerging findings of a microglial involvement in neuropathic pain, the role of a microglial CD40 signaling pathway has not been investigated in neuropathic pain.
The interaction between CD40, a 48 kD cell surface receptor primarily expressed by APC and CD40 ligand (CD154), a 34–39 kD surface protein primarily expressed by activated CD4+ T lymphocytes, has long been known to play critical roles in both humoral and cell-mediated immune responses [5, 6]. Increasingly, CD40-CD154 ligation has been linked to the pathogenesis of various CNS diseases. Elevated CD40 expression was observed during CNS diseases, such as multiple sclerosis, Alzheimer's disease, amyotrophic lateral sclerosis, and HIV-1 encephalitis, both in human patients and in animal models of these CNS diseases [7–11]. Blocking the CD40-CD154 pathway in the CNS resulted in reduced clinical manifestations of multiple sclerosis and Alzheimer's disease in the respective rodent models of these diseases [8, 12]. Microglia, the CNS resident cells of monocyte origin, are the primary cell type expressing CD40 in the CNS. Although resting microglia express minimal levels of CD40 [13, 14], upon stimulation, microglia express significantly elevated levels of CD40 both in vitro and in vivo [15–18]. Microglial CD40-downstream cytokine/chemokine production and the subsequent proinflammatory responses are suggested to be responsible for the pathogenesis of the CNS diseases mediated by CD40-CD154 pathway [19–23].
We have reported an increased infiltration of CD4+ T lymphocytes in the lumbar spinal cord following spinal nerve L5 transaction (L5Tx), a murine model of neuropathic pain [24]. These infiltrating CD4+ T lymphocytes were found to play a contributing role in the maintenance of L5Tx-induced neuropathic pain, while the underlying mechanisms remain unknown. We proposed that the observed infiltrating CD4+ T lymphocytes interact with spinal cord microglia via cell surface CD40-CD154 engagement, which further promotes microglial production of proinflammatory cytokines, thus maintaining long-term behavioral hypersensitivity. In this study, we set out to evaluate the role of CD40 pathway in the development of nerve injury-induced neuropathic pain using the L5Tx model.
Results
Mechanical sensitivity of CD40 KO mice
To investigate the involvement of CD40 in nerve injury-induced neuropathic pain, CD40 KO mice on the BALB/c background underwent L5Tx or sham surgery and were tested for mechanical sensitivity with von Frey filaments in comparison with WT BALB/c mice (Fig. 1). Similar basal levels of mechanical sensitivity were found between CD40 KO mice and the WT controls. Sham surgery did not induce significant hypersensitivity in CD40 KO mice. As expected, L5Tx significantly increased mechanical sensitivity as early as day 1 up to day 21 post-L5Tx in WT mice. CD40 KO mice developed similar mechanical hypersensitivity initially (day 1 post-L5Tx) which gradually and significantly attenuated over time. The 50% threshold force was significantly higher in CD40 KO mice from day 5 to day 21 post-L5Tx compared with the WT controls (Fig. 1: two-way repeated ANOVA, ptime<0.001, pgroup<0.001, and ptime×group<0.001). Thus, CD40 plays its role primarily in the maintenance of neuropathic pain post-L5Tx.
Figure 1.
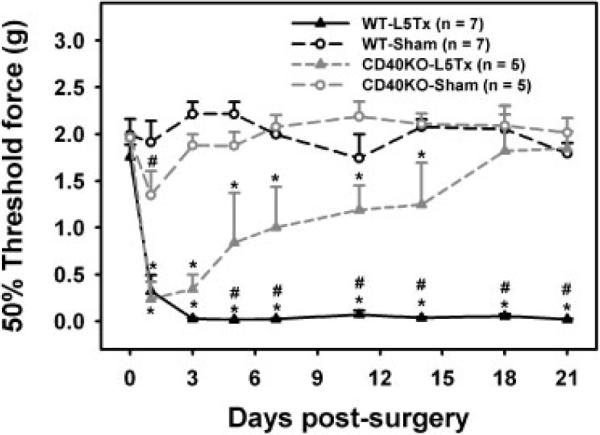
Decreased mechanical sensitivity of CD40 KO mice post-L5Tx. CD40 KO mice on BALB/c background and WT control mice were subjected to either L5Tx or sham surgery. All mice were tested for their mechanical sensitivity with von Frey filaments using the up-down method as described in the Materials and Methods. Data show mean+SEM. The numbers of animals used in each group are indicated in the graph (n). *p<0.05, compared between L5Tx and sham-operated mice within the same mouse strains; #p0.05, compared between CD40 KO and WT with the same surgery performed.
Mechanical sensitivity of CD40 KO-WT bone marrow chimeric mice
To determine the involvement of central versus peripheral CD40 in the development of L5Tx-induced neuropathic pain, reciprocal bone marrow chimeric mice were generated using CD40 KO mice and WT BALB/c mice. Mice from each group were subjected to either L5Tx or sham surgery and tested for their mechanical sensitivity with von Frey filaments as described for CD40 KO mice. Upon euthanization at 21 days post-surgery, blood and splenic cells were collected from all mice and tested for CD40 expression via flow cytometry to confirm the respective chimerism of each animal (Fig. 2A and data not shown). No significant differences in the basal levels of mechanical sensitivities were detected among all chimeric groups. As expected, sham surgery did not induce significant mechanical hypersensitivity in any of the groups of mice tested. WT→WT and CD40 KO→CD40 KO chimeras displayed similar mechanical sensitivities as respective non-chimeric WT and CD40 KO mice following L5Tx. WT→CD40 KO (no CNS CD40 expression), but not CD40 KO→WT (no peripheral CD40 expression) mice developed significantly reduced mechanical hypersensitivity post-L5Tx in a similar manner as it was observed in CD40 KO or CD40 KO→CD40 KO mice. CD40 KO→WT mice presented similar changes in mechanical sensitivity as WT or WT→WT mice post-L5Tx did (Fig. 2B: two-way repeated ANOVA, ptime<0.001, pgroup<0.001, and ptime×group<0.001). Together, these data indicate that CD40 expressed in the CNS compartment rather than the peripheral compartment plays a critical role in the maintenance of L5Tx-induced neuropathic pain.
Figure 2.

Generation of bone marrow chimeras and mechanical sensitivity of CD40 chimeric mice post-L5Tx. (A) CD40 expression of both splenic and blood leukocytes for all chimeric and donor mice was analyzed flow cytometrically post-experimental use. The average percentages of CD40+ leukocytes in total splenocytes for each group (including donor mice) are presented in the figure (mean+SEM). (B) All chimeric mice that underwent either L5Tx or sham surgery were tested for mechanical sensitivity as described in the Materials and methods (mean±SEM). The numbers of animals in each group are indicated as “n”. *p<0.05, compared between L5Tx and sham operated mice within the same type of chimeras, #p<0.05, compared between the indicated group and WT→WT chimeras post-L5Tx surgery.
Lumbar spinal cord microglial CD40 expression post-L5Tx
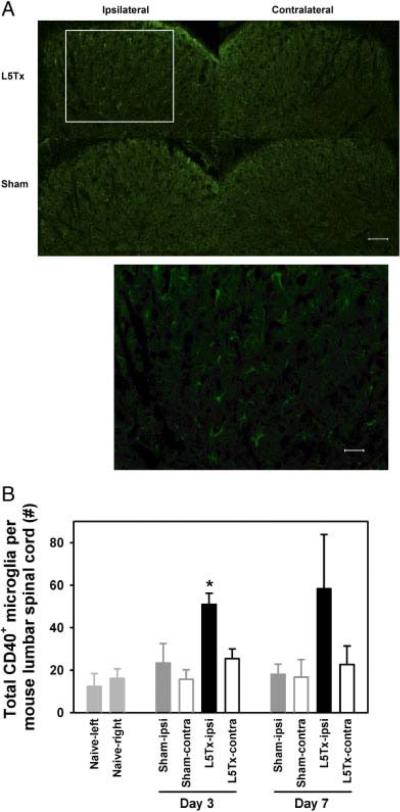
It is known that microglia are the main source of CD40 in the CNS. We first visualized CD40+ cells in the lumbar spinal cord via immunohistochemistry (IHC). At the spinal L5 dorsal horn level, CD40 expression was observed mainly on microglial-like cells (based on morphology) and was substantially greater in the ipsilateral side of L5Tx-operated mice than that in the contralateral side of L5Tx-operated mice and in either side of the lumbar spinal cord from sham-operated animals (Fig. 3A).
Figure 3.
Increased lumbar spinal cord microglial CD40 expression post-L5Tx. (A) L5 spinal cord dorsal horn sections from mice 3 days after either L5Tx or sham surgery were labeled for CD40 via IHC. Representative microphotographs are shown (20×, scale bar = 50 μm). Selected region (defined by the rectangular) from “L5Tx-ipsi” image was also shown as 40× (scale bar = 20 μm). (B) Mononuclear cells from lumbar spinal cord of pooled animals were collected and analyzed by flow cytometry using combination of mAb against CD45, CD11b, and CD40. Three independent experiments were conducted. The total numbers of CD40+ microglia (CD45+CD11b+) per mouse (mean+SEM). p<0.05, compared between “L5Tx-ipsi” group and all other groups within the same time point. ipsi, ipsilateral side and contra, contralateral side.
We then quantified lumbar spinal cord (CNS site of relevance following L5Tx) microglial CD40 expression by flow cytometry. Mononuclear cells were collected from both ipsilateral (side of injury) and contralateral (side without injury) lumbar spinal cord regions from pooled (four per treatment in each experiment and three independent experiments were performed) mice. CD45loCD11b+ cells were identified as microglia. Identical to our previously published results [25] at day 3 post-surgery, we observed a significant increase in the total number of microglia in the ipsilateral lumbar spinal cord post-L5Tx compared with the contralateral side of transected mice and either side of sham-operated mice, and microglia from the ipsilateral lumbar spinal cord displayed significant increases in both size (forward scatter (FSC)) and granularity (side scatter (SSC)) in SSC versus FSC dot plot (potential morphological changes). At day 7, these changes were still obvious although not statistically significant (data not shown). The total numbers of CD40+ microglia were further determined based on the total numbers of mononuclear cells collected from each treatment group. We observed that the number of CD40+ microglia significantly increased in the ipsilateral lumbar spinal cord 3 days post-L5Tx compared with the contralateral lumbar spinal cord and either side of lumbar spinal cord from sham-operated animals (Fig. 3B, one-way ANOVA with all groups on day 3, p=0.018). Similar trends were clearly seen at day 7 post-surgery (Fig. 3B) but not at 1-day post-L5Tx (data not shown). Expression of CD40 by splenocytes was also assessed by flow cytometry and no significant changes of CD40 expression were observed post-L5Tx (data not shown).
Proinflammatory cytokine production in both WT and CD40 KO mice post-L5Tx
To test the hypothesis that CD40-mediated downstream proinflammatory cytokines are involved in the CD40-dependent maintenance of L5Tx-induced neuropathic pain, we evaluated the production of proinflammatory cytokines: IL-1β, TNF-α, IL-6, and IL-12.
First, changes in the levels of these cytokines following L5Tx and sham surgeries were measured in the periphery (spleen and serum). In the WT mice, serum levels of these cytokines were either not detectable or not affected by either surgery. In the spleens of WT mice, IL-6 was not detectable. Both L5Tx and sham surgeries induced significantly increased IL-1β production from day 1 to day 7 post-surgery (Fig. 4A, two-way ANOVA, ptime<0.001, psurgery=0.490, and ptime×surgery=0.737), while TNF-α production was significantly elevated at both days 1 and 3 post-surgery only in animals subjected to L5Tx (Fig. 4B, two-way ANOVA, ptime=0.409, psurgery=0.014, and ptime×surgery=0.351). When CD40 KO mice were examined, no significant increases of splenic IL-1β and TNF-α (Fig. 4C and D) were observed as that in WT mice.
Figure 4.
Splenic proinflammatory cytokine expression post-L5Tx. WT BALB/c and CD40 KO mice were subjected to either L5Tx or sham surgery. Spleens were collected at selected times post-surgery and then processed for ELISA. Changes of splenic levels of IL-1β (A and C) and TNF-α (B and D) in both WT mice (A and B) and CD40 KO mice (C and D) are shown here. Data show mean+SEM (n = 9–11 per group for A and B; n = 7–8 per group for C and D). p<0.05, compared between animals that underwent sham surgery and that underwent L5Tx surgery; #p<0.05, compared between the indicated group and the naïve group. Sh, sham and Tx, L5Tx.
Changes in the above cytokines were also measured in the lumbar spinal cord in both WT and CD40 KO mice. In the WT mice, lumbar spinal cord IL-1β did not show significant changes following either L5Tx or sham surgeries, although it trended towards a surgery-induced elevation in the ipsilateral side. TNF-α was too low to be accurately analyzed (data not shown). Interestingly, IL-6 was significantly increased in the ipsilateral lumbar spinal cord with its peak observed at day 1 post-L5Tx (Fig. 5A, two-way ANOVA, ptime<0.0001, pgroup=0.0002, and ptime×group<0.0001). Lumbar spinal cord IL-6 was also significantly elevated in the CD40 KO mice post-L5Tx in a similar manner as it was in the WT mice (Fig. 5B, two-way ANOVA, ptime<0.001, pgroup<0.001, and ptime×group<0.001). Additionally, splenic or lumbar spinal cord levels of IL-12 were not significantly affected by L5Tx (data not shown).
Figure 5.
Lumbar spinal cord IL-6 expression post-L5Tx. WT BALB/c (A) and CD40 KO (B) mice were subjected to either L5Tx or sham surgery. Lumbar spinal cords (ipsi and contralateral sides) were collected at selected times post-surgery and processed for multiplex Luminex assay. Changes of lumbar spinal cord levels of IL-6 (mean+SEM, n = 5–10 for WT, n = 4 for KO). *p<0.05, compared between indicated and all other groups at the same time point; #p<0.05, compared between the indicated group and both naïve groups; @p<0.05, compared between the indicated groups and the contralateral groups at the same time point. Sh, sham; Tx, L5Tx; ipsi, ipsilateral side; and contra, contralateral side.
Discussion
It is well known that CD40-mediated responses are involved in various CNS diseases, such as multiple sclerosis, Alzheimer's disease, amyotrophic lateral sclerosis, and HIV-1 encephalitis [7–11]. Whether CD40 is associated with the pathogenesis of nerve injury-induced neuropathic pain has not been previously investigated. Here, for the first time, we demonstrated that CD40, particularly microglial CD40, plays a critical role in the maintenance of neuropathic pain. Considering the similarities between these diseases, for example, CNS microglial reactions and proinflammatory responses are believed to be essential in the manifestation of these diseases, we are not surprised about our findings. However, unlike the observation in animal models of multiple sclerosis and Alzheimer's disease, in which blockade of CD40-CD154 interaction significantly inhibited both the initiation and the progression of the diseases [8, 26], CD40 did not affect the initiation of L5Tx-induced neuropathic pain behavior in mice. Mice without functional CD40 in the CNS exhibited attenuated mechanical hypersensitivity only after day 3 post-L5Tx. These data indicate that unlike in multiple sclerosis and Alzheimer's disease, CD40 plays a role primarily in the maintenance phase of neuropathic pain, suggesting that different molecular/cellular pathways that require CD40-signalling may be associated with neuropathic pain. Together with our previous finding that lumbar spinal cord infiltrating CD4+ T lymphocytes contribute to the maintenance of L5Tx-induced neuropathic pain [24], our results support the hypothesis that infiltrating CD4+ T lymphocytes act in the etiology of neuropathic pain through the interaction between CD4+ T lymphocyte CD154 and microglial CD40. In addition, according to the observed significantly enhanced reduction of L5Tx-induced mechanical hypersensitivity in the CD40 KO mice compared with that in the CD4 KO mice [24], CD4 T-cell-independent microglial CD40-mediated pathways in L5Tx-induced mechanical hypersensitivity are most likely to exist simultaneously.
There is emerging evidence suggesting that there are substantial numbers of newly generated microglia that are derived from certain subset of peripheral monocytes present in the CNS following whole body irradiation due to inherent irradiation damage [27, 28]. Although thorough examination of our irradiation/transplantation regimen did not detect newly donor-derived microglia in the CNS previously [8], small numbers of donor-derived microglia (i.e. CD40+ microglia in WT→CD40 KO chimera and CD40− microglia in WT→CD40 KO chimeras) could be present in our study. However, based on the behavioral results in which the WT→CD40 KO chimeric mice mirrored CD40 KO mice, it is unlikely that these donor-derived microglia played a significant role in reversing/modifying the mechanical sensitivity of the animal. Further, the existence of donor-derived CD40+ microglia in BW→CD40 KO mice (that displayed reduced mechanical hypersensitivity) would just suggest that a significant decrease, and not a total depletion, in microglial CD40 expression is sufficient to reduce L5Tx-induced mechanical hypersensitivity. In addition, macrophages of the peripheral nervous system are radiosensitive and this population of macrophages in the chimeric mice will be primarily donor derived [29]. Thus, the reduced mechanical hypersensitivity in WT→CD40 KO mice is not due to the lack of CD40 expression in peripheral nervous system macrophages.
It is known that upon CD40 stimulation, microglia produce various proinflammatory mediators, including cytokines (such as IL-1β, IL-6, TNF-1α, IL-8, IL-10, IL-12, and IFN-γ), chemokines (such as MCP-1), and nitric oxide (NO) [19–23, 30]. Proinflammatory cytokines (such as IL-1β, IL-6, TNF-α, and IL-12) have been well accepted as critical players in neuropathic pain [31]. To further investigate whether CD40-mediated proinflammatory responses, particularly proinflammatory cytokines, contribute to the reduced mechanical hypersensitivity in CD40 KO mice and WT→CD40 KO chimeras, we examined the levels of selected proinflammatory cytokines (IL-1β, IL-6, TNF-α, and IL-12) in the lumbar spinal cord post-L5Tx. Surprisingly, no significant differences in the levels of any cytokines we measured were detected between WT and CD40 KO mice. Other mediators (such as MCP-1, IFN-γ, and NO) that can be produced by microglia have also been shown to be critical in the development of neuropathic pain [32–34]. Future studies will focus on these other selected CD40-downstream mediators.
Both WT and CD40 KO mice displayed a significant early increase of lumbar spinal cord IL-6 production. This observation suggests that L5Tx-induced spinal cord IL-6 may be involved in the induction of microglial CD40 that was observed at and after 3 days post-L5Tx. Owing to the possible non-specific activation of spinal cord microglia following repeated intrathecal injection of a neutralizing Ab, and the observed, unexplained inhibitory effects of isotype control Ab on L5Tx-induced mechanical hypersensitivity [35], IL-6 KO mice on the BALB/c background (currently not available) will be generated and used to test this hypothesis in the future studies.
Additionally, we did observe significant surgery-induced splenic IL-1β and L5Tx-induced TNF-α in WT mice and these increases were diminished in CD40 KO mice. Surgery-induced peripheral responses are not unexpected. We have previously shown that both L5Tx and sham surgeries equally induced slight but significant activation of peripheral CD4+ T lymphocytes [24]. However, whether L5Tx-induced elevation of splenic TNF-α plays a role in the development of neuropathic pain needs to be further determined. For example, L5Tx-induced mechanical sensitivity of CD40 KO mice can be examined following a peripheral TNF-α treatment.
In summary, our study is the first to demonstrate a critical role of microglial CD40 in the maintenance of peripheral nerve injury-induced mechanical sensitivity. Our results support the hypothesis that spinal cord infiltrating CD4+ T cells play a contributing role in the maintenance of neuropathic pain via an interaction between microglial CD40 and CD4+ T-cell CD154. Further testing of this hypothesis will advance our understanding of the role of CD4+ T cells/adaptive immunity in neuropathic pain. Despite previous findings that adoptive transfer of type I helper CD4+ T cells induced behavioral hypersensitivity in nude rats [36], association between adaptive immunity and neuropathic pain, to date, is still a rarely investigated area. In addition, further study of the downstream responses upon CD40 stimulation in both infiltrating CD4+ T cells and CNS microglia is essential in identifying new drug targets in treating neuropathic pain.
Materials and methods
Animals
Male and female BALB/c mice were purchased from National Cancer Institute (NCI, Frederick, MD, USA) and were allowed to habituate to the institutional animal facility for at least 1 wk before experimental use (8–9 wk old). Breeding pairs for BALB/c CD40 KO mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and bred in the animal facility of Dartmouth-Hitchcock Medical Center (DHMC) and University of New England (UNE). All mice were group-housed with food and water ad libitum and maintained on a 12-h light/dark cycle. The Institutional Animal Care and Use Committee (IACUC) at Dartmouth College and UNE approved all experimental procedures that were conducted in the respective institution.
L5Tx and assessment of mechanical sensitivity
WT or CD40 KO mice were randomly selected into either sham or L5Tx groups. L5Tx and sham surgeries were conducted by following the previously published procedures [24]. The tactile sensitivity response was measured with von Frey filaments (Stoelting, Wood Dale, IL, USA) using the up-down paradigm (detailed in [37]) as described previously [24]. The 50% threshold force needed for paw withdrawal was calculated and used to represent the mechanical sensitivity. It has been reported that mice with the same background but bred in different facilities could display behavioral differences [38]. Mechanical sensitivities of all types of mice used in this study were tested before and after surgery. No significant differences in the mechanical sensitivity at basal level were observed across all mice. Further, no significant differences in mechanical sensitivity after L5Tx were detected between CD40 KO mice bred in the DHMC and those bred in the UNE animal facility. Additionally, the levels of 50% threshold force for the WT mice before and after L5Tx are similar to those obtained in our previous study in which the same mouse strain was used [24].
Generation of irradiation bone marrow chimeric mice
Reciprocal irradiation bone marrow chimeric mice were generated using CD40 KO mice and WT BALB/c mice following an established protocol [8, 39]. Briefly, bone marrow cells were aseptically collected from both femur and tibia of donor mice, and then filtered through a 45-micron cell strainer (BD Biosciences, San Diego, CA, USA), washed, and resuspended in sterile PBS for injection. Recipient mice were lethally irradiated using a JL Shepard Cesium (γ) irradiator (Cs-137 source) (Irradiation Shared Resource, Norris Cotton Cancer Center, DHMC). Irradiation was given in two split doses at 500 rad each (i.e. total 1000 rad) with a 20-h interval. After the second dose, all recipient mice were intravenously injected with 5 × 106 bone marrow cells prepared from the donor mice. Engraftment takes place over the following 6–8wk of recovery. In the previous study by Becher et al. [8], it is shown that >95% engraftment of leukocytes was observed with this protocol: Briefly, (i) the chimerism of all major leukocyte populations in the periphery (peripheral blood, spleen and LN) and microglia in the CNS were thoroughly examined at 8wk after induction of chimerism with no other experimental treatments via flow cytometry using the CD45 congenic bone marrow chimeras; (ii) only small numbers of host-derived cells were found in the blood (2%) and LN/spleen (5%). The host-derived cells consisted primarily of CD3+ T cells. Less than 0.1% of host-derived cells were myeloid cells and B lymphocytes; and (iii) in the CNS, no host-derived microglia were detected. In this study, at 8wk post-bone marrow transplantation, chimeric mice were used as described in Results. After each experiment, all chimeric mice were euthanized and surface CD40 expression on both blood cells and splenocytes was evaluated via flow cytometry to ensure the chimerism.
Flow cytometry
As described previously, mononuclear cells for FACS were prepared from pooled (four mice) mouse lumbar spinal cord tissue using the discontinuous Percoll (Amersham, Piscataway, NJ, USA) gradients and stained for flow cytometry [24]. The total mononuclear cell number was determined using a hemacytometer with trypan blue (Sigma, St. Louis, MO, USA) before staining. All mAb were obtained from either BD Biosciences or eBioscience. Cell surface Fc receptors were blocked by anti-mouse-CD16/CD32 (2.4G2). The following combinations of mAb were used: APC-Cy7-anti mouse CD45 (30-F11), PE-Cy7-anti mouse CD11b (M1/70), and Biotin-anti mouse CD40 (1C10)/streptavidin-PE (1:50, eBioscience). Up to 10000 events from each sample were analyzed using a FACSCanto flow cytometer with Cell Quest software (BD Biosciences). Non-stained cells and proper isotype controls were included in each run as controls.
IHC
Tissue slices (15 μm) from L5 segments of lumbar spinal cord for IHC were prepared as described previously [24]. Fluorescent IHC for CD40 was conducted following a modified procedure [40]. Briefly, tissue slices were first blocked with 2% FBS/PBS/0.4% Triton X-100 and then incubated with Armenia hamster anti-mouse CD40 IgM (HM40-3, 1:50, eBioscience) overnight at 4°C. After wash with PBS, slices were incubated with FITC-anti Armenia hamster IgM (1:100, BD Biosciences) for 1 h at room temperature. After final wash, tissue slices were covered using Fluoromount-GT (VWR, Bristol, CT, USA) mounting medium. Slices were examined using a Nikon Eclipse E800 fluorescence microscope (Nikon Instruments, Melville, NY) with a SPOT RT Slider CCD microscope digital camera (Burlingame, CA, USA). Both “non-stained” and “no primary Ab” controls were included in each run and no significant fluorescent signal was detected from either controls.
Determination of tissue levels of cytokines
Spleen and lumbar spinal cord (divided as ipsilateral and contralater sides) were collected following transcardiac perfusion and homogenized in tissue lysis buffer. The supernatants were collected after centrifugation (10000 × g at 4°C for 15 min) and stored at −80°C until analyses. For ELISA assays, a lysis buffer (pH 8.0) containing 0.05M Tris-base, 0.12mM NaCl, 4mM Na2EDTA, 3mM NaN3, and protease inhibitor cocktail (Sigma) was used. IL-1β, IL-6, and TNF-α were determined with DuoSet ELISA kits (R&D Systems, Minneapolis, MN, USA) following the manufacturers' protocols. For multiplex Luminex assay, PBS supplied with 1mM Na2EDTA, 1% BSA (Sigma), and protease inhibitor cocktail (Sigma) was used as lysis buffer. Luminex assay was performed with mouse Fluorokine MAP base kit and mouse Fluorokine MAP bead sets for IL-1β, IL-6, and TNF-α (R&D Systems) following the manufacturers' protocols using a Luminex100 analyzer (Luminex, Austin, TX). All cytokine levels were normalized based on the protein concentrations of each sample that were determined with BCA protein assay (Pierce-Thermo Scientific, Rockford, IL, USA).
Statistical analyses
All data were graphed with SigmaPlot 10.0 (Systat Software, San Jose, CA, USA) and analyzed using SigmaStat 3.5 (Systat Software). Appropriate analyses of variance (ANOVA) were performed followed by Student–Newman–Keuls (SNK) post hoc test. All data are presented as mean±SEM when applicable. p<0.05 was considered as statistically significant.
Acknowledgements
The authors thank Rendall R. Strawbridge and Augustinius Ong in the Shared Resources for Cancer Research at Norris Cotton Cancer Center in DHMC for providing irradiation service; and Dr. Alice Givan and Gary Ward in the Englert Cell Analysis Laboratory in the Dartmouth Medical School for their help in flow cytometric analysis. The authors are grateful for the help for the multiplex Luminex assay from Dr. David A. Lawrence and Nancy Anderson in the Wadsworth Center, Albany, NY, USA. The authors also thank Nancy Nutile-McMenemy for her technical help in IHC. This work was supported by 5 RO1 DA11276 NIDA(PI DeLeo), Immunology Training grant T32 NIAID (PI Green), 1K01DA023503-01A1 and NIDA (PI Cao) from NIH, as well as the Dean's Research Fellowships from College of Osteopathic Medicine, UNE.
Abbreviations
- IHC
immunohistochemistry
- L5Tx
spinal nerve L5 transection
- PNS
peripheral nervous system
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Backonja MM. Defining neuropathic pain. Anesth. Analg. 2003;97:785–790. doi: 10.1213/01.ANE.0000062826.70846.8D. [DOI] [PubMed] [Google Scholar]
- 2.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 3.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 4.Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J. Neuroimmunol. 2001;116:29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- 5.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 7.Togo T, Akiyama H, Kondo H, Ikeda K, Kato M, Iseki E, Kosaka K. Expression of CD40 in the brain of Alzheimer's disease and other neurological diseases. Brain Res. 2000;885:117–121. doi: 10.1016/s0006-8993(00)02984-x. [DOI] [PubMed] [Google Scholar]
- 8.Becher B, Durell BG, Miga AV, Hickey WF, Noelle RJ. The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J. Exp. Med. 2001;193:967–974. doi: 10.1084/jem.193.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benveniste EN, Nguyen VT, O'Keefe GM. Immunological aspects of microglia: relevance to Alzheimer's disease. Neurochem. Int. 2001;39:381–391. doi: 10.1016/s0197-0186(01)00045-6. [DOI] [PubMed] [Google Scholar]
- 10.D'Aversa TG, Weidenheim KM, Berman JW. CD40-CD40L interactions induce chemokine expression by human microglia: implications for human immunodeficiency virus encephalitis and multiple sclerosis. Am. J. Pathol. 2002;160:559–567. doi: 10.1016/S0002-9440(10)64875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okuno T, Nakatsuji Y, Kumanogoh A, Koguchi K, Moriya M, Fujimura H, Kikutani H, Sakoda S. Induction of cyclooxygenase-2 in reactive glial cells by the CD40 pathway: relevance to amyotrophic lateral sclerosis. J. Neurochem. 2004;91:404–412. doi: 10.1111/j.1471-4159.2004.02727.x. [DOI] [PubMed] [Google Scholar]
- 12.Tan J, Town T, Mullan M. CD40-CD40L interaction in Alzheimer's disease. Curr. Opin. Pharmacol. 2002;2:445–451. doi: 10.1016/s1471-4892(02)00180-7. [DOI] [PubMed] [Google Scholar]
- 13.Havenith CE, Askew D, Walker WS. Mouse resident microglia: isolation and characterization of immunoregulatory properties with naive CD41 and CD81 T-cells. Glia. 1998;22:348–359. [PubMed] [Google Scholar]
- 14.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Matyszak MK, Denis-Donini S, Citterio S, Longhi R, Granucci F, Ricciardi-Castagnoli P. Microglia induce myelin basic protein-specific T-cell anergy or T-cell activation, according to their state of activation. Eur. J. Immunol. 1999;29:3063–3076. doi: 10.1002/(SICI)1521-4141(199910)29:10<3063::AID-IMMU3063>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Olson JK, Girvin AM, Miller SD. Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler's virus. J. Virol. 2001;75:9780–9789. doi: 10.1128/JVI.75.20.9780-9789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalpke AH, Schafer MK, Frey M, Zimmermann S, Tebbe J, Weihe E, Heeg K. Immunostimulatory CpG-DNA activates murine microglia. J. Immunol. 2002;168:4854–4863. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- 18.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 19.Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, Mattson MP, et al. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science. 1999;286:2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- 20.Tan J, Town T, Paris D, Placzek A, Parker T, Crawford F, Yu H, et al. Activation of microglial cells by the CD40 pathway: relevance to multiple sclerosis. J. Neuroimmunol. 1999;97:77–85. doi: 10.1016/s0165-5728(99)00053-3. [DOI] [PubMed] [Google Scholar]
- 21.Becher B, Blain M, Antel JP. CD40 engagement stimulates IL-12 p70 production by human microglial cells: basis for Th1 polarization in the CNS. J. Neuroimmunol. 2000;102:44–50. doi: 10.1016/s0165-5728(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 22.Chabot S, Williams G, Hamilton M, Sutherland G, Yong VW. Mechanisms of IL-10 production in human microglia-T-cell interaction. J. Immunol. 1999;162:6819–6828. [PubMed] [Google Scholar]
- 23.Ait-Ghezala G, Mathura VS, Laporte V, Quadros A, Paris D, Patel N, Volmar CH, et al. Genomic regulation after CD40 stimulation in microglia: relevance to Alzheimer's disease. Brain Res. Mol. Brain Res. 2005;140:73–85. doi: 10.1016/j.molbrainres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Cao L, DeLeo JA. CNS-infiltrating CD41 T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur. J. Immunol. 2008;38:448–458. doi: 10.1002/eji.200737485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao L, Tanga FY, Deleo JA. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience. 2009;158:896–903. doi: 10.1016/j.neuroscience.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, Obregon D, et al. Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice. Nat. Neurosci. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- 27.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, et al. Microglia in the adult brain arise from Ly-6ChiCCR21 monocytes only under defined host conditions. Nat. Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 28.Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat. Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 29.Monaco S, Gehrmann J, Raivich G, Kreutzberg GW. MHC-positive, ramified macrophages in the normal and injured rat peripheral nervous system. J. Neurocytol. 1992;21:623–634. doi: 10.1007/BF01191724. [DOI] [PubMed] [Google Scholar]
- 30.Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J. Biol. Chem. 2001;276:44527–44533. doi: 10.1074/jbc.M106771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc. Natl. Acad. Sci. USA. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy D, Zochodne DW. NO pain: potential roles of nitric oxide in neuropathic pain. Pain Pract. 2004;4:11–18. doi: 10.1111/j.1533-2500.2004.04002.x. [DOI] [PubMed] [Google Scholar]
- 34.Robertson B, Xu XJ, Hao JX, Wiesenfeld-Hallin Z, Mhlanga J, Grant G, Kristensson K. Interferon-gamma receptors in nociceptive pathways: role in neuropathic pain-related behaviour. Neuroreport. 1997;8:1311–1316. doi: 10.1097/00001756-199703240-00050. [DOI] [PubMed] [Google Scholar]
- 35.Arruda JL, Sweitzer S, Rutkowski MD, DeLeo JA. Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: possible immune modulation in neuropathic pain. Brain Res. 2000;879:216–225. doi: 10.1016/s0006-8993(00)02807-9. [DOI] [PubMed] [Google Scholar]
- 36.Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience. 2004;129:767–777. doi: 10.1016/j.neuroscience.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 37.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 38.Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, McRoberts JA. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J. Neurogenet. 2008;22:315–331. doi: 10.1080/01677060802357388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hickey WF, Vass K, Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J. Neuropathol. Exp. Neurol. 1992;51:246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Sweitzer SM, Hickey WF, Rutkowski MD, Pahl JL, DeLeo JA. Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: potential relationship to neuropathic pain. Pain. 2002;100:163–170. doi: 10.1016/s0304-3959(02)00257-9. [DOI] [PubMed] [Google Scholar]