Abstract
Juvenile hemochromatosis is an iron overload disorder caused by mutations in the genes encoding the major iron regulatory hormone hepcidin (HAMP)1 and hemojuvelin (HFE2)2. We have previously shown that hemojuvelin is a bone morphogenetic protein (BMP) co-receptor and that BMP signals regulate hepcidin expression and iron metabolism3,4. However, the endogenous BMP regulator(s) of hepcidin in vivo is unknown. Here, we show that in vitro, compared with soluble hemojuvelin (HJV.Fc), the homologous DRAGON.Fc more potently inhibits hepcidin induction by BMP-2 or BMP-4, but less potently inhibits BMP-6. In vivo, HJV.Fc or a neutralizing BMP-6 antibody inhibits hepcidin expression and increases serum iron, while DRAGON.Fc has no effect. Notably, Bmp6 null mice have a phenotype resembling hereditary hemochromatosis with reduced hepcidin expression and tissue iron overload. Finally, we demonstrate a physical interaction between HJV.Fc and BMP-6, and we show that BMP-6 increases hepcidin expression and reduces serum iron in mice. These data support a key role for BMP-6 as a ligand for HJV and an endogenous regulator of hepcidin expression and iron metabolism in vivo.
Secreted by the liver5, hepcidin inhibits intestinal iron absorption and macrophage iron release by decreasing cell surface expression of the iron exporter ferroportin6. Hepcidin is upregulated by iron administration5,7–8 and inhibited by anemia7. Hepcidin deficiency and unchecked ferroportin activity are the common pathogenic mechanisms underlying the genetic iron overload disorder hereditary hemochromatosis9. Hepcidin is also upregulated by inflammatory cytokines, and hepcidin excess is implicated in the pathogenesis of anemia of inflammation5,7–8,10–12.
Recently, a role for the BMP signaling pathway in hepcidin regulation was discovered3–4,13. BMPs are members of the TGF-β superfamily, which is comprised of over forty ligands14. BMP/TGF-β superfamily ligands initiate an intracellular signaling cascade by binding to a complex of type I and type II serine threonine kinase receptors. The activated receptor complex phosphorylates intracellular Smad proteins, which translocate to the nucleus to modulate gene expression. Reduction of hepatic BMP signaling by a liver-specific conditional knockout of the common BMP/TGF-β intracellular mediator Smad413, or by mutations in the BMP co-receptor HFE22–3,15,16, are associated with inappropriately low hepcidin expression and iron overload. We and others have shown that BMP signals positively increase hepcidin expression at the transcriptional level in vitro3–4,13,17–18. We have also shown that iron administration in vivo increases hepatic BMP signaling19, and that BMP administration in vivo increases hepcidin expression and reduces serum iron4. Conversely, inhibition of endogenous BMP signaling with soluble hemojuvelin (HJV.Fc) or with the small molecule BMP inhibitor Dorsomorphin inhibits hepcidin expression and increases serum iron in vivo4,19. Presumably, the mechanism by which HJV.Fc inhibits hepcidin is by binding to endogenously secreted BMP ligands and preventing their interaction with cell-surface signaling receptors4.
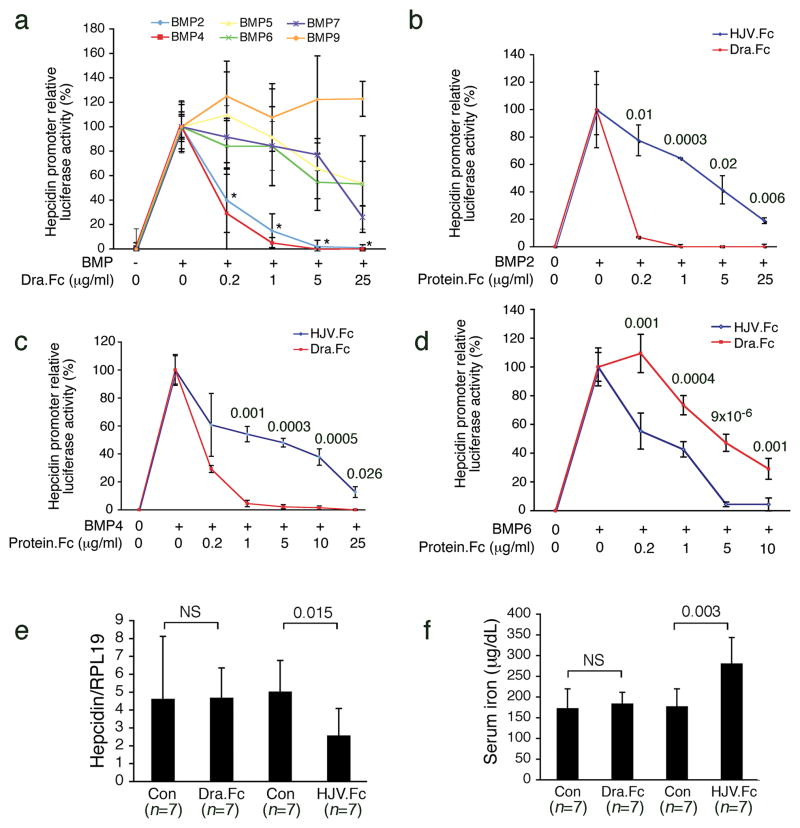
Hemojuvelin (also known as RGMc) is a member of the Repulsive Guidance Molecules family, including RGMa and DRAGON (RGMb), which share 50–60% amino acid identity20. Like Hemojuvelin, RGMa21 and DRAGON22 also bind BMP ligands and function as co-receptors for the BMP signaling pathway. To explore other BMP inhibitors as potential hepcidin lowering agents, we tested whether purified soluble DRAGON fused to the Fc portion of human IgG1 (DRAGON.Fc) inhibited BMP induction of hepcidin expression in a manner similar to HJV.Fc4. As shown in Figure 1a, DRAGON.Fc significantly inhibited hepcidin promoter induction by BMP-2 or BMP-4, but was less effective in inhibiting BMP-5, BMP-6, or BMP-7, and did not inhibit BMP-9. In comparison with HJV.Fc, DRAGON.Fc was significantly more potent against BMP-2 (Fig. 1b) and BMP-4 (Fig. 1c), but was less potent against BMP-6 (Fig. 1d). DRAGON.Fc also inhibited endogenous hepcidin mRNA expression in hepatoma-derived HepG2 cells, where basal hepcidin expression is dependent in part on endogenous BMP-2, BMP-4, and BMP-6 ligands4 (Supplementary Fig. 1).
Figure 1. DRAGON.Fc selectively inhibits BMP induction of hepcidin expression in vitro but does not affect hepcidin expression or iron metabolism in vivo.
(a–d) Hep3B cells were transfected with a hepcidin promoter luciferase reporter and control Renilla luciferase vector (pRL-TK), and incubated in the absence or presence of BMP ligands, either alone or in combination with 0.2 to 25 μg/mL purified DRAGON.Fc (Dra.Fc) or HJV.Fc as shown. Relative luciferase activity was calculated as the ratio of firefly to Renilla luciferase to control for transfection efficiency. The fold increase over baseline was generally between 40 to 150-fold for all BMP ligands for most experiments, although there was some variability. Results are reported as the mean +/− s.d. of the percent decrease in relative luciferase activity for cells treated with BMP ligands in combination with Dra.Fc or HJV.Fc compared with cells treated with BMP ligands alone, n = 6 to 11 per group. (a) Effects of Dra.Fc on BMP-2, BMP-4, BMP-5, BMP-6, BMP-7, and BMP-9 ligands. * For the indicated cells treated with BMP-2 or BMP-4 in combination with all concentrations of Dra.Fc, P < 0.001 compared with cells treated with BMP-2 or BMP-4 alone, and P ≤0.02 compared with cells stimulated with all other BMPs and treated with identical concentrations of Dra.Fc. (b–d) Head to head comparison of DRAGON.Fc and HJV.Fc for inhibiting BMP-2 (b), BMP-4 (c), and BMP-6 (d). Exact P values are shown. (e–f) Eight week-old male 129S6/SvEvTac mice received an intraperitoneal injection of purified soluble DRAGON.Fc (Dra.Fc) at 5 or10 mg/kg, HJV.Fc at 5 or 7 mg/kg, or an equal volume of isotonic saline (Con) three times weekly for three weeks. Results for both DRAGON.Fc and HJV.Fc doses were similar and were therefore combined into one group. (e) Total liver RNA was isolated and analyzed by quantitative real-time RT-PCR for hepcidin mRNA relative to Rpl19 mRNA as an internal control. (f) Measurement of serum iron. (e–f) Results are reported as the mean +/− s.d, n = 7 per group. Exact P values are shown.
We then tested whether DRAGON.Fc administration affected hepcidin expression and iron metabolism in vivo. HJV.Fc at a similar dose was used as a positive control. In contrast to HJV.Fc, DRAGON.Fc had no effect on hepatic hepcidin mRNA (Fig. 1e), splenic ferroportin (Supplementary Fig. 2a–b.), serum iron (Fig. 1f), serum transferrin saturation, liver iron content, or spleen iron content (Supplementary Fig. 2c–e) compared with mock treated control mice. Anti-BMP-2 activity in the serum of DRAGON.Fc treated mice was confirmed by the ability of this serum to inhibit BMP-2 induction of hepcidin promoter activity in vitro compared with serum from mock treated mice (Supplementary Fig. 2f).
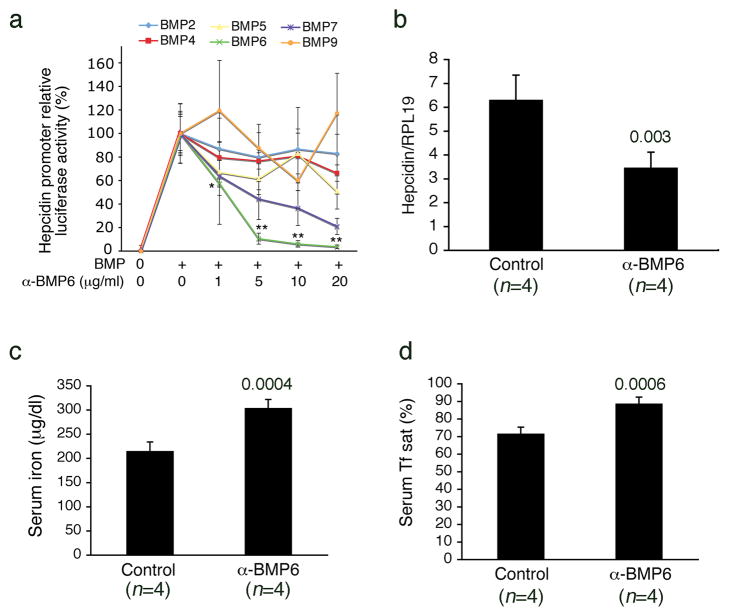
Since DRAGON.Fc had no effect in vivo despite its higher potency in vitro as an inhibitor of BMP-2 and BMP-4 compared with HJV.Fc, and since DRAGON.Fc was less potent at inhibiting BMP-6 compared with HJV.Fc, we hypothesized that the BMP-6 inhibiting properties of HJV.Fc are important for its effects as a hepcidin-lowering agent in vivo. We therefore tested whether a neutralizing BMP-6 antibody affected hepcidin expression and serum iron levels in mice. BMP-6 neutralizing antibody selectively inhibited BMP-6 induced hepcidin promoter activity in vitro, but had no significant effect on BMP-2, BMP-4, or BMP-9 (Fig. 2a). BMP-6 antibody exhibited some inhibitory activity against BMP-7 and to a lesser extent BMP-5 at higher concentrations, but significantly less compared with BMP-6 (Fig. 2a). This crossreactivity is not surprising since BMP-6, BMP-7, and BMP-5 have 71–80% amino acid identity and form a subfamily within the BMP ligands23. To minimize the effects of this cross-reactivity, we used the lowest effective dose of BMP-6 antibody for subsequent in vivo experiments. Cross-reactivity with BMP-7 was also less of a concern since BMP-7 is not a ligand for hemojuvelin, is not expressed in the liver and is not thought to be an important endogenous hepcidin regulator4,23. Treatment with BMP-6 antibody at 10 mg/kg for three days significantly reduced hepatic hepcidin mRNA expression by ~50%, and increased serum iron and transferrin saturation compared with mock treated control mice (Fig. 2b-d). As a control, BMP-6 antibody treatment also caused a trend toward reduced hepatic Id1 mRNA expression, another expected target of BMP-621, by ~60% (Supplementary Fig. 3). Similar results were found with a lower dose of BMP-6 antibody, although this dose was less effective (Supplementary Fig. 4)
Figure 2. Neutralizing BMP-6 antibody inhibits hepatic hepcidin expression and increases serum iron and transferrin saturation in vivo.
(a) Hep3B cells were transfected with a hepcidin promoter luciferase reporter and control pRL-TK as in Figure 1. Forty-eight hours after transfection, cells were incubated in the absence or presence of BMP ligands, either alone or in combination with 0.2 to 25 μg/mL neutralizing BMP-6 antibody as shown (n = 5 per group). Relative luciferase activity was calculated as in Figure 1. (b–d) Eight week-old male 129S6/SvEvTac mice received an intraperitoneal injection of neutralizing BMP-6 antibody at 10 mg/kg (α-BMP6, n = 4) or an equal volume of isotonic saline (Control, n = 4) daily for three days. (b) Total liver RNA was isolated and analyzed by quantitative real-time RT-PCR for hepcidin mRNA relative to Rpl19 mRNA as an internal control. (c and d) Measurement of serum iron (c) and transferrin saturation (d). Results are expressed as the mean +/− s.d. (a) * P = 0.036 for cells treated with 0.2 μg/mL BMP-6 antibody in combination with BMP-6 ligand compared with cells treated with BMP-6 alone. ** P ≤0.01 for all other concentrations of BMP-6 antibody compared with cells treated with BMP-6 alone and compared with cells stimulated with all other BMPs and treated with identical concentrations of BMP-6 antibody. (b–d) Exact P values are shown.
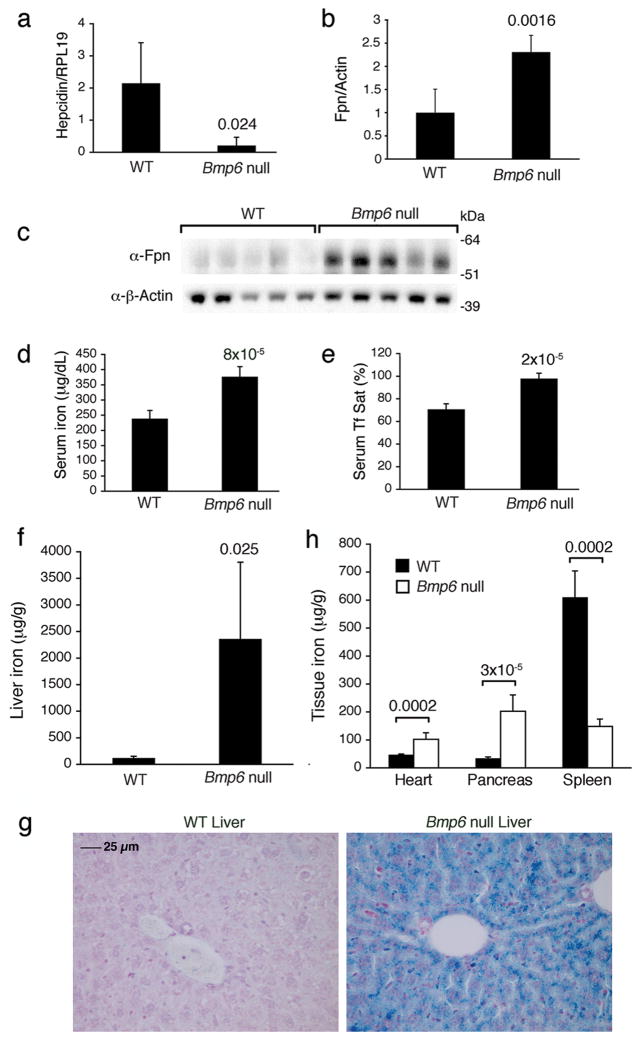
To validate the importance of endogenous BMP-6 in regulating hepcidin expression and iron metabolism in vivo, we examined 4 and 8 week-old Bmp6 null mice, which were previously generated by Solloway et al24. These Bmp6 null mice were described to have some mild delays in bone formation during development, but no other overt defects were found24. Compared with wildtype mice, 8-week-old Bmp6 null mice exhibited significantly reduced hepatic hepcidin mRNA expression by 10-fold, increased splenic ferroportin expression, and increased serum iron with serum transferrin saturation approaching 100% (Figs 3a-e). Liver iron content was significantly increased in Bmp6 null mice by 6-fold at four weeks and by 20-fold at eight weeks (Supplementary Fig. 5a and Fig. 3f,h). Significant iron accumulation was also evident in the heart and pancreas of Bmp6 null mice, while spleen iron content was reduced (Fig. 3g and Supplementary Fig. 5b). The degree of iron overload in the liver of 8 week-old Bmp6 null mice appears comparable to that reported in Hfe2−/− mice at a similar age15–16. Thus, Bmp6 null mice have a phenotype that resembles mouse models of juvenile hemochromatosis due to loss of the BMP co-receptor hemojuvelin.
Figure 3. Bmp6 null mice exhibit reduced hepatic hepcidin expression, increased spleen ferroportin expression, increased serum iron and transferrin saturation, increased liver, heart, and pancreas iron content, and reduced spleen iron content.
Eight-week-old male Bmp6 null mice (n = 5) and strain matched wildtype control mice (WT, n = 5) were analyzed for (a) hepcidin mRNA expression relative to Rpl19 mRNA expression by quantitative real-time RT-PCR, (b–c) ferroportin expression relative to β-actin expression by Western blot (c) followed by quantitation using IPLab Spectrum software (b), (d) serum iron, (e) serum transferrin saturation, (f) liver iron content, (g) heart, pancreas, and spleen iron content. (H) Perls Prussian blue staining of tissue iron in wildtype (WT) and a Bmp6 null mouse livers. Original magnification x 40. Results are expressed as mean +/− s.d. Exact P values are shown.
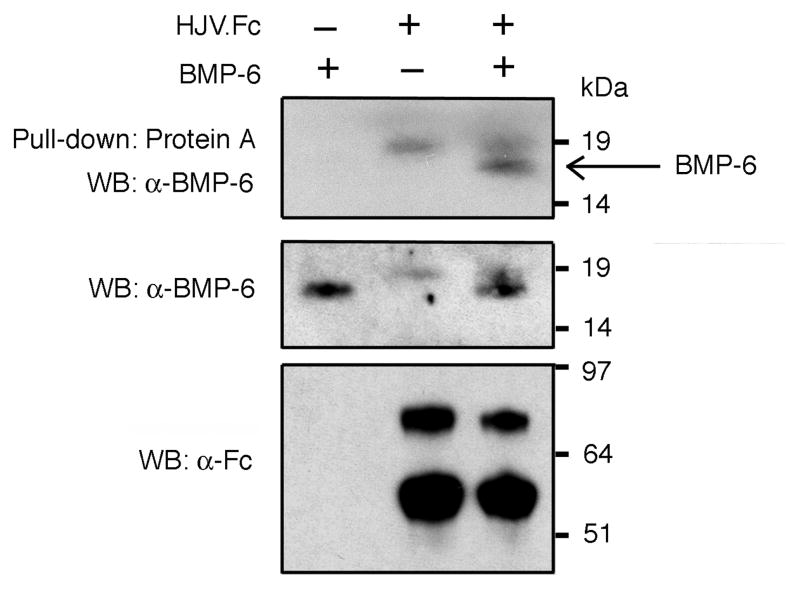
To further explore whether BMP-6 is a ligand for hemojuvelin, purified BMP-6 alone, HJV.Fc alone, or the combination of BMP-6 and HJV.Fc were incubated in solution followed by pull-down with Protein A beads. Western blot of eluates with BMP-6 antibody showed that an 18 kDa protein, corresponding to the predicted size of BMP-6 monomer, was pulled down in the presence of HJV.Fc (Fig. 4, arrow). No 18 kDa band was seen from solutions containing BMP-6 alone or HJV.Fc alone (Fig. 4). These data demonstrate a direct interaction between BMP-6 and HJV.Fc.
Figure 4. BMP-6 interacts with HJV.Fc.
BMP-6 alone, HJV.Fc alone, or BMP-6 in combination with HJV.Fc were incubated in solution. (Upper panel) BMP-6 bound to HJV.Fc was precipitated with protein A beads, and the eluted protein complex was analyzed by SDS-PAGE, followed by Western blot with BMP-6 antibody under reducing conditions. The band migrating at ~18 kDa present only in the lane containing BMP-6 in combination with HJV.Fc corresponds to the predicted size of BMP-6 monomer (arrow). (Lower panels) As a control to demonstrate input proteins, solution aliquots prior to Protein-A pull-down were also analyzed by Western blot with anti-Fc antibody and anti-BMP-6 antibody under reducing conditions. Control Westerns revealed the predicted bands migrating at 18 kDa for BMP-6 monomer and 75 and 60 kDa for HJV.Fc4. A background band at ~19 kDa was present in the lanes containing HJV.Fc probed with BMP-6 antibody suggesting nonspecific crossreactivity of this antibody with a component of the HJV.Fc solution.
Next, we examined the ability of exogenous BMP-6 to regulate hepcidin expression and iron metabolism in vivo. Mice were injected with a single dose of exogenous BMP-6 at 250 and 1000 μg/kg IP. As a positive control, BMP-6 administration significantly increased hepatic Id1 mRNA expression (Supplementary Fig. 6). BMP-6 administration also significantly increased hepatic hepcidin mRNA expression (Fig. 5a), and caused a dose dependent reduction in serum iron (Fig 5b) and serum transferrin saturation (Fig 5c).
Figure 5. BMP-6 administration in mice increases hepcidin mRNA expression and reduces serum iron.
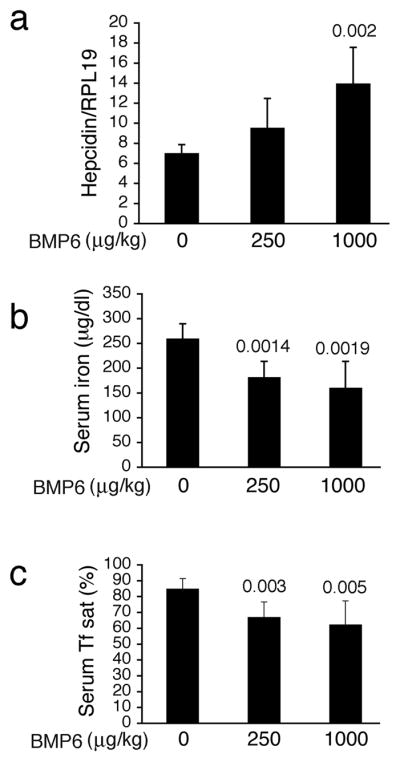
Eight-week-old male 129S6/SvEvTac mice received an intraperitoneal injection of BMP-6 at 250 μg/kg (n = 6) or 1000 μg/kg (n = 7) or an equal volume of vehicle alone (n = 6). Six hours after injection, blood and livers were harvested. (a) Total liver RNA was isolated and analyzed by quantitative real-time RT-PCR for hepcidin mRNA relative to Rpl19 mRNA as an internal control. (b–c) Measurement of serum iron (b) and transferrin saturation (c). Results are reported as the mean +/− s.d. Exact P values are shown where significant.
Together, these data suggest that BMP-6 is a ligand for hemojuvelin and that BMP-6 is a key endogenous regulator of hepcidin expression and iron metabolism in vivo. Numerous other BMP ligands have been shown to regulate hepcidin when added exogenously in vitro or in vivo, including BMP-2, BMP-4, BMP-5, BMP-7, and BMP-93–4,13,17. We have also previously shown that endogenous BMP-2, BMP-4, and BMP-6 all contribute to basal hepcidin expression HepG2 cells4. Thus, it is not surprising that inhibition of BMP-2 and BMP-4 by DRAGON.Fc inhibits hepcidin expression in these cells. However, it is well-described that cell-based assays do not accurately mirror the in vivo regulation of hepcidin by iron, since iron increases hepcidin expression in vivo, but decreases hepcidin expression in vitro under most conditions5,10,25. One reason for this discrepancy may be that the complement of BMP ligands and receptors that regulate hepcidin expression in vitro are different from those that regulate hepcidin expression in response to iron in vivo. We hypothesize that HJV.Fc and BMP-6 antibody inhibit hepcidin expression in vivo by binding to endogenously secreted BMP-6 and preventing its interaction with cell-surface signaling receptors. We hypothesize that DRAGON.Fc does not affect hepcidin expression in vivo because 1) it is a less potent inhibitor of BMP-6 compared with HJV.Fc and does not significantly inhibit BMP-6 at the doses used in this study, and 2) in contrast to the in vitro experiments, endogenous BMP-2 and BMP-4 ligands do not significantly contribute to hepcidin regulation in vivo. Indeed, the liver is presumably the source of the endogenous BMPs that regulate hepcidin, and a recent study reported that hepatic Bmp6 mRNA levels vary concordantly with hepcidin mRNA levels in response to dietary iron content. In contrast, Bmp2 mRNA levels are only upregulated slightly under extreme iron overload conditions and Bmp4 mRNA levels are not modulated by dietary iron26. We also did not find changes in hepatic Bmp2 and Bmp4 mRNA levels in Bmp6 null mice despite significant iron overload (Supplementary Fig. 7). Interestingly, Bmp2 and Bmp4 mRNA were increased in the bone of Bmp6 null mice by 2.5–6-fold (P. Simic, I. Orlic, V. Kufner, and S.V., unpublished data), suggesting that BMP-2 and BMP-4 are differentially regulated in different tissues and may compensate for the loss of BMP-6 function in some tissues, but do not compensate for the lack of BMP-6 to regulate hepcidin expression and iron metabolism.
The Bmp6 null mice contain a neomycin cassette, raising the possibility that expression of neighboring genes might also be affected, as described in other mouse models27. The closest neighboring gene to Bmp6 is the gene encoding thioredoxin domain-containing protein 5 (Txndc5) separated by 547 base pairs. Txndc5 mRNA is present in Bmp6 null mice with similar expression levels compared with wildtype mice as measured by RT-PCR (Supplementary Fig. 7). Although we cannot rule out small changes in Txndc5 mRNA levels by this technique, our interventional data showing that BMP-6 administration increases hepcidin and reduces serum iron, while endogenous BMP-6 inhibition reduces hepcidin expression and increases serum iron, lend further support to the hypothesis that it is the loss of BMP-6 that is the major cause of the iron overload phenotype in Bmp6 null mice.
Our data suggest that selective BMP-6 inhibitors may be effective agents for treating anemia of inflammation due to hepcidin excess. The lack of any other notable phenotype in Bmp6 null mice suggests that a more selective inhibitor may be better tolerated with fewer off-target effects. Additionally, BMP-6-like agonists may be an alternative treatment strategy for managing iron overload disorders in patients resistant to current therapies. Although no human patients with BMP6 mutations have yet been described, our data also suggests that BMP6 mutations or BMP6 gene variants may function as another cause of hereditary hemochromatosis or a modifier of disease penetrance.
METHODS
cDNA
cDNA encoding codon optimized DRAGON.Fc was generated by GenScript Corporation (Piscataway, NJ 08854), based on the human DRAGON protein sequence upstream of the predicted GPI anchor (UniProtKB/Swiss-Prot accession Q6NW40, amino acids 1-409) and the human IgG1 Fc sequence (from the Signal pIg plus vector (R&D Systems) and GenBank AF150959).
Production and purification of DRAGON.Fc and HJV.Fc
cDNA encoding DRAGON.Fc was transfected using 239fectin (Invitrogen) into Freestyle 293-F cells (Invitrogen) according to the manufacturer’s instructions. Transfected cells were cultured in GIBCO Freestyle 293 Expression medium (Invitrogen) shaking at 110 RPM in a humidified 8% CO2 incubator at 37 °C. Seven days after transfection, cells were pelleted by centrifugation and DRAGON.Fc was purified from the media via one-step Protein A affinity chromatography using Hi-Trap rProtein A FF columns (Amersham Biosciences) as previously described21. HJV.Fc was produced as previously described4. To determine purity and to quantify protein concentration, DRAGON.Fc and HJV.Fc were subjected to reducing SDS-PAGE followed by Bio-safe Coomassie blue staining (Bio-Rad) as well as Western blot with anti-HJV antibody3, anti-DRAGON antibody20, and goat anti-human Fc antibody (Jackson ImmunoResearch Laboratories) as previously described3,20. Protein concentration was also quantified by the bovine serum albumin protein assay (Pierce).
Production of BMP-6
Purified recombinant human BMP-6 was prepared as previously described28. Lyophilized BMP-6 was dissolved in 20 mM sodium acetate, 5% mannitol solution, pH 4.0 for animal injections.
Luciferase Assay
Hepcidin promoter luciferase reporter assays in hepatoma-derived Hep3B cells were carried out using the Dual-Luciferase Reporter Assay System (Promega) as previously described3,4 with the following modifications. Hep3B cells transfected with the hepcidin promoter luciferase reporter3 and control Renilla luciferase vector (pRL-TK) were serum starved in α-MEM with L-glutamine (Invitrogen) supplemented with 1% FBS for 6 hours, followed by stimulation with 25 ng/mL BMP-2 (kindly provided by Vicki Rosen, Harvard School of Dental Medicine), BMP-4, BMP-6, or BMP-7, 50 ng/mL BMP-5 or 5 ng/mL BMP-9 (R&D Systems) either alone or with 0.2–25 μg/mL of DRAGON.Fc, HJV.Fc or anti-BMP6 antibody for 16 hrs. Relative concentrations of BMP ligands were chosen to elicit similar degrees of hepcidin promoter luciferase activity, as previously described4. All experiments were performed at least three times. Some repetitions used a mouse homologue of DRAGON.Fc20 which had similar results compared with the human DRAGON.Fc homologue.
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee at the Massachusetts General Hospital and the Institutional Animal Care Committee and the Ministry of Science and Technology at the University of Zagreb School of Medicine. Eight-week-old 129S6/SvEvTac mice (Taconic) were housed in the Massachusetts General Hospital rodent facility and fed on the Prolab 5P75 Isopro RMH 3000 diet with 380 parts per million iron. Bmp6 null mice on a mixed 129Sv/C57 background24, kindly provided by Elizabeth J. Robertson, were housed at the University of Zagreb School of Medicine and maintained on standard GLP diet (4RF21, Mucedola, Italy) with 180 mg/kg iron.
For DRAGON.Fc and HJV.Fc experiments, 8-week-old 129S6/SvEvTac mice (Taconic) received an intraperitoneal injection of DRAGON.Fc at doses of 5 or10 mg/kg, HJV.Fc at doses of 5 or 7 mg/kg, or an equal volume of isotonic saline three times per week for three weeks. Twenty-four hours after the last injection, mice were sacrificed and blood and livers were harvested for measurement of iron parameters and hepcidin expression.
For BMP-6 antibody injection experiments, 8-week-old 129S6/SvEvTac mice received an intraperitoneal injection of monoclonal anti-human BMP-6 antibody (R&D Systems) at 10 mg/kg daily for three days or at 5 mg/kg three times weekly for three weeks. Control mice received an intraperitoneal injection of an equal volume of isotonic saline using the same dosing regimen. Based on the assumption that BMP-6 antibody would function by a similar mechanism as HJV.Fc protein, by binding and sequestering endogenously produced BMP-6 ligand, we chose the expected minimal effective dose of BMP-6 antibody for these in vivo experiments based on our in vitro data using this antibody and our experience with HJV.Fc protein. Twelve hours after the last injection, mice were sacrificed and blood and livers were harvested for measurement of iron parameters and hepcidin expression.
For BMP-6 injection experiments, 8-week-old 129/SvEvTac mice received an intraperitoneal injection of BMP-6 at 250 or 1000 mcg/kg or an equal volume of vehicle alone (20 mM sodium acetate, 5% mannitol solution, pH 4.0). Six hours after injection, mice were sacrificed and blood, livers, and spleen were harvested for measurement of iron parameters and hepcidin expression. A time point of six hours was chosen to capture both an increase in hepcidin expression and a resulting decrease in serum iron based on prior in vitro3 and in vivo4 data studying the effects of BMP-2 on hepcidin expression and serum iron, as well as preliminary dose curve and time curve data studying the effects of BMP-6 on hepcidin expression and serum iron in vivo (data not shown).
For Bmp6 null mouse experiments, 4-week-old and 8-week-old Bmp6 null mice and wildtype control mice of a similar age were sacrificed and blood, livers, heart, pancreas, and spleen were harvested for measurements of iron parameters and hepcidin expression.
Quantitative real-time RT-PCR and RT-PCR
Total RNA was isolated from mouse livers using the RNeasy kit (QIAGEN) according to the manufacturer’s instructions. Real-time quantification of Hamp1 mRNA transcripts relative to Rpl19 was performed using 2-step quantitative real-time RT-PCR as previously described3–4,29. Real time quantification of Id1 mRNA was performed using primers summarized in Supplementary Table 1. Real time quantification of Bmp2, Bmp4, and Bmp6 mRNA from livers of Bmp6 null versus wildtype mice was performed using previously described primers26. Txndc5 mRNA was amplified from the livers of Bmp6 null versus wildtype mice using primers summarized in Supplementary Table 1.
Western blot
For ferroportin assays, spleen membrane preparations were prepared as previously described4. Protein concentrations were determined by BCA assay (Pierce). After solubilization in 1× Laemmli buffer for 30 minutes at room temperature, 20 μg of protein per sample were resolved by reducing SDS-PAGE using pre-cast NuPAGE Novex 4–12% Bis-Tris gels (Invitrogen) and transferred onto PDVF membranes (liquid transfer method). The blots were saturated with 10% non-fat milk in tris buffered saline (TBS) containing 0.1% Tween (TBS-T) and probed overnight at 4°C with 2.5 μg/ml ferroportin antibody (diluted in TBS-T with 5% non-fat milk)30. Following wash with TBS-T, the blots were incubated with 1:5000 diluted peroxidase-coupled goat anti-rabbit IgG (Sigma) for 1 hour. Detection was performed with the enhanced chemiluminescence ECL® method (Perkin Elmer). Blots were stripped and re-probed for β-actin expression as a loading control as previously described3. Chemiluminescence was quantitated using IPLab Spectrum software version 3.9.5 r2 (Scanalytics).
Serum and tissue iron measurements
Serum was collected and analyzed for iron concentration and unsaturated iron-binding capacity as previously described4. Total iron binding capacity and transferrin saturation were calculated as previously described4. Quantitative measures of nonheme iron was performed on liver, spleen, heart, and pancreas as previously described4.
Histology
Tissues from Bmp6 null and wildtype mice were fixed in 2% paraformaldehyde followed by 2% ethanol and embedded in paraffin. Sections at 5 μm were deparaffinized in Xylene and hydrated to distilled water. Sections were then placed in staining solution with equal volumes of 2% potassium ferrocyanide (Electron Microscopy Sciences, Hatfield, PA) and 2% hydrochloric acid, for 60 min at room temperature. The sections were then rinsed in distilled water, counterstained in Safranin O, 0.2% (Electron Microscopy Sciences, Hatfield, PA) for 2 min, and washed in 1% acetic acid, before being dehydrated in 95% alcohol, absolute alcohol, cleared in Xylene, and mounted in DPX.
Pull-down assay
BMP-6 (1 μg, R & D Systems) alone, HJV.Fc (5 μg) alone, or BMP-6 (1 μg) in combination with HJV.Fc (5 μg) were incubated in 500 μL TBS (Tris.HCl, 50 mM, NaCl 150 mM, 0.2% Tween-20, pH 7.4) at 4 °C overnight. The solutions were then incubated for 3 h at 4 °C with Protein-A beads (PIERCE Biotechnology), which had been blocked with 1% BSA. The beads were washed and proteins were eluted with reducing 1× Laemmli sample buffer. Eluted protein was separated by SDS-PAGE and subjected to Western blot analysis under reducing conditions using a rabbit polyclonal BMP6 antibody (1:500)28. Western blot analysis of the above solutions prior to Protein-A pull down was also performed under reducing conditions using rabbit polyclonal BMP-6 antibody (1:500) as above and goat anti-human Fc antibody (Jackson ImmunoResearch Laboratories) as previously described3.
Statistics
A two-tailed Student’s t test with P < 0.05 was used to determine statistical significance.
Supplementary Material
Acknowledgments
We thank Dr. Vicki Rosen for kindly providing BMP-2 for our study. E.C. was supported in part by the Associazione Modenese per le Malattie del Fegato. S.V. was supported in part by the Croatian Ministry of Science, education, and sport. H.Y.L. was supported in part by NIH grants RO1 DK-69533 and RO1 DK-071837. J.L.B. was supported in part by NIH grant K08 DK-075846 and by a Claflin Distinguished Scholar Award from the Massachusetts General Hospital.
Footnotes
Accession numbers.
HAMP, hepcidin (Homo sapiens), NM_021175, GeneID: 57817. Hamp1, hepcidin (Mus musculus), NM_032541, GeneID: 84506. HFE2 transcript variant a, hemojuvelin, (Homo sapiens), NM_213653, GeneID: 148738. Id1, inhibitor of DNA binding, (Mus musculus) NM_010495, GeneID: 15901. Bmp6, bone morphogenetic protein 6, (Mus musculus), NM_007556, GeneID: 12161. Bmp2, bone morphogenetic protein 2, (Mus musculus), NM_007553, GeneID: 12156. Bmp4, bone morphogenetic protein 4, (Mus musculus), NM_007554, GeneID: 12159. Txndc5, thioredoxin domain containing 5, (Mus musculus), NM_145367, GeneID: 105245. Rpl19, ribosomal protein L19, (Mus musculus), NM_009078, GeneID: 19921.
Author contributions.
B.A.J., E.C., Y.X., H.Y.L, and J.L.B. designed experiments. B.A.J., E.C., Y.X., S.A.F., S.C., L.G., and J.L.B. performed experiments. B.A.J., E.C., Y.X., S.A.F. and J.L.B. analyzed data. M.D.K., A.P., S.V., and H.Y.L. contributed vital resources and reagents. B.A.J., E.C., Y.X., A.P., S.V., H.Y.L. and J.L.B. wrote or edited the paper. J.L.B. conceived and oversaw the entire project.
References
- 1.Roetto A, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33:21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 2.Papanikolaou G, et al. Mutations in HFE2 cause iron overload in chromosome 1q linked juvenile hemochromatosis. Nat Genet. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 3.Babitt JL, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 4.Babitt JL, et al. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117:1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pigeon C, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth E, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 7.Nicolas G, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemeth E, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pietrangelo A. Hereditary hemochromatosis. Biochim Biophys Acta. 2006;1763:700–710. doi: 10.1016/j.bbamcr.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Nemeth E, et al. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 11.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 12.Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–230. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang RH, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 15.Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest. 2005;115:2187–2191. doi: 10.1172/JCI25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115:2180–2186. doi: 10.1172/JCI25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci U S A. 2006;103:10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verga Falzacappa MV, Casanovas G, Hentze MW, Muckenthaler MU. A bone morphogenetic protein (BMP)-responsive element in the hepcidin promoter controls HFE2-mediated hepatic hepcidin expression and its response to IL-6 in cultured cells. J Mol Med. 2008;86:531–540. doi: 10.1007/s00109-008-0313-7. [DOI] [PubMed] [Google Scholar]
- 19.Yu PB, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samad TA, et al. DRAGON: a member of the repulsive guidance molecule-related family of neuronal- and muscle-expressed membrane proteins is regulated by DRG11 and has neuronal adhesive properties. J Neurosci. 2004;24:2027–2036. doi: 10.1523/JNEUROSCI.4115-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babitt JL, et al. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. J Biol Chem. 2005;280:29820–29827. doi: 10.1074/jbc.M503511200. [DOI] [PubMed] [Google Scholar]
- 22.Samad TA, et al. DRAGON, a bone Morphogenetic protein co-receptor. J Biol Chem. 2005;280:14122–14129. doi: 10.1074/jbc.M410034200. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y, Babitt JL, Sidis Y, Chung RT, Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood. 2008;111:5195–5204. doi: 10.1182/blood-2007-09-111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solloway MJ, et al. Mice lacking Bmp6 function. Dev Genet. 1998;22:321–339. doi: 10.1002/(SICI)1520-6408(1998)22:4<321::AID-DVG3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Gehrke SG, et al. Expression of hepcidin in hereditary hemochromatosis: evidence for a regulation in response to the serum transferrin saturation and to non-transferrin-bound iron. Blood. 2003;102:371–376. doi: 10.1182/blood-2002-11-3610. [DOI] [PubMed] [Google Scholar]
- 26.Kautz L, et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112:1503–1509. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 27.Nicolas G, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simic P, et al. Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J Biol Chem. 2006;281:25509–25521. doi: 10.1074/jbc.M513276200. [DOI] [PubMed] [Google Scholar]
- 29.Xia Y, et al. Repulsive guidance molecule RGMa alters utilization of bone morphogenetic protein (BMP) type II receptors by BMP2 and BMP4. J Biol Chem. 2007;282:18129–18140. doi: 10.1074/jbc.M701679200. [DOI] [PubMed] [Google Scholar]
- 30.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.