Abstract
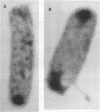
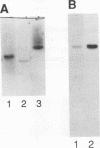
Verotoxin-producing Escherichia coli strains of the serotype O157:H7 belong to a class of gastrointestinal pathogens that adhere to epithelial cells in a characteristic pattern known as attaching and effacing. Recent insight into the nature of E. coli O157:H7 adhesion was provided by the cloning and sequencing of the chromosomal eaeA (for E. coli attaching and effacing) gene homolog (G. Beebakhee, M. Louie, J. De Azavedo, and J. Brunton, FEMS Microbiol. Lett. 91:63-68, 1992, and J. Yu and J. B. Kaper, Mol. Microbiol. 6:411-417, 1992) and isolation of a 60-MDa plasmid referred to as pO157 (I. Toth, M. L. Cohen, H. S. Rumschlag, L. W. Riley, E. H. White, J. H. Carr, W. W. Bond, and I. K. Wachsmuth, Infect. Immun. 58:1223-1231, 1990, and S. Tzipori, H. Karch, K. I. Wachsmuth, R. M. Robins-Browne, A. D. O'Brien, H. Lior, M. L. Cohen, J. Smithers, and M. M. Levine, Infect. Immun. 55:3117-3125, 1987) and an approximately 94-kDa outer membrane protein (94-kDa OMP; P. Sherman, F. Cockerill III, R. Soni, and J. Brunton, Infect. Immun. 59:890-899, 1991). In this study, we examined the gene products of both eaeA and pO157 in relation to the 94-kDa OMP and as candidate effectors for O157:H7 attachment-effacement. Peptide sequencing and immunoassay demonstrated that the C. coli O157:H7 eaeA gene product is distinct from the 94-kDa OMP. Using ultrastructural analyses, we found that both parent and pO157 plasmid-cured O157:H7 strains demonstrated attaching and effacing adhesion to host epithelial cells and reacted equally well to rabbit antiserum raised against the 94-kDa OMP. By both transmission electron microscopy and light microscopy, E. coli HB101 transformed separately with the cloned eaeA gene and the pO157 plasmid did not form attaching and effacing lesions on cultured epithelial cells in vitro and rabbit intestinal tissues in vivo. Since additional determinants may mediate the attaching and effacing phenotype, we examined transposon TnphoA mutants constructed from E. coli O157:H7 strain CL8. Two TnphoA mutants were found deficient in bacterial factors that are necessary for O157:H7 attachment-effacement and likely distinct from the eaeA gene product.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi S., Larocco M., Murray B. E., Cleary T. G. The adherence of verocytotoxin-producing Escherichia coli to rabbit intestinal cells. J Med Microbiol. 1992 Nov;37(5):304–309. doi: 10.1099/00222615-37-5-304. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Beebakhee G., Louie M., De Azavedo J., Brunton J. Cloning and nucleotide sequence of the eae gene homologue from enterohemorrhagic Escherichia coli serotype O157:H7. FEMS Microbiol Lett. 1992 Feb 1;70(1):63–68. doi: 10.1016/0378-1097(92)90563-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Krämer C., Schmidmayr W., Chen-Schmeisser U., Henning U. Primary structure of major outer-membrane protein I (ompF protein, porin) of Escherichia coli B/r. Biochem J. 1982 Apr 1;203(1):33–43. doi: 10.1042/bj2030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Schmidmayr W., Krämer C., Chen-Schmeisser U., Henning U. Primary structure of major outer membrane protein II (ompA protein) of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4592–4596. doi: 10.1073/pnas.77.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. A., Mitchell J. B. Viability measurements in mammalian cell systems. Anal Biochem. 1989 May 15;179(1):1–7. doi: 10.1016/0003-2697(89)90191-7. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Calderwood S. B., Donohue-Rolfe A., Keusch G. T., Kaper J. B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect Immun. 1990 Jun;58(6):1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Kaper J. B. Enteropathogenic Escherichia coli. Infect Immun. 1992 Oct;60(10):3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dytoc M., Gold B., Louie M., Huesca M., Fedorko L., Crowe S., Lingwood C., Brunton J., Sherman P. Comparison of Helicobacter pylori and attaching-effacing Escherichia coli adhesion to eukaryotic cells. Infect Immun. 1993 Feb;61(2):448–456. doi: 10.1128/iai.61.2.448-456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Jerse A. E., Kaper J. B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991 Dec;59(12):4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Yu J., Tall B. D., Kaper J. B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Heesemann J., Laufs R., O'Brien A. D., Tacket C. O., Levine M. M. A plasmid of enterohemorrhagic Escherichia coli O157:H7 is required for expression of a new fimbrial antigen and for adhesion to epithelial cells. Infect Immun. 1987 Feb;55(2):455–461. doi: 10.1128/iai.55.2.455-461.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989 Jan;2(1):15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Baldini M. M., Kaper J. B., McNeish A. S. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect Immun. 1987 Jan;55(1):78–85. doi: 10.1128/iai.55.1.78-85.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Donnenberg M. S., Kaper J. B., Finlay B. B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992 Oct;11(10):3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. M., Soni R. Adherence of Vero cytotoxin-producing Escherichia coli of serotype O157:H7 to human epithelial cells in tissue culture: role of outer membranes as bacterial adhesins. J Med Microbiol. 1988 May;26(1):11–17. doi: 10.1099/00222615-26-1-11. [DOI] [PubMed] [Google Scholar]
- Sherman P., Cockerill F., 3rd, Soni R., Brunton J. Outer membranes are competitive inhibitors of Escherichia coli O157:H7 adherence to epithelial cells. Infect Immun. 1991 Mar;59(3):890–899. doi: 10.1128/iai.59.3.890-899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P., Fleming N., Forstner J., Roomi N., Forstner G. Bacteria and the mucus blanket in experimental small bowel bacterial overgrowth. Am J Pathol. 1987 Mar;126(3):527–534. [PMC free article] [PubMed] [Google Scholar]
- Sherman P., Soni R., Petric M., Karmali M. Surface properties of the Vero cytotoxin-producing Escherichia coli O157:H7. Infect Immun. 1987 Aug;55(8):1824–1829. doi: 10.1128/iai.55.8.1824-1829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P., Soni R., Yeger H. Characterization of flagella purified from enterohemorrhagic, vero-cytotoxin-producing Escherichia coli serotype O157:H7. J Clin Microbiol. 1988 Jul;26(7):1367–1372. doi: 10.1128/jcm.26.7.1367-1372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Observations on the pathogenic properties of the K88, Hly and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971 Nov;4(4):467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Manoil C., Mekalanos J. J. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J Bacteriol. 1989 Apr;171(4):1870–1878. doi: 10.1128/jb.171.4.1870-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh V. L., O'Brien A. D. Adherence and colonization mechanisms of enteropathogenic and enterohemorrhagic Escherichia coli. Microb Pathog. 1992 Apr;12(4):245–254. doi: 10.1016/0882-4010(92)90043-n. [DOI] [PubMed] [Google Scholar]
- Toth I., Cohen M. L., Rumschlag H. S., Riley L. W., White E. H., Carr J. H., Bond W. W., Wachsmuth I. K. Influence of the 60-megadalton plasmid on adherence of Escherichia coli O157:H7 and genetic derivatives. Infect Immun. 1990 May;58(5):1223–1231. doi: 10.1128/iai.58.5.1223-1231.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Karch H., Wachsmuth K. I., Robins-Browne R. M., O'Brien A. D., Lior H., Cohen M. L., Smithers J., Levine M. M. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun. 1987 Dec;55(12):3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadolkowski E. A., Burris J. A., O'Brien A. D. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1990 Aug;58(8):2438–2445. doi: 10.1128/iai.58.8.2438-2445.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Kaper J. B. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992 Feb;6(3):411–417. doi: 10.1111/j.1365-2958.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]