The secreted molecule sonic hedgehog (Shh) is essential for many developmental processes in vertebrates, including the induction of motor neurons (reviewed in Ingham, 1998; Wicking et al., 1999). Three hedgehog genes, shh (Krauss et al., 1993), tiggy-winkle hedgehog (twhh; Ekker et al., 1995) and echidna hedgehog (ehh; Currie and Ingham, 1996) are expressed in various tissues in zebrafish embryos. However, only mutations in shh have been identified thus far (Schauerte et al., 1998). Therefore, the precise roles of the three zebrafish hedgehog genes in inducing particular cell types such as motor neurons remain unclear (Beattie et al., 1997; Chandrasekhar et al., 1998).
We showed previously that embryos homozygous for a deletion of shh (Schauerte et al., 1998) exhibit characteristic deficits in branchiomotor neuron (BMN) populations in the zebrafish hindbrain (Chandrasekhar et al., 1998). We now demonstrate that knockdown of shh function by morpholino (MO) injection phenocopies the shh loss-of-function motor neuron phenotype. Furthermore, our studies using a morpholino targeted against twhh indicate that Shh and Twhh cooperatively induce all branchiomotor neurons in zebrafish.
We injected control (con) or gene-specific MOs (Nasevicius and Ekker, 2000) into 1–8 cell stage embryos obtained either from sonic-you (syut4) heterozygotes (syut4:shh deletion allele; Schauerte et al., 1998; Table 1) or from wild-type fish (Table 2). All fish carried an islet1-GFP transgene that is expressed in all branchiomotor neurons (nV, nVII, nX BMNs), except the nIX (Fig. 1A; Higashijima et al., 2000). In all experiments summarized in Table 1, except the shh MO injections, syut4 homozygous mutant embryos were unambiguously identified on the basis of U-shaped somites and curled trunks (van Eeden et al., 1996; Schauerte et al., 1998). Uninjected and control (con) MO-injected embryos exhibit the wild-type branchiomotor neuron (BMN) phenotype (Fig. 1A) and the syu mutant BMN phenotype (Fig. 1E) in approximately 3:1 Mendelian ratios (Table 1). In contrast, injection of increasing amounts of shh MO from ~6 to 25 ng per embryo into syut4 incross embryos leads to a decrease in the wild-type BMN phenotype from 75% to 21% and a concomitant increase in the syu mutant BMN phenotype (Fig. 1F) from 16% to 41% (Table 1). Because a majority of 25 ng shh MO-injected wild-type embryos also develop U-shaped somites (Nasevicius and Ekker, 2000), the embryos scored for the syu mutant BMN phenotype following shh MO injection are composed of syut4 +/+, +/−, and −/− genotypes, which were not distinguished from one another. Injection of increasing amounts of shh MO from ~6 to 25 ng per embryo into wild-type embryos leads to a decrease in the wild-type BMN phenotype from 97% to 27% and a concomitant increase in the syu mutant BMN phenotype (Fig. 1F) from 0% to 19% (Table 2). In addition, the number of embryos exhibiting a reduced BMN phenotype, in which motor neuron loss is a subset of the loss seen in syu mutants, increases from 3% (6.25 ng shh MO) to 54% (25 ng shh MO) (Fig. 1C, D; Table 2).
Table 1.
Injection of Control, shh, and twhh MOs into Embryos from syut4 +/− Incrossesa
| Percent embryos exhibiting particular BMNb phenotype |
||||||
|---|---|---|---|---|---|---|
| Morpholino | Amount | Number of Embryosc | Wild-typed | Reducede | sonic-youf | “Double mutant”g |
| None | — | 75 (3) | 81% | 0% | 19% | — |
| con MO | 25.0 ng | 211 (6) | 73% | 0% | 27% | — |
| shh MO | 6.25 ng | 63 (2) | 75% | 9% | 16% | — |
| shh MO | 12.5 ng | 118 (3) | 38% | 43% | 19% | — |
| shh MO | 25.0 ng | 96 (3) | 21% | 38% | 41% | — |
| twhh MO | 25.0 ng | 232 (5) | 74% | 0% | 1% | 25% |
| twhh MO | 25.0 ng | 102 (3) | 67%h | 0% | 27%h | 6% |
| twhh RNA | 1 ng | |||||
Phenotypes were scored at 48 hours post-fertilization.
BMNs, Branchiomotor Neurons.
Depending upon the genotype of the transgenic fish used in these experiments, 75–100% of the embryos contained GFP-expressing BMNs. Only GFP-expressing embryos were included in these analyses. Number of experiments is indicated in parantheses.
BMNs were found in similar numbers and locations to those described previously for wild-type embryos (Fig. 1A, B; Chandrasekhar et al., 1997, 1998; Higashijima et al., 2000).
Complete or severe loss of nV motor neurons in rhombomere 3, and a partial loss of nX motor neurons in the caudal hindbrain (Fig. 1C, D).
Complete loss of nV motor neurons in rhombomere 3, severe loss of nX motor neurons, and substantial loss of nVII motor neurons, as described for sonic-you mutant embryos (Fig. 1E, F; Chandrasekhar et al., 1998).
Complete or severe loss of all GFP-expressing motor neurons in the hindbrain (Fig. 1G, H).
21% (14/68) of wild-type and 35% (12/34) syu mutant embryos co-injected with twhh MO and twhh RNA contained excessive numbers of BMNs that were displaced dorsally within the rhombomeres. This phenotype is similar to the shh gain-of-function BMN phenotype described previously (Chandrasekhar et al., 1998).
Table 2.
Injection of Control, shh, and twhh MOs into Embryos from Wild-Type Fisha
| Percent embryos exhibiting particular BMNb phenotype |
||||||
|---|---|---|---|---|---|---|
| Morpholino | Amount | Number of Embryosc | Wild-typed | Reducede | sonic-youf | “Double mutant”g |
| None | — | 76 (2) | 100% | 0% | 0% | — |
| con MO | 25.0 ng | 53 (2) | 100% | 0% | 0% | — |
| shh MO | 6.25 ng | 70 (2) | 97% | 3% | 0% | — |
| shh MO | 12.5 ng | 35 (2) | 60% | 40% | 0% | — |
| shh MO | 25.0 ng | 157 (4) | 27% | 54% | 19% | — |
| twhh MO | 25.0 ng | 57 (2) | 100% | 0% | 0% | — |
| shh MO | 12.5 ng | 86 (2) | 2% | 7% | 63%h | 28% |
| twhh MO | 12.5 ng | |||||
Phenotypes were scored at 48 hours post-fertilization.
BMNs, Branchiomotor Neurons.
Depending upon the genotype of the transgenic fish used in these experiments, 75–100% of the embryos contained GFP-expressing BMNs. Only GFP-expressing embryos were included in these analyses. Number of experiments is indicated in parantheses.
BMNs were found in similar numbers and locations to those described previously for wild-type embryos (Fig. 1A, B; Chandrasekhar et al., 1997, 1998; Higashijima et al., 2000).
Complete or severe loss of nV motor neurons in rhombomere 3, and a partial loss of nX motor neurons in the caudal hindbrain (Fig. 1C, D).
Complete loss of nV motor neurons in rhombomere 3, severe loss of nX motor neurons, and substantial loss of nVII motor neurons, as described for sonic-you mutant embryos (Fig. 1E, F; Chandrasekhar et al., 1998).
Complete or severe loss of all GFP-expressing motor neurons in the hindbrain (Fig. 1G, H).
Many embryos in this group contained far fewer motor neurons compared to syu mutants, but did not exhibit as severe a loss as seen in “double mutant” embryos.
FIG. 1.
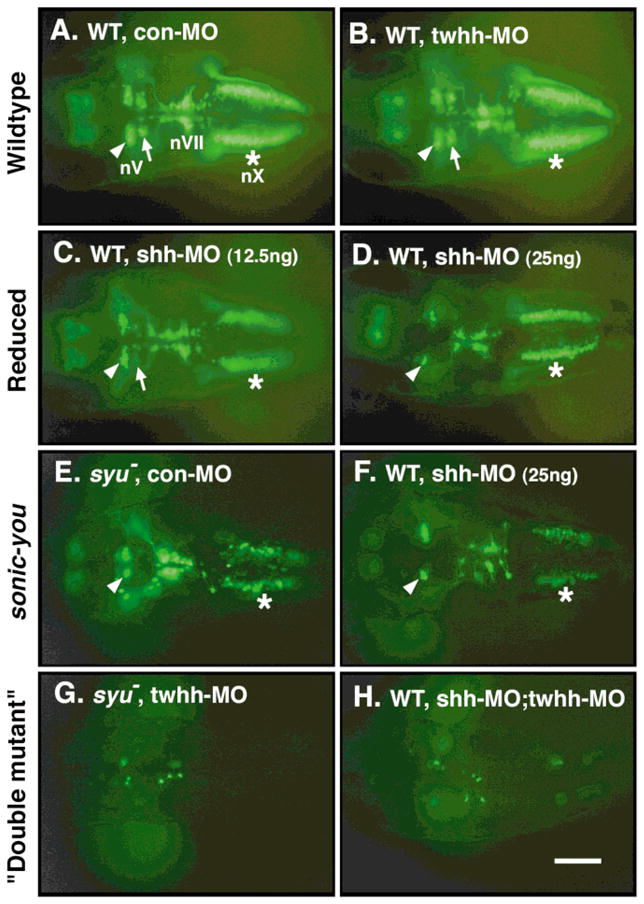
Shh and Twhh act cooperatively in branchiomotor neuron (BMN) induction in zebrafish. All panels show dorsal views of the hindbrain with anterior to the left. The images are fluorescent micrographs of live, 48 HPF (Hours Post Fertilization) embryos embedded in 3% methycellulose, and shows the distribution of GFP-expressing BMNs. (A, B) In wild-type embryos injected with either control MO (A) or twhh MO (B), the development of BMNs is unaffected. The nV motor neurons are found in rhombomere 2 (r2) (arrowhead) and r3 (arrow), the nVII motor neurons are found in r6 and r7, and the nX motor neurons (asterisk) are found in the caudal hindbrain. (C, D) In many wild-type embryos injected with shh MO, the nV motor neurons in r3 are either greatly reduced in number (arrow in C) or missing (D), whereas the nV neurons in r2 (arrowheads) are unaffected. The nX neurons (asterisks) are also slightly reduced in number. (E) In syut4 homozygotes injected with control MO, the nV neurons in r3 are greatly reduced or missing, the nV neurons in r2 are unaffected (arrowhead), the nVII neurons are slightly reduced, and the nX neurons are greatly reduced in number (asterisk). This BMN phenotype is identical to that described previously using immunohistochemistry (Chandrasekhar et al., 1998). (F) Many wild-type embryos injected with shh MO exhibit the same BMN phenotype as syu mutant embryos (E). (G, H) Most (95%) syu mutant embryos injected with twhh MO (G) and many (28%) wild-type embryos co-injected with shh MO and twhh MO (H) exhibit an almost complete loss of GFP-expressing BMNs throughout the hindbrain. Scale bar = 100 μm.
These results demonstrate that injection of shh MO leads to the loss of specific populations of BMNs, and that this loss is either a subset of or identical to the deficits resulting from a deletion of shh (Chandrasekhar et al., 1998). Taken together with our previous observations on the effect of shh MO on somite morphology, fin, and eye development (Nasevicius and Ekker, 2000), these results suggest strongly that shh MO injection generates the shh loss-of-function phenotype.
Because shh, twhh, and ehh are all expressed in midline tissues in the zebrafish embryo, we also investigated the role of twhh in BMN induction using a twhh-specific morpholino (Nasevicius and Ekker, 2000). Injecting 25 ng per embryo of twhh MO has no effect on BMN development in wild-type embryos obtained either from syut4 heterozygotes or from wild-type fish (Fig. 1B; Table 1, 2). In contrast, injection of twhh MO into syu mutants leads to an almost complete loss of GFP-expressing cells from the hindbrain (Fig. 1G; Table 1), generating a “double mutant” phenotype. Because twhh MO injection has no effect on somite development (Nasevicius and Ekker, 2000), the twhh MO-injected embryos exhibiting near-total loss of BMNs could be unambiguously identified as “double mutants” because they developed U-shaped somites and curled trunks characteristic of syu mutants. When shh MO and twhh MO are co-injected into embryos from wild-type fish, over 90% of the injected embryos display either the syu mutant BMN phenotype or the “double mutant” phenotype (Fig. 1H; Table 2). The dramatic loss of BMNs upon injection of twhh MO into syu mutants or co-injection of shh and twhh MOs into wild-type embryos suggest strongly that twhh is necessary for motor neuron induction in the zebrafish hindbrain.
The hindbrains of twhh MO-injected wild-type (n = 171) and syu mutant (n = 61) embryos exhibit no signs of cell death, even though the mutant hindbrains contain few GFP-expressing cells (Fig. 2A, B). Furthermore, hoxb1 is expressed normally in rhombomere 4 in twhh MO-injected syu mutants (n = 3) that were examined by epifluorescence prior to in situ hybridization to confirm that GFP-expressing hindbrain cells were missing (Fig. 2C, D). These results demonstrate that the extensive loss of BMNs in twhh MO-injected syu mutants does not result from aberrant development or degeneration of the hindbrain.
FIG. 2.
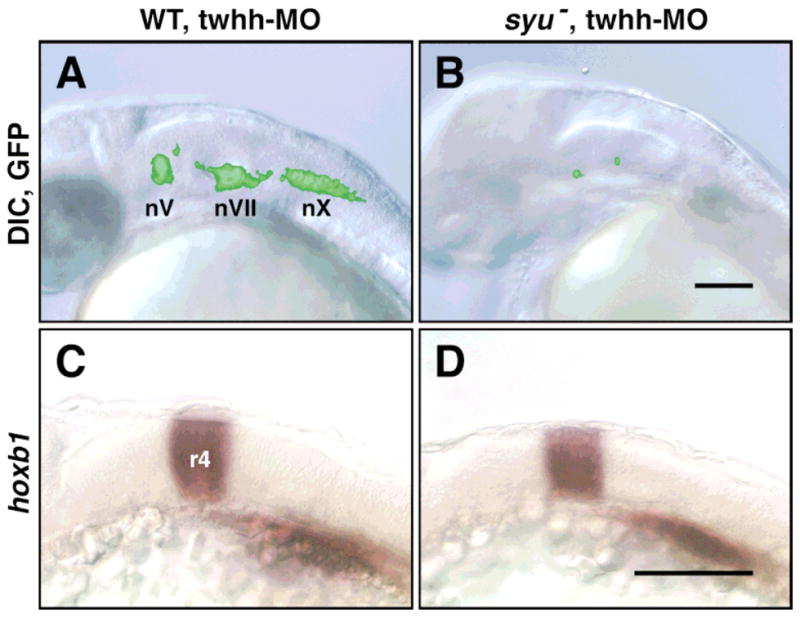
Hindbrain development and patterning are not affected in “double mutants.” All panels show side views of the hindbrain with anterior to the left. (A, B) Live, 30 HPF embryos were embedded in 3% methylcellulose and photographed using DIC optics and GFP epifluorescence. The fluorescent images of the GFP-expressing cells were subsequently superimposed on the DIC images using Photoshop software. In a twhh MO-injected wild-type embryo (A), the GFP-expressing BMNs (nV, nVII, nX) are found in normal numbers at characteristic locations. In contrast, in a twhh MO-injected syu “double mutant” (B), very few GFP-expressing cells are found in an otherwise healthy hindbrain. (C, D) Twhh MO-injected embryos were examined under epifluorescence at 23 HPF to select wild-type (n = 5) and “double mutant” (n = 3) embryos, which were processed for hoxb1 in situ hybridization. Hoxb1 is expressed normally in rhombomere 4 in twhh MO-injected wild-type (C) and syu mutant embryos (D). Scale bars = 100 μm.
We successfully rescued the “double mutant” motor neuron phenotype of twhh MO-injected syu mutants by co-injecting synthetic, full-length twhh RNA (Ekker et al., 1995; Table 1). BMN cell numbers are unchanged or slightly higher in 100% (68/68) of wild-type embryos co-injected with twhh MO and twhh RNA, relative to twhh MO-injected wild-type embryos (n=53) (Fig. 3A, B). In contrast, BMN cell numbers are dramatically higher in 82% (28/34) of syu mutant embryos co-injected with twhh MO and twhh RNA, relative to twhh MO-injected syu mutants (n = 11) (Fig. 3C, D). Furthermore, the organization of the BMNs in the rescued mutant embryos is similar to that in wild-type embryos. These results suggest strongly that the dramatic loss of BMNs in twhh MO-injected syu mutants results from the specific loss of Twhh activity.
FIG. 3.
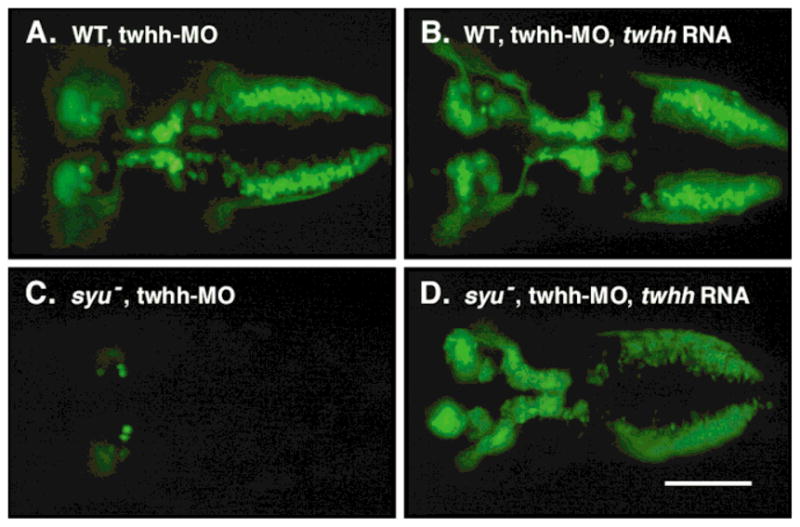
Twhh RNA injection rescues the BMN defects caused by twhh MO injection. All panels show dorsal views with anterior to the left. Live, 48 HPF embryos were embedded in methylcellulose and viewed under GFP epifluorescence. (A) In a twhh MO-injected wild-type embryo, BMN development is normal. (B) In a twhh MO; twhh RNA-injected wild-type embryo, BMN numbers are variably increased, and many GFP-expressing cells are located more dorsally (not shown). (C) In a twhh MO-injected syu mutant, BMNs are almost completely missing. (D) In a twhh MO; twhh RNA-injected syu mutant, BMN loss is prevented, and many GFP-expressing cells are located at ectopic, dorsal locations (not shown). Scale bar = 100 μm.
We have shown that twhh MO has a synergistic effect on BMN induction when injected into syu mutants, or when co-injected with shh MO into wild-type embryos. However, injecting twhh MO alone into wild-type embryos has no effect on BMN induction (Figs. 1B, 3A), somite, fin, and eye development (Nasevicius and Ekker, 2000), or on the expression of Hh-induced genes such as patched (Nasevicius and Ekker, 2000), neurogenin1, and nk2.2 (S.B. and A.C., unpublished results). These observations suggest that Twhh represents a subset of Hh-mediated signaling, and that its contribution becomes apparent only when Shh activity is either missing or greatly reduced. Because Shh and Twhh have similar biological activities (Chandrasekhar et al., 1998; Currie and Ingham, 1996; Ekker et al., 1995; Lauderdale et al., 1998), our results further suggest that subsets of zebrafish BMNs are sensitive to, and therefore induced by, different concentrations of total hedgehog activity, rather than different hedgehog proteins.
Acknowledgments
We are indebted to Shinichi Higashijima and Hitoshi Okamoto for sharing the islet1-GFP strain before publication. We thank Moe Baccam for fish care. This work was supported by a NSF-MGE fellowship (SB), a University of Missouri Research Board grant (AC), and grants from the NIH (SCE, AC). Experiments reported by Etheridge et al. (2001) in this issue also suggest strongly that Shh and Twhh function synergistically during branchiomotor neuron development.
LITERATURE CITED
- Beattie CE, Hatta K, Halpern ME, Liu H, Eisen JS, Kimmel CB. Temporal separation in the specification of primary and secondary motoneurons in zebrafish. Dev Biol. 1997;187:171–182. doi: 10.1006/dbio.1997.8604. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar A, Moens CB, Warren JT, Jr, Kimmel CB, Kuwada JY. Development of branchiomotor neurons in zebrafish. Development. 1997;124:2633–2644. doi: 10.1242/dev.124.13.2633. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar A, Warren JT, Jr, Takahashi K, Schauerte HE, van Eeden FJM, Haffter P, Kuwada JY. Role of sonic hedgehog in branchiomotor neuron induction in zebrafish. Mech Dev. 1998;76:101–115. doi: 10.1016/s0925-4773(98)00101-4. [DOI] [PubMed] [Google Scholar]
- Currie PD, Ingham PW. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature. 1996;382:452–455. doi: 10.1038/382452a0. [DOI] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Etheridge LA, Wu T, Liang JO, Ekker SC, Halpern ME. Floor plate develops upon depletion of Tiggy-winkle and Sonic hedgehog. Genesis. 2001 doi: 10.1002/gene.1056. (this issue) [DOI] [PubMed] [Google Scholar]
- Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J Neurosci. 2000;20:206–218. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW. Transducing Hedgehog: the story so far. The EMBO Jour. 1998;17:3505–3511. doi: 10.1093/emboj/17.13.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Lauderdale JD, Pasquali SK, Fazel R, van Eeden FJM, Schauerte HE, Haffter P, Kuwada JY. Regulation of netrin-1a Expression by Hedgehog Proteins. Mol Cell Neurosci. 1998;11:194–205. doi: 10.1006/mcne.1998.0015. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene “knockdown” in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Schauerte HE, van Eeden FJM, Fricke C, Odenthal J, Strahle U, Haffter P. Sonic hedgehog is not required for the induction of medial floor plate cells in the zebrafish. Development. 1998;125:2983–2993. doi: 10.1242/dev.125.15.2983. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, Granato M, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Warga RM, Allende ML, Weinberg ES, Nusslein-Volhard C. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development. 1996;123:153–164. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- Wicking C, Smyth I, Bale A. The hedgehog signalling pathway in tumorigenesis and development. Oncogene. 1999;18:7844–7851. doi: 10.1038/sj.onc.1203282. [DOI] [PubMed] [Google Scholar]


