Abstract
Phase II enzymes are induced primarily through the common electrophile response element (EpRE) signaling. Studies performed in different cell types and with different inducer appear to indicate variation in the upstream signaling pathways involved in the induction of these phase II genes. Nonetheless, whether variation in signaling among phase II genes in the same cell with the same inducer is unclear. This study is designed to answer this question using human bronchial epithelial cells (HBE1 cells) as a model and screening with a variety of protein kinase inhibitors with varying degrees of specificity. Two electrophiles, 4-hydroxynonenal (HNE) and acrolein, induced the expression of phase II genes (GCLC, GCLM, NQO1, NQO2, HO-1, and GSTM-1). Nrf2 silencing significantly decreased the induction of all of these genes, confirming the involvement of Nrf2-EpRE signaling. ERK and p38MAPK inhibitors had no effect, while a JNK inhibitor abrogated the GCLC and GCLM induction by HNE, but not that by acrolein. Among the PKC inhibitors used, one eliminated gene induction by HNE and acrolein, while two others showed no effects. One PI3K inhibitor decreased the induction of GCLM, NQO1, NQO2 and HO-1, but not GCLC and GST-M1; on the other hand, the inhibitory effects of another PI3K inhibitor on gene induction seems to be gene- and inducer- specific. In conclusion, our data suggest that although phase II genes are coordinately induced through Nrf2-EpRE signaling by electrophiles, the upstream signaling pathways involved are gene- and inducer- specific. It is also suggested that commercial kinase inhibitors may produce non-specific effects on phase II gene expression via mechanisms unrelated to their purported specificity.
Keywords: detoxification, electrophiles, Nrf2, oxidative stress, phase II enzyme
Introduction
Phase II enzymes play important roles in maintaining redox homeostasis, resistance against oxidative stress, and detoxification and elimination of toxins. The expression of many phase II enzymes, such as nicotinamide adenine dinucleotide phosphate: quinone oxidoreductase (NQO-1), glutathione S-transferases (GST), heme oxygenase-1 (HO-1), and glutamate-cysteine ligase (GCL), can be up-regulated in response to stimuli such as oxidative stress (Chen and Kunsch, 2004). This inducible feature makes these enzymes critical for protection against toxic insults and implies a promising strategy to prevent and/or treat diseases through modulating the expression level of these enzymes. Indeed, in the past decade there has been an extensive study on the mechanism especially the signaling pathways involved in the induction of phase II genes, aiming to develop effective ways to boost the antioxidant/detoxifying capacity and prevent diseases such as cancer. The purpose of this investigation was to ascertain whether the signaling pathways for phase II genes within a single cell type were the same or different using screening with protein kinase inhibitors. It is important to note that many commercial protein kinase inhibitors do not have absolute specificity of action. Nonetheless, using a combination of these inhibitors (each at concentrations previously shown to significantly inhibit their target) allows screening that can determine similarity or differences in signaling. This approach was used in this study as the principal goal was not to identify the signaling pathways for each phase II gene but to determine whether or not the same pathways were involved in their induction. Regardless, with those few inhibitors that have well-defined specificity, more definitive statements regarding the pathways can be made.
Studies have reported that the expression of phase II genes is usually coordinately up-regulated in response to oxidative stress, and this involves the activation of an electrophile response element (EpRE, also called antioxidant response element) in their promoter regions (Lee and Surh, 2005). The activation of EpRE is triggered primarily by the binding of a transcription factor called NF-E2-related factor 2 (Nrf2). Under resting condition, Nrf2 is retained in the cytosol by associating with cytoskeleton-bound Kelch-like erythroid cell-derived protein with cap ‘n’ collar homology–associated protein 1 (Keap1). Upon exposure to electrophilic compounds, especially those producing oxidative stress, Nrf2 is released from Keap1 and translocated into the nucleus, where it forms heterodimers with other transcription factors such as Jun family proteins and binds to EpRE to promote the transcription of phase II genes [for recent reviews, see (Kobayashi and Yamamoto, 2005, Katoh, et al., 2005, Nguyen, et al., 2004)].
Despite the common downstream EpRE–Nrf2 pathway, a variety of upstream signaling pathways have been reportedly implicated in the induction of phase II genes. These include extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), protein kinase C (PKC), and phosphatidylinositol 3-kinase (PI3K). Even for the induction of a single gene, various upstream signaling pathways were involved. For instance, for the induction of the catalytic unit of GCL (GCLC), the involvement of ERK, p38MAPK, JNK, and PI3K have been reported (Zipper and Mulcahy, 2000, Dickinson, et al., 2002, Kim, et al., 2005). It is suggested that the upstream signaling pathways involved in the induction of Nrf2–EpRE-regulating genes may be gene-, inducer-, and cell type-dependent. Besides EpRE, the potential involvement in phase II gene induction of other DNA cis-elements, such as AP-1-binding site, stress response element, cAMP response element, and NF-κB binding site, may also contribute to the complexity of upstream signaling pathways. Obviously, more evidence is required to define the variety of signaling pathways in the induction of phase II genes.
Some α, β-unsaturated aldehydes derived from lipid peroxidation, such as 4-hydroxynonenal (HNE), acrolein, and 15-deoxy-12,14-prostaglandin J2, are potent inducers of phase II genes (Zhang and Forman, 2008, Siow, et al., 2007, Kawamoto, et al., 2000). In this study, we examined the signaling pathways involved in the induction of phase II genes in human bronchial epithelial cells (HBE1 cell), aiming to define the question whether the variety of signaling pathways is associated with inducers and genes or not.
Materials and methods
Reagents
Unless otherwise noted, all chemicals were from Sigma (St. Louis, Missouri). Antibodies and small interfering RNAs were from Santa Cruz (Santa Cruz, California). TRIzol reagent was from Life Technologies (Grand Island, New York). DNA-free reagent was from Ambion (Austin, Texas). TaqMan Reverse Transcription Reagent and SYBR Green polymerase chain reaction (PCR) Master mix were from Applied Biosystems (Foster City, California). FuGENE 6 transfection reagent was from Roche (Indianapolis, Indiana).
Cell culture and treatment
HBE1 cell line was cultured in collagen-coated dishes in 5% CO2 at 37 ° C as described by (Harper, et al., 2001). Acrolein was diluted in 1× phosphate buffered saline, and HNE was diluted in ethanol. Cells were treated when the confluence was about 90% and the final concentration of ethanol in the medium was 0.05% in HNE exposure and controls.
Measurement of mRNA content
The mRNA contents of GCLC, glutamate-cysteine ligase modifier subunit (GCLM), NQO-1, NQO-2, glutathione S-transferase mu 1 (GST-M1), glutathione S-transferase pi 1 (GST-P1-1), and HO-1 were determined with real-time PCR method, using the protocol described previously (Zhang, et al., 2005). The primers used are as follows: NQO-1, sense 5′-TCTCGGCTCACTGCAACCTCT-3′, antisense, 5′-GCACTTTGGGAGGCTGAGGTA-3′; NQO2, sense 5′-CGCTCCTTTCCGTAACCAC-3′, antisense 5′-CACAGAGTTATTGCCCGAAGT; GST-P1-1, sense AGTCCAATACCATCCTGCGTC-3′, antisense 5′-GGTCTCCCACAATGAAGGTC; GSTM1, sense 5′-CTGCCCTACTTGGATTGATGGG- 3′, antisense 5′-TGGATTGTAGCAGATCATGC-3′; HO-1 sense 5′-TCTCTTGGCTGGCTTCCTTAC-3′, antisense 5′-GCTTTTGGAGGTTTGAGACA-3′; and GCLC and glyceraldehyde 3-phosphate dehydrogenase primers are the same as described previously (Dickinson, et al., 2004).
Nrf2 siRNA transient transfection
Transfection of Nrf2 siRNA was performed using FuGENE 6 transfection reagent by following the procedure provided with the transfection reagent. HBE1 cells at about 60–80% confluence were transfected with 50 nM Nrf2 or control siRNA (Santa Cruz Biotechnology, Santa Cruz, CA). Cell medium was replaced certain time after transfection, and cells were treated with or without HNE or acrolein as indicated in the figures before being collected for mRNA measurement.
Statistical analysis
A comparative ΔΔCT method was used for the relative mRNA quantification as described before (Zhang, et al., 2005). All data were expressed as the mean ± standard error. Sigma Stat software (Systat Software Inc, San Jose, CA) was used for statistical analysis, and statistical significance was accepted when P < 0.05. The one-way analysis of variance and Tukey test were used for the comparison of mRNA level.
Results
Induction of phase II genes by HNE and acrolein
To determine the induction of phase II genes, HBE1 cells were treated with HNE or acrolein for 12 h and the mRNAs of seven phase II genes, including GCLC, GCLM, NQO-1, NQO-2, HO-1, GST-M1, and GST-P1-1, were measured by real-time PCR. As shown in Table 1, except for GST-P1-1, the mRNAs of these genes were significantly increased by HNE or acrolein treatment. At 20 µM, acrolein increased the mRNA of GCLC, GCLM, NQO-1, NQO-2, HO-1, and GST-M1 by 2.36-, 3.24-, 1.40-, 3.39-, 32.33-, and 2.51-fold, respectively, whereas 15 µM of HNE increased the mRNAs by 2.17-, 2.56-, 1.83-, 1.86-, 1.94-, and 2.25-fold, respectively.
Table 1.
Induction of phase II genes by HNE and acrolein
| HNE | Acrolein | |||
|---|---|---|---|---|
| Gene | 5 µM | 15 µM | 5 µM | 20 µM |
| GCLC | 1.67 ± 0.27 | 2.17 ± 0.29 | 1.65 ± 0.28 | 2.36 ± 0.61 |
| GCLM | 1.82 ± 0.24 | 2.56 ± 0.32 | 2.16 ± 0.39 | 3.24 ± 0.33 |
| NQO-1 | 1.57 ± 0.15 | 1.83 ± 0.25 | 1.75 ± 0.36 | 1.84 ± 0.32 |
| NQO-2 | 1.61 ± 0.25 | 1.86 ± 0.28 | 1.61 ± 0.29 | 3.39 ± 0.61 |
| HO-1 | 1.57 ± 0.29 | 1.94 ± 0.35 | 2.54 ± 0.26 | 32.33 ± 5.31 |
| GST-M1 | 1.72 ± 0.38 | 2.25 ± 0.48 | 1.64 ± 0.27 | 2.51 ± 0.39 |
| GST-P1-1–11 | 0.91 ± 0.33 | 0.87 ± 0.21 | 0.94 ± 0.16 | 0.89 ± 0.19 |
GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modifier subunit; NQO, nicotinamide adenine dinucleotide phosphate: quinone oxidoreductase; HO, heme oxygenase; GST-M1, glutathione S-transferase mu 1.
HBE1 cells were treated with HNE or acrolein for 12 h and the mRNA levels of phase II genes were determined as described in Materials and methods. Data were shown as the fold induction ± standard error, and all data were statistically significant compared with vehicle control (P < 0.05, N = 3).
Nrf2–EpRE involvement in HNE and acrolein-mediated phase II gene induction
To confirm the involvement of Nrf2–EpRE signaling in the HNE or acrolein induction of the afore-mentioned phase II genes, Nrf2 protein was knocked out with pretreatment of 50 nM siRNA. Nrf2 silencing significantly suppressed the HNE- or acrolein-induced increases in the mRNAs of phase II genes and also decreased the basal expression of these genes (Table 2) confirming that Nrf2–EpRE signaling is involved in the basal and HNE- or acrolein-mediated expression of phase II genes. The induction of HO-1 gene by HNE or acrolein, however, was not completely eliminated with Nrf2 siRNA pretreatment, suggesting either that other cis-elements were also responsible for the gene induction or that the residual Nrf2 was sufficient for some responsiveness.
Table 2.
Effects of Nrf2 silencing on HNE- or acrolein-mediated phase II gene induction
| Gene | HNE (15 µM) | Acrolein (10 µM) | ||||
|---|---|---|---|---|---|---|
| Control- SiRNA + H |
Nrf2-SiRNA | Nrf2-SiRNA + H | Control- SiRNA + A |
Nrf2-SiRNA | Nrf2-SiRNA + A | |
| GCLC | 2.27 ± 0.35* | 0.57 ± 0.22* | 1.07 ± 0.34** | 1.82 ± 0.17* | 0.72 ± 0.09* | 0.85 ± 0.13** |
| GCLM | 2.44 ± 0.29* | 0.68 ± 0.14* | 0.87 ± 0.24** | 2.12 ± 0.27* | 0.86 ± 0.11 | 0.95 ± 0.14** |
| NQO-1 | 1.77 ± 0.23* | 0.71 ± 0.17* | 0.97 ± 0.33** | 1.76 ± 0.28* | 0.36 ± 0.19* | 0.57 ± 0.23** |
| NQO-2 | 1.79 ± 0.22* | 0.76 ± 0.43 | 1.12 ± 0.27** | 2.32 ± 0.33* | 0.79 ± 0.38 | 1.06 ± 0.32** |
| HO-1 | 1.91 ± 0.29* | 0.74 ± 0.18 | 0.98 ± 0.25** | 12.71 ± 2.8* | 0.76 ± 0.12* | 3.43 ± 1.17*,** |
| GST-M1 | 2.38 ± 0.31* | 0.55 ± 0.27* | 1.13 ± 0.29** | 1.79 ± 0.25* | 0.52 ± 0.33* | 0.92 ± 0.28** |
GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modifier subunit; NQO-2, nicotinamide adenine dinucleotide phosphate: quinone oxidoreductase; HO, heme oxygenase.
HBE1 cells were transfected with 50 nM of Nrf2 siRNA for 24 h before being treated with 15 µM HNE or 10 µM acrolein for 12 h. The mRNA levels of phase II genes were determined as described in Materials and methods. Data were shown as the fold induction compared with the vehicle control of the control-siRNA group.
P < 0.05, N = 3 compared with the vehicle control of the control-siRNA group.
P < 0.05, N = 3 compared with HNE or acrolein of the control-siRNA group.
Upstream signaling pathways involved in the induction of phase II genes by HNE and acrolein
Based on studies using a variety of cell types, many signaling pathways including MAPK, PKC, and PI3K have been reported to be involved in Nrf2–EpRE-regulating induction of phase II genes. To determine whether the signaling pathways involved in phase II gene induction in a single cell type were the same or different, HBE1 cells were pretreated with kinase inhibitors for 1 h before being exposed to 15 µM HNE or 20 µM acrolein, and then the mRNAs of GCLC, NQO1, NQO2, GST-M1, and HO-1 were determined by real-time PCR. Although the JNK inhibitor (JNKi) eliminated HNE-mediated induction of GCLC and GCLM genes (Dickinson, et al., 2002), it showed no inhibitory effect on acrolein-mediated induction of GCLC, GCLM, and other phase II genes. Also, we did not find any effect of PD98059 (ERK1/2 pathway inhibitor) and SB203580 (p38MAPK inhibitor) on HNE- or acrolein-mediated induction of NQO-1, NQO-2, GST-M1, and HO-1 (data not known). Thus, JNK involvement in phase II gene induction in HBE1 cells was unique to HNE-induced increases in GCLC and GCLM.
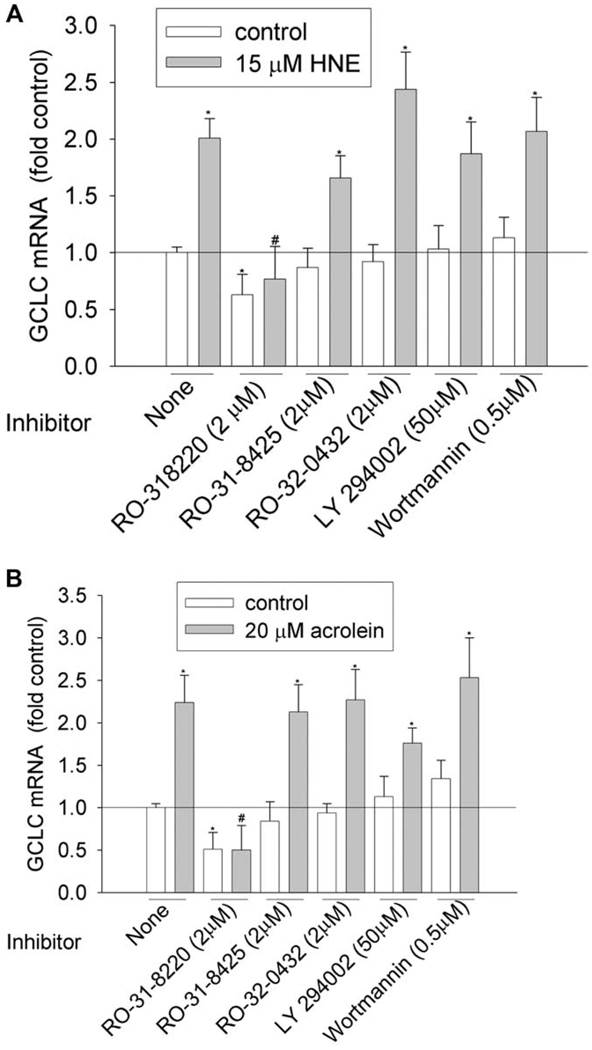
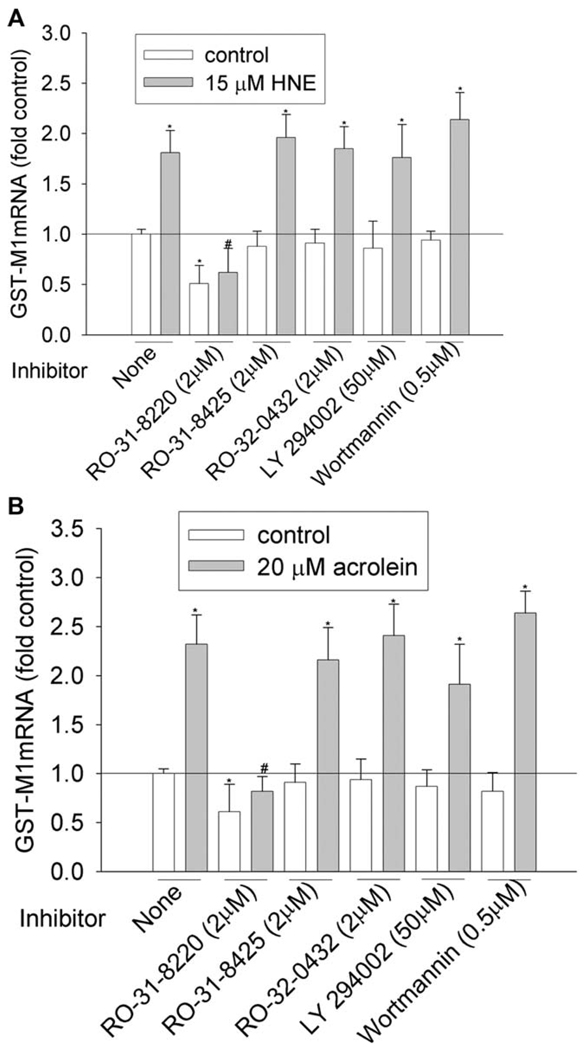
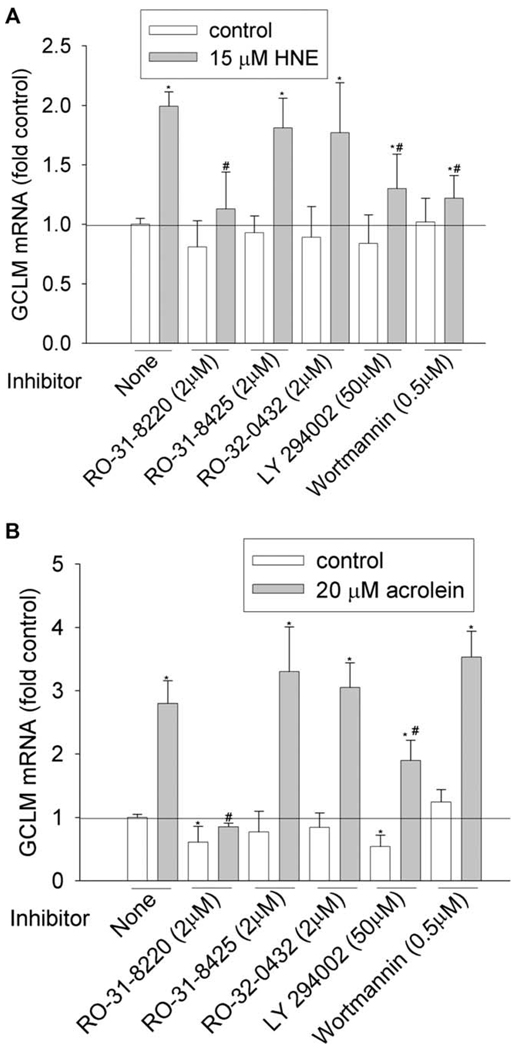
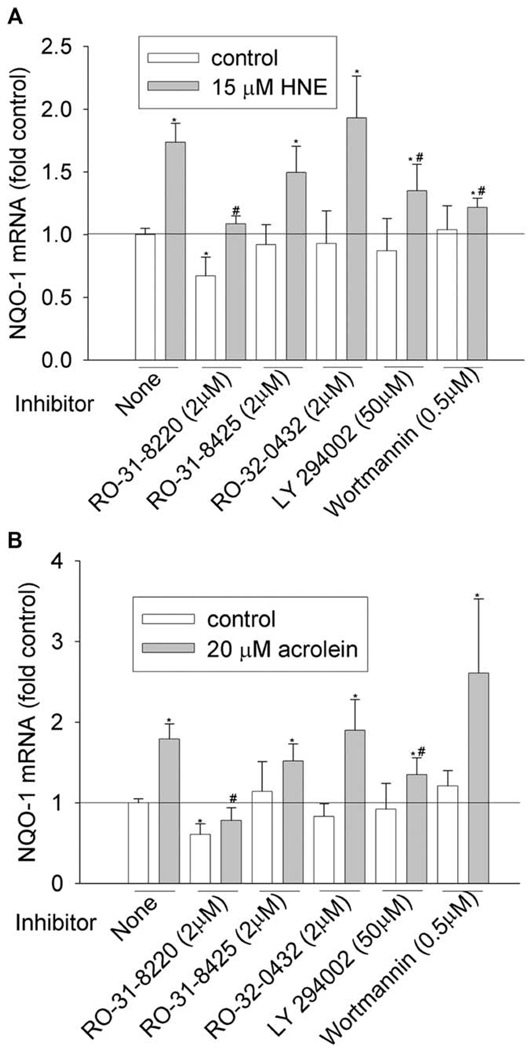
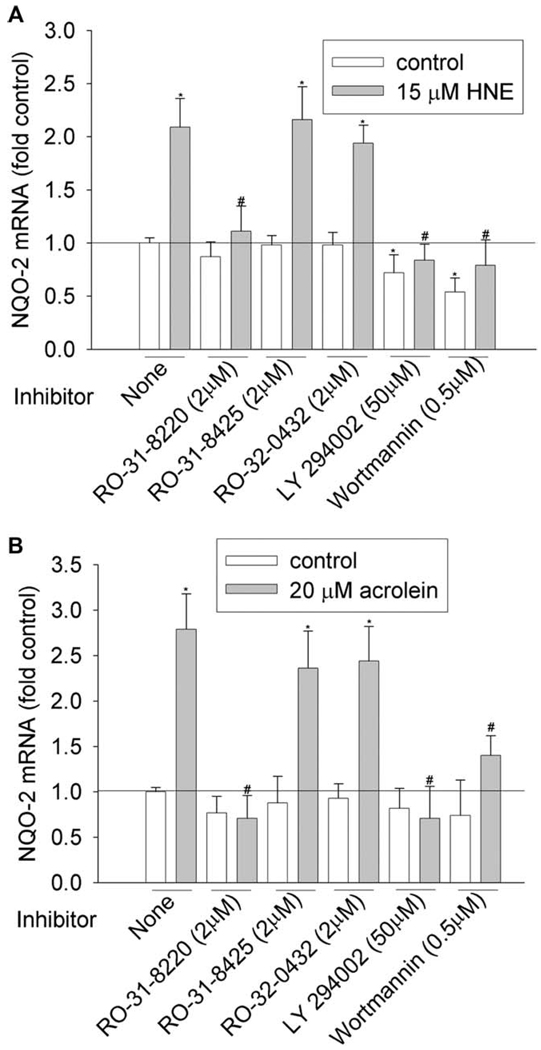
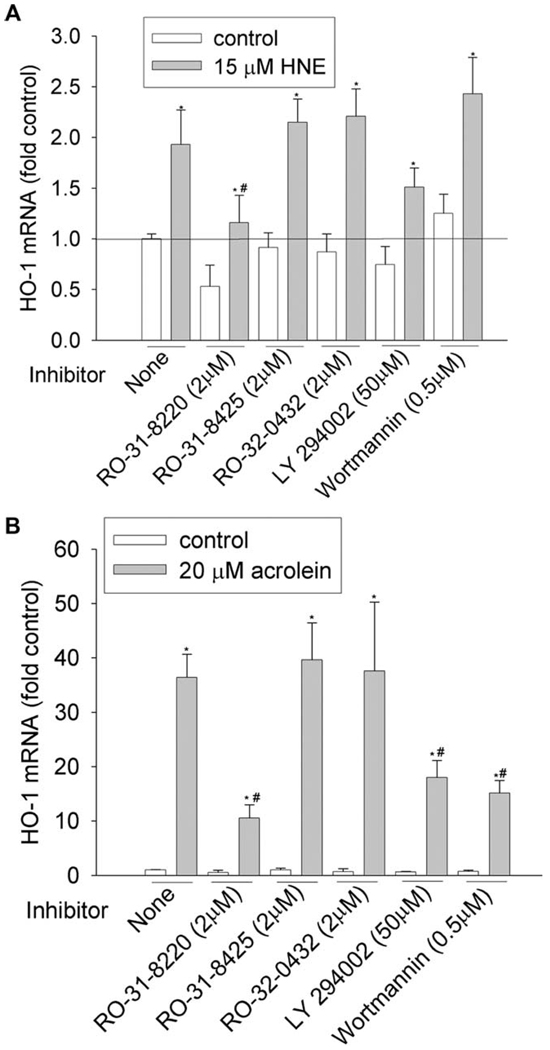
We then investigated the involvement of PKC and PI3K pathways by using commercially available inhibitors. To achieve the effective inhibitory concentration, a concentration higher than the half maximal inhibitory concentration (IC50) was used (Table 3). PKC family have 10 isozymes, which belong to three subfamilies: classic (α, β I, β II, and γ), novel (δ, ε, η, and θ), and atypical PKC (ξ and λ). As shown in Figure 1–Figure 6 and Table 4, pan-PKC inhibitor RO-31-8220 (2 µM) completely blocked HNE- or acrolein-induced expression of GCLC, NQO1, NQO2, and GST-M1 and partially reduced the induction of HO-1 mRNA. The inhibitory effect of RO-31-8220 was dose-dependent and at less than 1 µM it showed no effects (data not shown). Classic PKC inhibitors RO-31-8425 (2 µM) and RO-32-0432 (2 µM) had no inhibitory effects on the phase II gene inductions by HNE or acrolein (Figure 1–Figure 6 and Table 4).
Table 3.
Kinase inhibitors and concentrations used in the study
| Inhibitors | IC50 | Concentration used (µM) | Kinases inhibited |
|---|---|---|---|
| RO-318220 | PKC-α, -β, -γ (5–27 nM) | 0.5–2 | PKC-α, -β, -γ |
| PKC-ξ (100 nM) | PKC-ξ | ||
| PKA (900 nM) | PKA | ||
| GSK3 (38 nM) | GSK3 | ||
| MSK1 (8 nM) | MSK1 | ||
| P70 S6K (15 nM) | P70 S6K | ||
| RSK2 (3 nM) | RSK2 | ||
| PKC (8–39 nM) | PKA-α, -β1, -β2, -γ, -ε | ||
| PKA (2–8 µM) | |||
| RO-318425 | Phosphorylase kinase (1.3 µM) | 2 | Phosphorylase kinase |
| MLCK (2.7 µM) | |||
| RO-320432 | PKCs (1–10 µM) | 2 | PKC-α, -β1, -β2, -γ, -ε |
| LY294002 | PI3K (10 µM) | 50 | PI3K |
| Wortmannin | PI3K (50–450 nM) | 0.5–5 | PI3K |
PKC, protein kinase C; PKA, protein kinase A; GSK3, glycogen synthase kinase 3; MSK1, mitogen- and stress-activated kinase 1; P70 S6K, p70 ribosomal S6 kinase; RSK2, ribosomal S6 kinase 2; MLCK, myosin light chain kinase; PI3K, phosphatidylinositol 3-kinase.
Figure 1.
Effects of PKC and PI3K inhibitors on GCLC induction by HNE (A) and acrolein (B). HBE1 cells were pretreated with inhibitors for 1 h before being treated with HNE or acrolein. After 12 h, cells were collected and the mRNA levels of GCLC were determined with RT-real time PCR assay. *P < 0.05 compared with vehicle control, N = 3; **P < 0.05 compared with HNE or acrolein, N = 3.
Figure 6.
Effects of PKC and PI3K inhibitors on GST-M1 induction by HNE (A) and acrolein (B). HBE1 cells were pretreated with inhibitors for 1 h before being treated with HNE or acrolein. After 12 h, cells were collected and the mRNA levels of GST-M1 were determined with RT-real time PCR assay. *P < 0.05 compared with vehicle control, N = 3; ** P < 0.05 compared with HNE or acrolein, N = 3.
Table 4.
Effects of kinase inhibitors on phase II gene induction by HNE and acrolein
| GCLC | GCLM | NQO1 | NQO2 | HO-1 | GST-M1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitors | H | A | H | A | H | A | H | A | H | A | H | A |
| RO-318220 | −62% | −78% | −43% | −70% | −38% | −56% | −47% | −75% | −40% | −71% | −66% | −65% |
| RO-318425 | – | – | – | – | – | – | – | – | – | – | – | – |
| RO-320432 | – | – | – | – | – | – | – | – | – | – | – | – |
| LY294002 | – | – | −35% | −32% | −22% | −40% | −60% | −75% | – | −51% | – | – |
| Wortmannin | – | – | −39% | – | −30% | – | −62% | −50% | – | −58% | – | – |
GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modifier subunit; NQO, nicotinamide adenine dinucleotide phosphate: quinone oxidoreductase; HO, heme oxygenase, H, HNE; A, acrolein.
Data were shown as the percentage of decrease in mRNA with inhibitor pretreatment compared with the corresponding treatment (HNE or acrolein).
To examine the involvement of the PI3K pathway, two PI3K inhibitors, LY294002 and wortmannin, were used. Pretreatment with 50 µM of LY294002 decreased HNE- or acrolein-caused gene induction of GCLM, NQO-1, NQO-2, and HO-1 but had no effect on GCLC and GST-M1 induction. However, pretreatment of cells with 0.5 µM of wortmannin decreased HNE-mediated induction of GCLM, NQO-1, NQO2, and HO-1 and acrolein-mediated induction of NQO-2 and HO-1, but it had no effects on HNE-mediated induction of GCLC and GST-M1 and acrolein-mediated induction of GCLC, GCLM, NQO1, and GST-M1. Increasing wortmannin to 5 µM did not increase its inhibitory effect but actually increased the expression of some phase II genes such as HO-1 (data not shown).
Discussion
α, β-Unsaturated aldehydes derived from lipid peroxidation products or industrial processes were initially considered as mediators of oxidative stress, but it is now clear that these electrophilic compounds are also potent signaling molecules and can regulate the expression of genes, especially those with antioxidant and detoxifying functions. We reported here that HNE and acrolein, two commonly encountered α, β-unsaturated aldehydes, increased the mRNA levels of the phase II genes, including GCLC, GCLM, NQO-1, NQO-2, HO-1, and GST-M1 (Table 1), which play important roles in maintaining redox homeostasis, removing oxidants, and metabolizing toxicants. These results confirm previous findings that nonlethal concentrations of HNE and acrolein can induce antioxidant and detoxifying genes. Regardless, the expression of GST-P1-1 was not increased, suggesting that not all phase II genes are induced by HNE or acrolein.
It has been well-established that the transcription factor Nrf2 plays a critical role in phase II gene induction in response to various stimuli. The current study showed that Nrf2 silencing significantly decreased the induction of phase II genes by HNE or acrolein (Table 2), indicating the involvement of Nrf2–EpRE signaling. This finding also reveals the key role of Nrf2 in the coordinate induction of phase II genes. In addition, we found that Nrf2 silencing also decreased the basal mRNAs of phase II genes, as observed in Nrf2 silenced mice, suggesting the involvement of Nrf2 in the basal expression of phase II genes. Nonetheless, acrolein was more potent in inducing HO-1 than was HNE, and this induction was decreased by only 60% with Nrf2 silencing, compared with the complete elimination of HNE induction by Nrf2 silencing. For the other phase II genes, the effect of Nrf2 silencing was the same for acrolein and HNE induction. These data suggest that acrolein and HNE may follow different pathways in up-regulating HO-1 gene expression.
Many upstream signaling pathways such as MAPKs, PKC, and PI3K have been reported to be involved in Nrf2-mediating phase II gene regulation and the variation in these pathways is assumed to be gene-, inducer-, or cell-dependent. In this study, we showed that in HBE1 cells, the upstream signaling pathways involved in phase II was gene specific. For example, JNK was involved in GCLC and GCLM induction by HNE (Dickinson, et al., 2002) but not associated with the induction of other phase II genes by HNE; PI3K pathway was involved in the induction of phase II genes other than GCLC and GST-M1 (Table 4). The inducer-dependent difference in signaling pathways was also observed. Although JNK was involved in HNE-mediated GCLC and GCLM induction (Dickinson, et al., 2002), it was not involved in acrolein-mediated GCLC and GCLM induction (data not shown).
The HNE- and acrolein-mediated phase II gene induction might share a common upstream signaling pathway, as demonstrated by the effect of pretreatment with pan-PKC inhibitor RO-318220, which eliminated the induction of all the genes (Table 4). The inhibitory effect of RO-318220 on phase II gene induction is dose-dependent, and it showed no effect at less than 1 µM (data not shown), concentration at which RO-31-8220 inhibited only classic and novel PKCs. Combined with our findings that inhibitors against classic and novel PKCs, RO-318425 and RO-320432, had no inhibitory effects on phase II gene induction, it could be concluded that classic and novel PKC may not be involved and that the inhibitory effect of RO-318220 was due to its inhibition of atypical PKCs. Indeed, using siRNA, we previously found that atypical PKC-ξ was associated with acrolein-mediated HO-1 induction (Zhang and Forman, 2008). RO-318220 is a nonspecific inhibitor and many kinases could be inhibited by it (Table 3); therefore, the possibility exists that the inhibitory effect of RO-318220 on phase II gene induction may also be due to its inhibition of kinases other than atypical PKCs. Interestingly, RO-318220 also significantly decreased the basal expression of phase II genes. But again, whether it acted through atypical PKCs as it did in HO-1 induction by acrolein or through inhibiting other pathways needs further study.
As Nrf2–EpRE signaling was involved in phase II gene induction by HNE and acrolein, it was assumed that these pathways regulate gene expression through targeting of the transcription factors that bind to EpRE. The EpRE–protein complex is composed of multiple nuclear proteins, such as Nrf2 (Zipper and Mulcahy, 2000, Wild et al., 1999), c-Jun (Dickinson, et al., 2002, 2003, Jeyapaul and Jaiswal, 2000, Rahan, 2000, Zhang et al., 2006), JunD (Zipper and Mulcahy, 2000, Wild et al., 1999, Dickinson et al., 2003), cAMP response element binding protein (CREB)-binding protein (CBP) (Katoh et al., 2001, Shen et al., 2004), and others. All of these proteins are potential targets of upstream kinases; for example, Nrf2 activity (binding to EpRE, same as following) has been reportedly regulated by PKC [23] and PI3K [24], and c-Jun and JunD by JNK (Dickinson, et al., 2002). We previously found that atypical PKC was involved in Nrf2 activation by acrolein (Zhang and Forman, 2008), suggesting that the RO-318220 inhibition HO-1 induction could act through its inhibition of Nrf2 activation. Regardless, whether this is the case for other phase II genes or if other component proteins in the EpRE complex are also targets remains to be determined.
In summary, we showed that although Nrf2–EpRE signaling was involved in the induction of all phase II genes by HNE and resveratrol, the upstream signaling pathways involved vary dependent on both the gene and the inducers.
Figure 2.
Effects of PKC and PI3K inhibitors on GCLM induction by HNE (A) and acrolein (B). HBE1 cells were pretreated with inhibitors for 1 h before being treated with HNE or acrolein. After 12 h, cells were collected and the mRNA levels of GCLM were determined with RT-real time PCR assay. *P < 0.05 compared with vehicle control, N = 3; **P < 0.05 compared with HNE or acrolein, N = 3.
Figure 3.
Effects of PKC and PI3K inhibitors on NQO-1 induction by HNE (A) and acrolein (B). HBE1 cells were pretreated with inhibitors for 1 h before being treated with HNE or acrolein. After 12 h, cells were collected and the mRNA levels of NQO-1 were determined with RT-real time PCR assay. *P < 0.05 compared with vehicle control, N = 3; **P < 0.05 compared with HNE or acrolein, N = 3.
Figure 4.
Effects of PKC and PI3K inhibitors on NQO-2 induction by HNE (A) and acrolein (B). HBE1 cells were pretreated with inhibitors for 1 h before being treated with HNE or acrolein. After 12 h, cells were collected and the mRNA levels of NQO-2 were determined with RT-real time PCR assay. *P < 0.05 compared with vehicle control, N = 3; **P < 0.05 compared with HNE or acrolein, N = 3.
Figure 5.
Effects of PKC and PI3K inhibitors on HO-1 induction by HNE (A) and acrolein (B). HBE1 cells were pretreated with inhibitors for 1 h before being treated with HNE or acrolein. After 12 h, cells were collected and the mRNA levels of HO-1 were determined with RT-real time PCR assay. *P < 0.05 compared with vehicle control, N = 3; ** P < 0.05 compared with HNE or acrolein, N = 3.
Acknowledgment
This work was supported by grant 14RT-0059 from the California Tobacco Related Diseases Research Program and ES05511 from the National Institutes of Health.
Footnotes
Reprints and permissions: http://www.sagepub.co.uk/journalsPermissions.nav
References
- Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H: quinone oxidoreductase-1 gene expression. J Biol Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- Chen XL, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004;10:879–891. doi: 10.2174/1381612043452901. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Iles KE, Nobuo Watanabe, Takeo Iwamoto, Hongqiao Zhang, David Krzywanski M, et al. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic Biol Med. 2002;33:974–987. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Iiles KE, Zhang H, Blank V, Forman HJ. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J. 2003;17:473–475. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Iles KE, Wigley AF, Forman HJ. Analysis of transcription factor remodeling in phase II gene expression with curcumin. Methods Enzymol. 2004;378:302–318. doi: 10.1016/S0076-6879(04)78023-4. [DOI] [PubMed] [Google Scholar]
- Harper R, Wu K, Chang MM, Yoneda K, Pan R, Reddy SP, et al. Activation of nuclear factor-kappa b transcriptional activity in airway epithelial cells by thioredoxin but not by N-acetyl-cysteine and glutathione. Am J Respir Cell Mol Biol. 2001;25:178–185. doi: 10.1165/ajrcmb.25.2.4471. [DOI] [PubMed] [Google Scholar]
- Jeyapaul J, Jaiswal AK. Nrf2 and c-Jun regulation of antioxidant response element (ARE)-mediated expression and induction of gamma-glutamylcysteine synthetase heavy subunit gene. Biochem Pharmacol. 2000;59:1433–1439. doi: 10.1016/s0006-2952(00)00256-2. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Itoh K, Yoshida E, Miyagishi M, Fukamizu A, Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–868. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Iida K, Kang MI, Kobayashi A, Mizukami M, Tong KI, et al. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch Biochem Biophys. 2005;433:342–350. doi: 10.1016/j.abb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Yoshiyuki K, Nakamura Y, Naito Y, Torii Y, Kumagai T, Osawa T, et al. Cyclopentenone prostaglandins as potential inducers of phase II detoxification enzymes. 15-deoxy-delta(12,14)-prostaglandin j2-induced expression of glutathione S-transferases. J Biol Chem. 2000;275:11291–11299. doi: 10.1074/jbc.275.15.11291. [DOI] [PubMed] [Google Scholar]
- Kim J-Y, Yim J-H, Cho J-H, Kim J-H, Ko J-H, Kim S-M, et al. Adrenomedullin Regulates Cellular Glutathione Content via Modulation of {gamma}-Glutamate-cysteine Ligase Catalytic Subunit Expression. Endocrinology. 2005;147:1357–1364. doi: 10.1210/en.2005-0895. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Molecular mechanisms activating the nrf2-keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224:171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546:181–184. doi: 10.1016/s0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med. 2004;37:433–441. doi: 10.1016/j.freeradbiomed.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Rahman I. Regulation of nuclear factor-kappa B, activator protein-1, and glutathione levels by tumor necrosis factor-alpha and dexamethasone in alveolar epithelial cells. Biochem Pharmacol. 2000;60:1041–1049. doi: 10.1016/s0006-2952(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Shen G, Hebbar V, Nair S, Xu C, Li W, Lin W, et al. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J Biol Chem. 2004;279:23052–23060. doi: 10.1074/jbc.M401368200. [DOI] [PubMed] [Google Scholar]
- Siow RC, Ishii T, Mann GE. Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep. 2007;12:11–15. doi: 10.1179/135100007X162167. [DOI] [PubMed] [Google Scholar]
- Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- Zhang H, Dickinson DA, Liu RM, Forman HJ. 4-Hydroxynonenal increases gamma-glutamyl transpeptidase gene expression through mitogen-activated protein kinase pathways. Free Radic Biol Med. 2005;38:463–471. doi: 10.1016/j.freeradbiomed.2004.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Forman HJ. Acrolein induces heme oxygenase-1 through PKCdelta and PI3K in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2008;38:483–490. doi: 10.1165/rcmb.2007-0260OC. [DOI] [PubMed] [Google Scholar]
- Zhang H, Honglei Liu, Karen EIles, Rui-Ming Liu, Edward Postlethwait M, Yannick Laperche, et al. 4-Hydroxynonenal Induces Rat Gamma-glutamyl Transpeptidase through MAPK Mediated EpRE/Nrf2 Signaling. Am J Respir Cell Mol Biol. 2006;34:174–181. doi: 10.1165/rcmb.2005-0280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipper LM, Mulcahy RT. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem Biophys Res Commun. 2000;278:484–492. doi: 10.1006/bbrc.2000.3830. [DOI] [PubMed] [Google Scholar]