Abstract
Several diseases of the airways have a strong component of allergic inflammation in their cause, including allergic rhinitis, asthma, polypoid chronic rhinosinusitis, eosinophilic bronchitis, and others. Although the roles played by antigens and pathogens vary, these diseases have in common a pathology that includes marked activation of epithelial cells in the upper airways, the lower airways, or both. Substantial new evidence indicates an important role of epithelial cells as both mediators and regulators of innate immune responses and adaptive immune responses, as well as the transition from innate immunity to adaptive immunity. The purpose of this review is to discuss recent studies that bear on the molecular and cellular mechanisms by which epithelial cells help to shape the responses of dendritic cells, T cells, and B cells and inflammatory cell recruitment in the context of human disease. Evidence will be discussed that suggests that secreted products of epithelial cells and molecules expressed on their cell surfaces can profoundly influence both immunity and inflammation in the airways.
Keywords: Epithelium, innate immunity, adaptive immunity, airway inflammation, immune regulation
Epithelial cells play important roles in host defense, inflammation, and regulation of immune responses. This review highlights new data suggesting that epithelial cells mediate innate immune responses and regulate adaptive immune responses involving dendritic cells (DCs), T cells, and B cells, three cell types that are of central importance in allergic and other inflammatory diseases of the airways. We have included information regarding the relevance of these new findings to asthma and other allergic diseases, where available. A picture emerges in which epithelial cells mediate innate host defense through secretion of dozens of distinct antimicrobial products. When innate immune responses fail, epithelial cells direct early adaptive immune responses by helping to program the DC response to antigen exposure. Epithelial cells also directly influence the response of antigen-specific T and B cells through the release of cell subtype–specific chemokines and the expression of soluble and cell surface–expressed molecules that regulate differentiation, proliferation, activation, and survival of T and B lymphocytes. Epithelial activation is a characteristic of asthma, rhinitis, chronic rhinosinusitis, chronic obstructive pulmonary disease, and other airways diseases. In some cases, deficiencies in the ability of the epithelium to maintain the immunologic and physical barrier might play a role in susceptibility to these diseases.
EPITHELIAL CELLS AND INNATE IMMUNITY
Accumulating evidence indicates that epithelial cells play important roles in the initiation, maintenance, and regulation of both innate and adaptive immune responses in the airways. Epithelial roles in innate immunity have been known for decades, since Sir Alexander Fleming reported in 1922 that lysozyme and other mucosal substances prevent the growth of bacteria and other microorganisms. In addition to their mucociliary clearance function, epithelial cells are now known to kill or neutralize microorganisms through the production of several families of molecules, including enzymes (lysozyme, phospholipases, peroxidases, and complement components), permeabilizing peptides (eg, defensins, cathelicidins, bacterial permeability-increasing protein [BPI], and palate, lung, and nasal epithelium clone [PLUNC]), collectins (eg, SP-A, SP-D, and MBL), pentraxins (eg, PTX-3 and CRP), protease inhibitors (eg, secretory leukocyte proteinase inhibitor [SLPI] and elafin), small molecules (ROS, thiocyanate, and nitric oxide), binding/neutralizing proteins (eg, mucins, serum amyloid A [SAA], and lactoferrin) and others.1 Recent studies indicate that the production of many of these substances is initiated by pathogen-recognition receptors (see Fig 1), including Toll-like receptors (TLRs; TLR1 through TLR10), nucleotide-binding oligomerization domain [NOD]–like receptor [NLR] family receptors (eg, NOD1), helicases (eg, retinoic acid-inducible gene I [RIG-I] and melanoma differentiation-associated gene 5 [MDA5]), the double-stranded RNA binding kinase PKR, and others. It is now quite clear that epithelial cells routinely protect the airways from colonization or infection by most microorganisms.
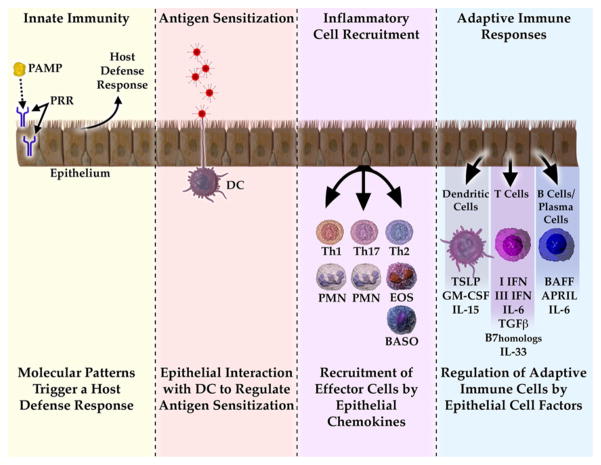
FIG 1.
Model summarizing the influence of epithelial cells on innate and adaptive immune responses in the airways. Epithelial cells express pattern-recognition receptors and release antimicrobial products into the airways. They also interact with interepithelial DCs and subepithelial DCs to alter the ability of DCs to skew T cells. During inflammatory and immune responses, epithelial cells release specific chemokines that recruit subsets of granulocytes and T cells that are appropriate to the particular immune response. Finally, epithelial cells regulate the adaptive immune response by expression of soluble and cell-surface molecules that alter the function of DCs, T cells, and B cells in the airways. PAMP, Pathogen-associated molecular pattern; PRR, pathogen-recognition receptor; PMN, polymorphonuclear leukocyte; EOS, eosinophil; BASO, basophil; APRIL, a proliferation-inducing ligand.
An important component of the epithelial armamentarium in innate immunity is the maintenance of barrier function. There is evidence for deficient barrier function in keratinocytes of patients with atopic dermatitis and to some extent in airway epithelial cells of patients with asthma because of insufficient expression of the epidermal differentiation complex, a cassette of genes that includes the protease inhibitor serine protease inhibitors of the Kazal type (SPINK5), profilaggrin, and other genes that either form or protect tight junctions and other structures involved in epithelial integrity.2–4 Reduced barrier function might increase susceptibility to sensitization and lower the threshold of antigen exposures required to drive local antigen-dependent inflammation. Recently, null mutations in profilaggrin have been linked to asthma incidence, as well as severity, suggesting that altered barrier function in either the skin or the airways is an important risk factor for allergic diseases.
Epithelial cells also play an important role in the initiation of adaptive immune responses in the airways. Epithelial cells can trigger and modify the activation and differentiation of DCs, B cells, and T cells. When the innate immune functions of epithelium mentioned briefly above fail, adaptive immune responses to potential pathogens are necessary and lifesaving. In addition, epithelial restraint of inflammatory immune responses might be helpful to prevent excessive or unnecessary damage to the airways. Although responses of cells armed with antigen-specific immunoglobulins (eg, mast cells, basophils, eosinophils, and neutrophils) can play a role in the adaptive response, they will not be considered here.
EPITHELIAL EFFECTS ON DCs
There is a highly meshed network of DCs within the respiratory epithelium. A subset of these DCs, referred to as intraepithelial DCs, has a distinct phenotype, expressing aE-β7, Fc receptors, langerin, and tight junction proteins (claudin-1, claudin-7, and Zona occludens 2 [ZO 2]).5,6 Intraepithelial DCs extend processes into the airway lumen between epithelial cells, presumably to collect antigenic material from the mucosal surface. These cellular processes interact with epithelial cells through the unusual membrane-associated chemokine fractalkine (CX3CL1), which is primarily expressed by epithelial cells.7 Fractalkine and its receptor, CX3CR1, have been shown to mediate DC-epithelial interactions in the gut8 and are increased in asthmatic airways.9 Mucosal M cells are a type of specialized epithelial cell found in the intestine that have high permeability and permit antigen tissue entry for access to subepithelial DCs. Analogous cells do not appear to be found in normal human airway or in bronchus-associated lymphoid tissue, which is generally only present in inflamed human airways.10 There is growing evidence that epithelial cells play a role in the recruitment and local survival of DCs because they produce the chemokine CCL20 (macrophage inflammatory protein 3α [MIP-3α]) and the cytokine GM-CSF, respectively, which promote these processes.11,12
The nature of the immune response that occurs after DC exposure to antigen (eg, TH1, TH2, regulatory T cell [Treg], and TH17) is determined by the state of activation of DCs and the context in which they present antigen to T cells (eg, level and type of costimulatory molecules and cytokine pattern expressed by the DCs). Although factors associated with antigen, such as the presence of TLR ligands, can have a profound outcome on the nature of the DC response, it is now clear that epithelial cells can also influence the subsequent DC activation status. The epithelial-derived factor thymic stromal lymphopoietin (TSLP) has been shown to skew DCs so that they activate formation of TH2 cells. TSLP is a 4-helix bundle cytokine related to IL-7 that binds to a specific receptor comprised of the IL-7 receptor α chain and the TSLP receptor.13 Production of TSLP occurs primarily by keratinocytes in the skin and epithelial cells in the airways. Besides activating DCs, epithelial TSLP has been shown recently to activate cytokine secretion by mast cells.14 Increased TSLP expression has been demonstrated in both atopic dermatitis and asthma.15,16 A feature of its effects on DCs is that it activates costimulation processes without triggering DC generation of IL-12, a cytokine with potent TH1-skewing activity. Epithelial expression of TSLP is triggered by the TLR3 ligand double-stranded RNA, by rhinovirus, and by TH2 cytokines, and the process involves activation of nuclear factor κB (NF-κB) and IRF-3 in the case of double-stranded RNA and signal transducer and activator of transcription 6 in the case of IL-4.17 Other epithelial-derived factors that are likely to influence the T cell–skewing characteristics of DCs are type I IFN (IFN-α and IFN-β, which skew toward TH1) and type III IFN (IFN-λ, IL-28, and IL-29, which skew toward Treg).18,19 In asthmatic patients the type I and type III IFN airway epithelial interferon response to rhinovirus infection is impaired and correlated with worse respiratory symptoms.20,21 This impaired epithelial response could lead to reduced TH1 and Treg responses in asthma. Epithelial cells are an inducible source of the IL-1 family members IL-1F9 and IL-33, the latter a ligand for ST2, a receptor that strongly induces TH2 cell skewing.22 The potential role of these molecules in TH2 diseases in human airways is under investigation.
EPITHELIAL EFFECTS ON T CELLS
Epithelial cells can shape the tissue response during adaptive immune effector responses to conform to the nature of the T-cell response and the leukocytes required. They release chemokines that attract neutrophils for TH1 and TH17 responses and eosinophils and basophils for TH2 responses. They also release chemokines that attract the T cells themselves: TH1 (CXCL10/IFN-inducible protein [IP]-10 and CXCL9/monokine induced by IFN-γ [MIG] in response to IFN-γ) and TH2 (CCL1/I-309, CCL22/macrophage-derived chemokine [MDC], and CCL17/thymus and activation-regulated chemokine [TARC] in response to IL-4 and IL-13).23 Epithelial cells produce CXCL8/IL-8 and CXCL1–3/growth-related oncogene (GRO)-α–γ in response to IL-17 to recruit neutrophils.24,25 TH17 cells comprise a relatively recently recognized subset of T cells that are thought to mediate immunity to extracellular organisms, as well as several auto-immune diseases.26 The chemokines responsible for TH17 recruitment have not been evaluated, but it is reasonable to expect that they will be partly epithelial cell derived. As discussed above, epithelial TSLP can influence the phenotype of outgoing DCs to modify the differentiation of the T cells to which they present antigen in draining lymph nodes. Epithelial cells might participate in T-cell responses in other ways because they express receptors for other factors that drive the T-cell differentiation process, such as IL-31 that might regulate both TH1 and TH2 cells, TGF-β that skews toward TH17, and IFN-λ that might promote Treg responses.27,28 It has not been tested whether epithelial cells have receptors for other such factors that influence the nature of the T-cell response, such as IL-25 that skews toward TH2, TSLP that skews toward TH2, or IL-23 that skews to TH17. However, IL-22 appears to be a prominent cytokine produced by TH17 cells, and epithelial cells (keratinocytes) display a robust response to this cytokine.29
Several recent studies have shown that airway epithelial cells express high levels of certain B7 family members, notably B7-H1 and B7-DC (also known as PD-L1 and PD-L2).30–32 These molecules are important regulators of T-cell activation, function, survival, and differentiation. Although B7-H1 and B7-DC levels are increased in chronic rhinosinusitis and after viral infections, their relevance to immune regulation and disease pathogenesis awaits clarification. Epithelial cells also express Fas ligand, which can limit lymphocyte survival,33 and CD40, which can stimulate epithelial production of RANTES, monocyte chemoattractant protein 1, IL-8, and intercellular adhesion molecule 1 when cross-linked.34,35 A picture is emerging that suggests that epithelial cells can shape the nature of an adaptive immune response by altering the phenotype of both DCs and T cells.
EPITHELIAL EFFECTS ON B CELLS
Studies in the 1970s demonstrated that IgA- and IgE-expressing B cells are found in the airways and that these cells produce IgE and IgA specific to known inhaled antigens. Several studies have shown that levels of aeroallergen-specific IgE are much higher in the airways than in the serum when normalized to total IgE or albumin. A survey study found that 19% of patients with rhinitis and polyposis had specific IgE in the nose, although not in the serum.36 Recent studies concluded that the majority of the total body aeroallergen-specific antibodies of the IgE and IgA isotypes are produced in the airways and that systemic sensitization largely reflects spillover of immunoglobulins from the mucosal site of their production into the circulation.37 It has been suggested that IgE is tightly regulated in this way to avoid the danger of anaphylaxis that accompanies the presence of high concentrations of circulating IgE. It is now clear that antigen-specific B cells are activated and undergo class-switch recombination (CSR) in the gut and airways. It thus becomes important to consider the role that local factors in the mucosae play in the recruitment, differentiation, activation, and survival of B cells. Epithelial cells, especially in the gut, have been shown to release chemokines that attract B cells in general and IgA-secreting B and plasma cells in particular. These chemokines include CCL25/thymus-expressed chemokine (TECK), CCL28/mucosae-associated epithelial chemokine (MEC), CXCL13/B lymphocyte chemoattractant (BLC), and CXCL12/stromal cell-derived factor (SDF)-1α. In many cases studies have been restricted to the mucosal epithelium of the intestine.38 In the airways epithelial production of CCL28 has been largely of interest because it attracts eosinophils and TH2 cells through CCR3 and CCR10.39 However, this chemokine is also likely to be an important epithelial-derived chemokine that attracts B cells in both the gut and the airways.40,41 CXCL12 is another known B cell–attracting chemokine that has nonetheless been of interest in airways disease for another reason; it has recently also been shown to play a role in the recruitment of epithelial stem cells to injured trachea as part of the repair process mediated by keratinocyte growth factor.42,43
Recent studies indicate that epithelial cells produce several factors that can modify the differentiation of B cells, much in the same way that has been described above for DCs and T cells. Epithelial cells have long been known to be a rich source of IL-6 and TGF-β, cytokines that have profound B cell–activating properties. In addition, recent studies indicate that epithelial cells produce B-cell–activating factor of the TNF family (BAFF)/B lymphocyte stimulator (BLyS), or TNFSF13B, which is a member of the TNF superfamily that is essential for B-cell development through the BAFF receptor and that can mediate B-cell CSR through another receptor, transmembrane activator and CAML interactor (TACI).44 More studies are needed to determine the relative importance of epithelial-derived BAFF (and the related molecule APRIL) versus other sources of BAFF on local CSR and plasma cell differentiation of B cells in the airways. In the intestine it has been concluded that epithelial BAFF is the major trigger for regulating immunoglobulin class switching and that this process is promoted further by epithelial-derived TSLP and regulated by the protease inhibitor SLPI.45
Epithelial cells perform a well-known role in the transport of IgA and IgM across the epithelium into mucosal secretions.46,47 This process is likely to be of importance both in innate and adaptive immunity because natural IgA antibodies (ie, those not generated by somatic hypermutation) can be produced locally in mucosal tissues, in some cases without the participation of T cells. Mucosal B cells produce dimeric IgA or pentameric IgM with monomers joined by the J chain. These multimers bind to the polymeric immunoglobulin receptor (pIgR), which transports them across epithelial cells into the airway lumen. This process occurs to a significant extent in airway mucosal glands, as well as in the lamina propria of the intestine and conducting airways.48 During the process of transport of IgA (or IgM) by pIgR, the transported antibodies are covalently linked to a portion of pIgR that becomes the secretory component to produce the secretory forms sIgA or sIgM. This process is quite important in mucosal immunity, as well as in the neutralization of potential antigens in the gut and airways (immune exclusion). pIgR and secretory component have important immunologic roles beyond immunoglobulin transport. It has been established that pIgR expression and function is regulated by numerous cytokines, hormones, and pathogen-associated molecular patterns.46 There are reports suggesting that defective epithelial transport of IgA might play a role in mucosal airways diseases, such as chronic obstructive pulmonary disease, chronic rhinosinusitis, and asthma.49–51 More information is needed to determine the role of local B-cell responses in inflammatory disease, protective immunity, and immune exclusion (neutralization of antigens) in the airways.
It should be clear from this brief review that several lines of evidence now support the concept that epithelial cells are primary innate immune effector cells that also regulate the adaptive immune responses in the airways at the level of DCs, T cells, and B cells. Epithelial facilitation of adaptive immune responses probably occurs when antigen exposure is high and accompanied by a ligand for a pattern-recognition receptor. A high load of antigen could reflect the failure of epithelial innate immune responses to clear the antigen source or could reflect high ambient exposure in inhaled air. Once adaptive responses are mobilized, it is clear that epithelial cells regulate them at every phase of the response (sensitization, effector responses, and termination). Finally, airway diseases associated with excessive or aberrant innate or adaptive immune responses might in some cases initially result from inappropriate responses of epithelium.
Abbreviations
- BAFF
B-cell–activating factor of the TNF family
- CSR
Class-switch recombination
- DC
Dendritic cell
- pIgR
Polymeric immunoglobulin receptor
- TLR
Toll-like receptor
- Treg
Regulatory T cell
- TSLP
Thymic stromal lymphopoietin
Footnotes
Disclosure of potential conflict of interest: R. P. Schleimer has received grant support from the National Institutes of Health and has served as an expert witness in patent infringement cases. P. C. Avila has consulting arrangements with Genentech and has received grant support from Genentech, Novartis, GlaxoSmithKline, AstraZeneca, and Aventis. The rest of the authors have declared that they have no conflict of interest.
References
- 1.Avila PC, Schleimer RP. Airway epithelium. In: Kay AB, Kaplan AP, Bousquet J, Holt P, editors. Allergy and allergic diseases. 2. Oxford: Blackwell Publishing; 2008. [Google Scholar]
- 2.Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol. 2004;4:978–88. doi: 10.1038/nri1500. [DOI] [PubMed] [Google Scholar]
- 3.Kabesch M, Carr D, Weiland SK, von Mutius E. Association between polymorphisms in serine protease inhibitor, kazal type 5 and asthma phenotypesin a large German population sample. Clin Exp Allergy. 2004;34:340–5. doi: 10.1111/j.1365-2222.2004.01860.x. [DOI] [PubMed] [Google Scholar]
- 4.Hudson TJ. Skin barrier function and allergic risk. Nat Genet. 2006;38:399–400. doi: 10.1038/ng0406-399. [DOI] [PubMed] [Google Scholar]
- 5.Gong JL, McCarthy KM, Telford J, Tamatani T, Miyasaka M, Schnee-berger EE. Intraepithelial airway dendritic cells: a distinct subset of pulmonary dendritic cells obtained by microdissection. J Exp Med. 1992;175:797–807. doi: 10.1084/jem.175.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–72. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 7.Lucas AD, Chadwick N, Warren BF, Jewell DP, Gordon S, Powrie F, et al. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am J Pathol. 2001;158:855–66. doi: 10.1016/S0002-9440(10)64034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 9.Rimaniol AC, Till SJ, Garcia G, Capel F, Godot V, Balabanian K, et al. The CX3C chemokine fractalkine in allergic asthma and rhinitis. J Allergy Clin Immunol. 2003;112:1139–46. doi: 10.1016/j.jaci.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 10.Richmond I, Pritchard GE, Ashcroft T, Avery A, Corris PA, Walters EH. Bronchus associated lymphoid tissue (BALT) in human lung: its distribution in smokers and non-smokers. Thorax. 1993;48:1130–4. doi: 10.1136/thx.48.11.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol. 2003;28:648–54. doi: 10.1165/rcmb.2002-0095OC. [DOI] [PubMed] [Google Scholar]
- 12.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–64. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 13.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 14.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–8. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 16.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–90. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 17.Kato A, Favoreto S, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–7. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogge L, D’Ambrosio D, Biffi M, Penna G, Minetti LJ, Presky DH, et al. The role of Stat4 in species-specific regulation of Th cell development by type I IFNs. J Immunol. 1998;161:6567–74. [PubMed] [Google Scholar]
- 19.Mennechet FJ, Uze G. Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood. 2006;107:4417–23. doi: 10.1182/blood-2005-10-4129. [DOI] [PubMed] [Google Scholar]
- 20.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–47. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–6. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Nickel R, Beck LA, Stellato C, Schleimer RP. Chemokines and allergic disease. J Allergy Clin Immunol. 1999;104:723–42. doi: 10.1016/s0091-6749(99)70281-2. [DOI] [PubMed] [Google Scholar]
- 24.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–52. [PubMed] [Google Scholar]
- 25.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175:404–12. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 27.Chattopadhyay S, Tracy E, Liang P, Robledo O, Rose-John S, Baumann H. Interleukin-31 and oncostatin-M mediate distinct signaling reactions and response patterns in lung epithelial cells. J Biol Chem. 2007;282:3014–26. doi: 10.1074/jbc.M609655200. [DOI] [PubMed] [Google Scholar]
- 28.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 30.Kurosawa S, Myers AC, Chen L, Ni J, Plitt JR, Heller NM, et al. Expression of the costimulatory molecule B7-H2 by human airway epithelial cells. Am J Respir Cell Mol Biol. 2003;28:563–73. doi: 10.1165/rcmb.2002-0199OC. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Myers AC, Chen L, Pardoll DM, Truong-Tran QA, Lane AP, et al. Constitutive and inducible expression of B7 family of ligands by human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;33:280–9. doi: 10.1165/rcmb.2004-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanciu LA, Bellettato CM, Laza-Stanca V, Coyle AJ, Papi A, Johnston SL. Expression of programmed death-1 ligand (PD-L) 1, PD-L2, B7-H3, and inducible costimulator ligand on human respiratory tract epithelial cells and regulation by respiratory syncytial virus and type 1 and 2 cytokines. J Infect Dis. 2006;193:404–12. doi: 10.1086/499275. [DOI] [PubMed] [Google Scholar]
- 33.Hamann KJ, Dorscheid DR, Ko FD, Conforti AE, Sperling AI, Rabe KF, et al. Expression of Fas (CD95) and Fas L(CD95L) in human airway epithelium. Am J Respir Cell Mol Biol. 1998;19:537–42. doi: 10.1165/ajrcmb.19.4.3100. [DOI] [PubMed] [Google Scholar]
- 34.Propst SM, Denson R, Rothstein E, Estell K, Schwiebert LM. Proinflammatory and Th2-derived cytokines modulate CD40-mediated expression of inflammatory mediators in airway epithelia: implications for the role of epithelial CD40 in airway inflammation. J Immunol. 2000;165:2214–21. doi: 10.4049/jimmunol.165.4.2214. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka H, Maeda K, Nakamura Y, Azuma M, Yanagawa H, Sone S. CD40 and IFN-gamma dependent T cell activation by human bronchial epithelial cells. J Med Invest. 2001;48:109–17. [PubMed] [Google Scholar]
- 36.Shatkin JS, Delsupehe KG, Thisted RA, Corey JP. Mucosal allergy in the absence of systemic allergy in nasal polyposis and rhinitis: a meta-analysis. Otolaryngol Head Neck Surg. 1994;111:553–6. doi: 10.1177/019459989411100503. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida T, Usui A, Kusumi T, Inafuku S, Sugiyama T, Koide N, et al. A quantitative analysis of cedar pollen-specific immunoglobulins in nasal lavage supported the local production of specific IgE, not of specific IgG. Microbiol Immunol. 2005;49:529–34. doi: 10.1111/j.1348-0421.2005.tb03758.x. [DOI] [PubMed] [Google Scholar]
- 38.Hieshima K, Kawasaki Y, Hanamoto H, Nakayama T, Nagakubo D, Kanamaru A, et al. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J Immunol. 2004;173:3668–75. doi: 10.4049/jimmunol.173.6.3668. [DOI] [PubMed] [Google Scholar]
- 39.John AE, Thomas MS, Berlin AA, Lukacs NW. Temporal production of CCL28 corresponds to eosinophil accumulation and airway hyperreactivity in allergic airway inflammation. Am J Pathol. 2005;166:345–53. doi: 10.1016/S0002-9440(10)62258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol. 2003;170:3799–805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- 41.English K, Brady C, Corcoran P, Cassidy JP, Mahon BP. Inflammation of the respiratory tract is associated with CCL28 and CCR10 expression in a murine model of allergic asthma. Immunol Lett. 2006;103:92–100. doi: 10.1016/j.imlet.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Gomperts BN, Belperio JA, Fishbein MC, Keane MP, Burdick MD, Strieter RM. Keratinocyte growth factor improves repair in the injured tracheal epithelium. Am J Respir Cell Mol Biol. 2007;37:48–56. doi: 10.1165/rcmb.2006-0384OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomperts BN, Belperio JA, Rao PN, Randell SH, Fishbein MC, Burdick MD, et al. Circulating progenitor epithelial cells traffic via CXCR4/CXCL12 in response to airway injury. J Immunol. 2006;176:1916–27. doi: 10.4049/jimmunol.176.3.1916. [DOI] [PubMed] [Google Scholar]
- 44.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol. 2006;177:7164–72. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 46.Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 47.Brandtzaeg P, Carlsen HS, Halstensen TS. The B-cell system in inflammatory bowel disease. Adv Exp Med Biol. 2006;579:149–67. doi: 10.1007/0-387-33778-4_10. [DOI] [PubMed] [Google Scholar]
- 48.Fagarasan S, Honjo T. Regulation of IgA synthesis at mucosal surfaces. Curr Opin Immunol. 2004;16:277–83. doi: 10.1016/j.coi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Pilette C, Durham SR, Vaerman JP, Sibille Y. Mucosal immunity in asthma and chronic obstructive pulmonary disease: a role for immunoglobulin A? Proc Am Thorac Soc. 2004;1:125–35. doi: 10.1513/pats.2306032. [DOI] [PubMed] [Google Scholar]
- 50.Chee L, Graham SM, Carothers DG, Ballas ZK. Immune dysfunction in refractory sinusitis in a tertiary care setting. Laryngoscope. 2001;111:233–5. doi: 10.1097/00005537-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Daele JJ. Chronic sinusitis in children. Acta Otorhinolaryngol Belg. 1997;51:285–304. [PubMed] [Google Scholar]