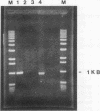
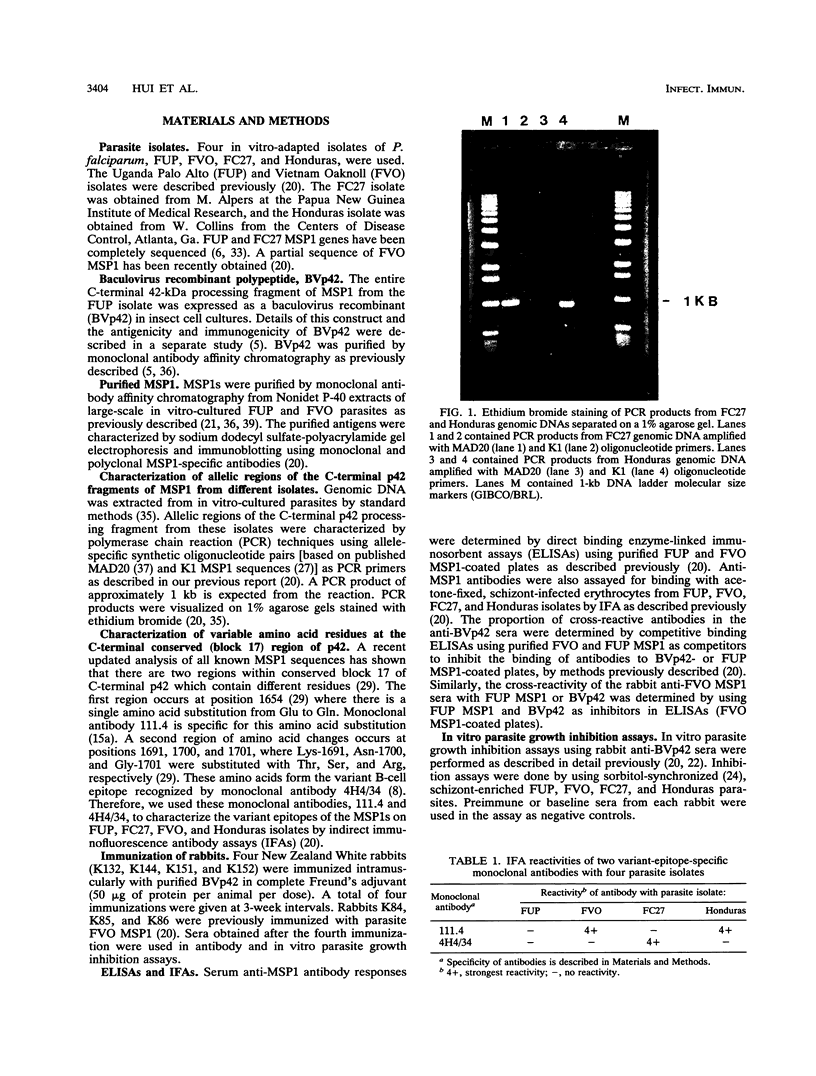
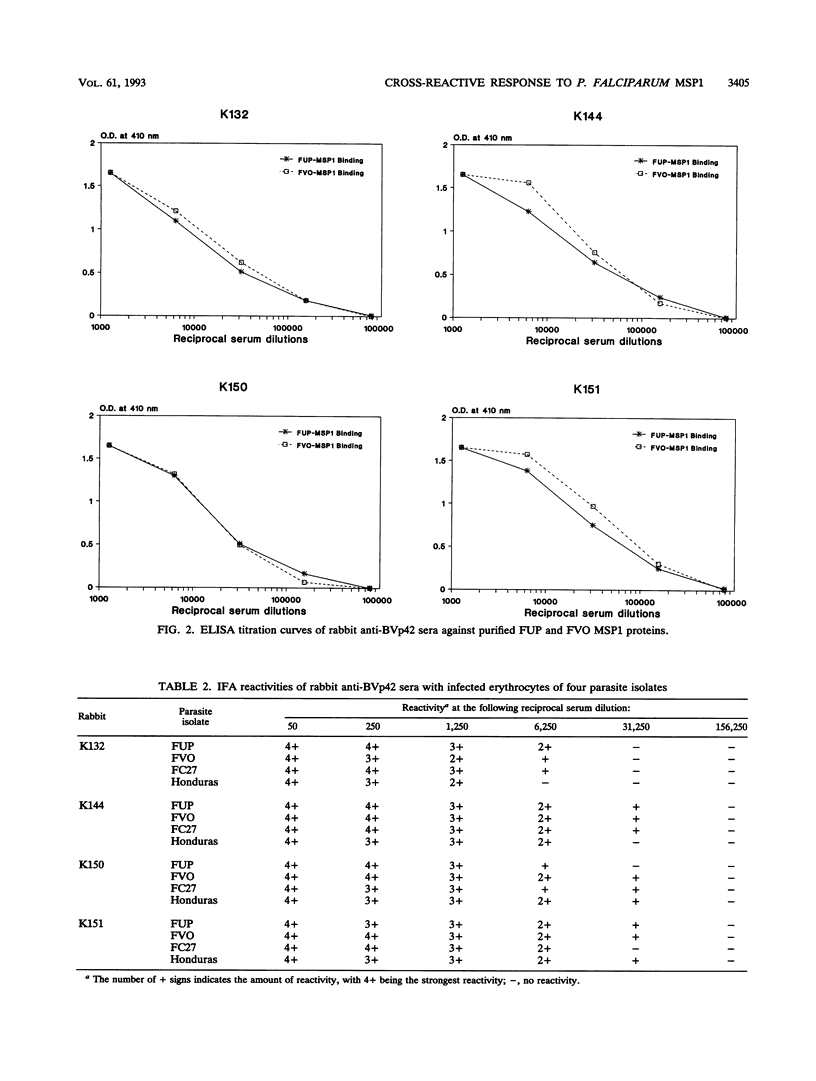
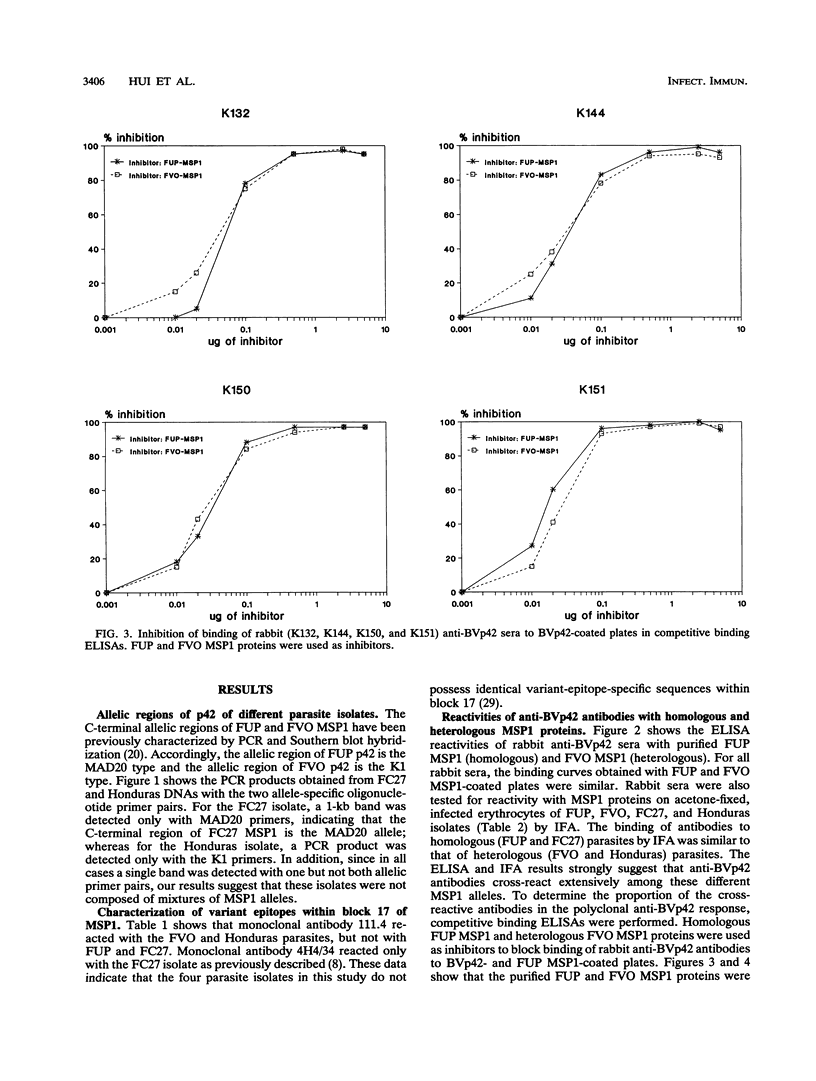
Abstract
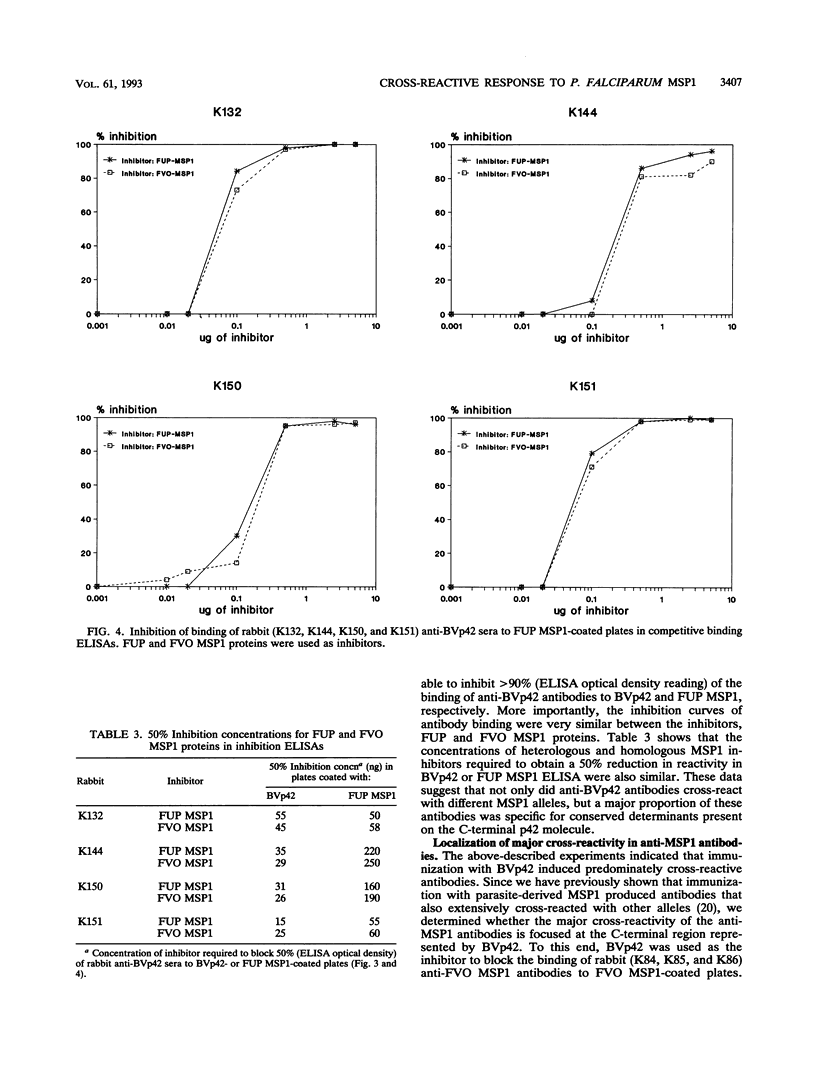
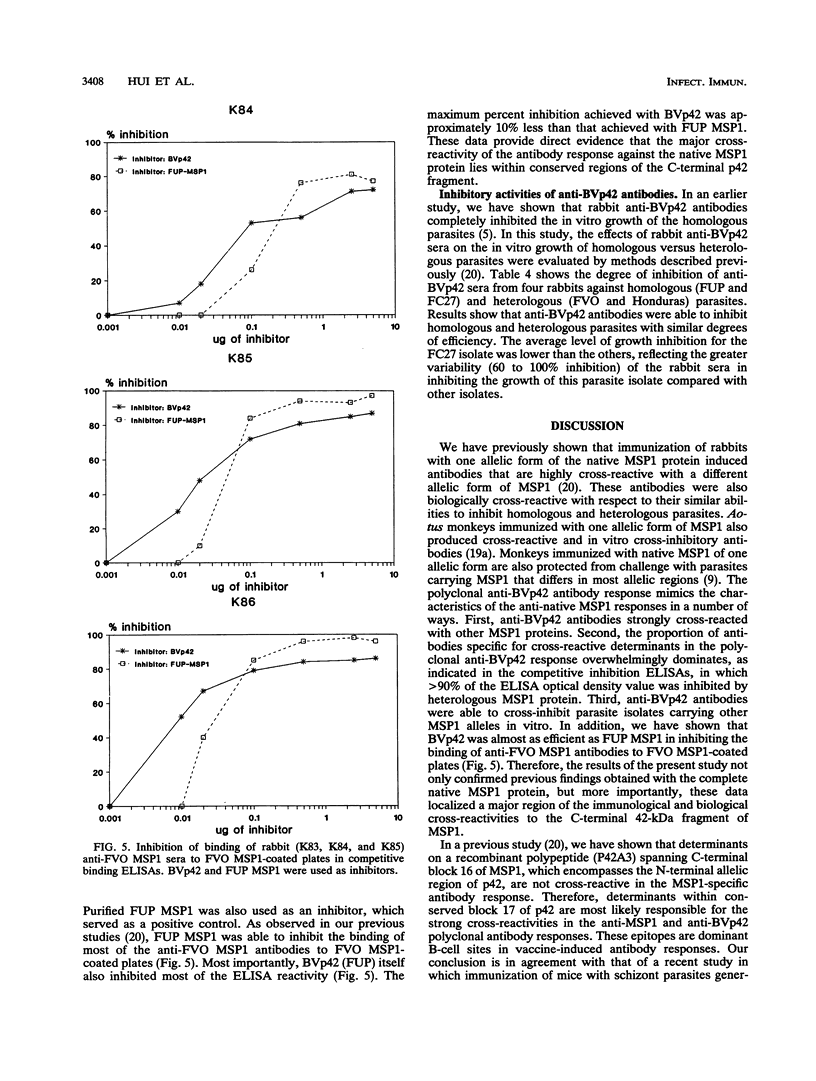
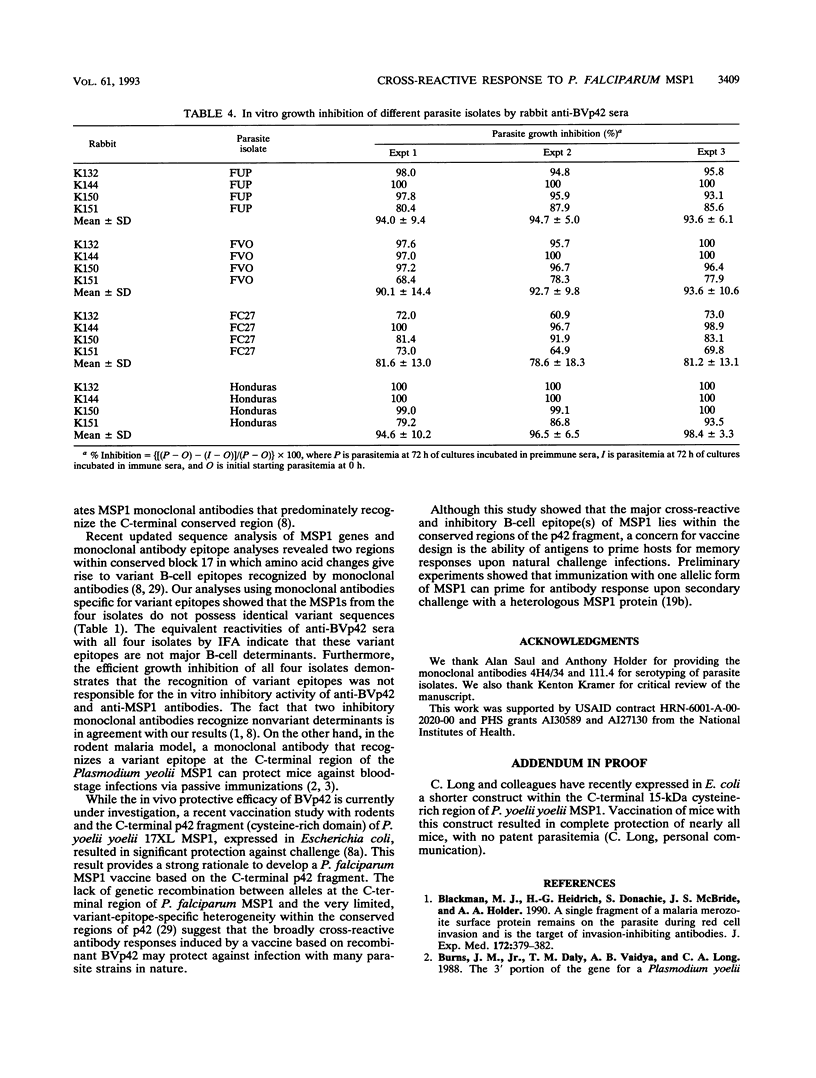
The roles of allelic and conserved epitopes in vaccine-induced immunity to the C-terminal 42-kDa fragment of the Plasmodium falciparum merozoite surface protein 1 (MSP1) were investigated. The C-terminal fragment of MSP1 was expressed as a baculovirus recombinant protein, BVp42. Rabbits were immunized with BVp42, and antibodies were tested for reactivity to MSP1s of the homologous and heterologous allelic forms, represented by the FUP, FVO, FC27, and Honduras parasite isolates, by enzyme-linked immunosorbent assay and indirect immunofluorescence antibody assay. Despite the fact that allelic sequences accounted for approximately 50% of the BVp42 molecule, anti-BVp42 antibodies cross-reacted extensively with parasites carrying heterologous MSP1 alleles. Enzyme-linked immunosorbent inhibition assays confirmed that an overwhelming majority of the anti-BVp42 antibodies were cross-reactive, suggesting that determinants within conserved block 17 are dominant B-cell epitopes in the anti-BVp42 response. Moreover, the BVp42 polypeptide could inhibit (> 90%) the cross-reactivity of anti-MSP1 antibodies in animals immunized with the complete native MSP1 protein. Anti-BVp42 antibodies were equally effective in inhibiting the in vitro growth of parasites carrying homologous or heterologous MSP1 alleles. Serotyping by monoclonal antibodies indicated that the immunological and biological cross-reactivities were not caused by identical variant-specific amino acid substitutions within conserved block 17. These results should provide the impetus to develop a vaccine based on the C-terminal conserved region(s) of MSP1 against parasites of diverse genetic makeup.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackman M. J., Heidrich H. G., Donachie S., McBride J. S., Holder A. A. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990 Jul 1;172(1):379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. M., Jr, Parke L. A., Daly T. M., Cavacini L. A., Weidanz W. P., Long C. A. A protective monoclonal antibody recognizes a variant-specific epitope in the precursor of the major merozoite surface antigen of the rodent malarial parasite Plasmodium yoelii. J Immunol. 1989 Apr 15;142(8):2835–2840. [PubMed] [Google Scholar]
- Certa U., Rotmann D., Matile H., Reber-Liske R. A naturally occurring gene encoding the major surface antigen precursor p190 of Plasmodium falciparum lacks tripeptide repeats. EMBO J. 1987 Dec 20;6(13):4137–4142. doi: 10.1002/j.1460-2075.1987.tb02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. P., Gibson H. L., Lee-Ng C. T., Barr P. J., Hui G. S. A carboxyl-terminal fragment of Plasmodium falciparum gp195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J Immunol. 1992 Jul 15;149(2):548–555. [PubMed] [Google Scholar]
- Chang S. P., Kramer K. J., Yamaga K. M., Kato A., Case S. E., Siddiqui W. A. Plasmodium falciparum: gene structure and hydropathy profile of the major merozoite surface antigen (gp195) of the Uganda-Palo Alto isolate. Exp Parasitol. 1988 Oct;67(1):1–11. doi: 10.1016/0014-4894(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Cheung A., Leban J., Shaw A. R., Merkli B., Stocker J., Chizzolini C., Sander C., Perrin L. H. Immunization with synthetic peptides of a Plasmodium falciparum surface antigen induces antimerozoite antibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8328–8332. doi: 10.1073/pnas.83.21.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Cooper L. T., Saul A. J. Mapping of the region predominantly recognized by antibodies to the Plasmodium falciparum merozoite surface antigen MSA 1. Mol Biochem Parasitol. 1992 Apr;51(2):301–312. doi: 10.1016/0166-6851(92)90080-4. [DOI] [PubMed] [Google Scholar]
- Daly T. M., Long C. A. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993 Jun;61(6):2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger H. M., Caspers P., Matile H., Schoenfeld H. J., Stueber D., Takacs B. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect Immun. 1991 Oct;59(10):3498–3503. doi: 10.1128/iai.59.10.3498-3503.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983 Nov 1;158(5):1647–1653. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Hall R., Osland A., Hyde J. E., Simmons D. L., Hope I. A., Scaife J. G. Processing, polymorphism, and biological significance of P190, a major surface antigen of the erythrocytic forms of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Apr;11:61–80. doi: 10.1016/0166-6851(84)90055-0. [DOI] [PubMed] [Google Scholar]
- Heidrich H. G., Miettinen-Baumann A., Eckerskorn C., Lottspeich F. The N-terminal amino acid sequences of the Plasmodium falciparum (FCB1) merozoite surface antigens of 42 and 36 kilodalton, both derived from the 185-195-kilodalton precursor. Mol Biochem Parasitol. 1989 May 1;34(2):147–154. doi: 10.1016/0166-6851(89)90006-6. [DOI] [PubMed] [Google Scholar]
- Herrera M. A., Rosero F., Herrera S., Caspers P., Rotmann D., Sinigaglia F., Certa U. Protection against malaria in Aotus monkeys immunized with a recombinant blood-stage antigen fused to a universal T-cell epitope: correlation of serum gamma interferon levels with protection. Infect Immun. 1992 Jan;60(1):154–158. doi: 10.1128/iai.60.1.154-158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera S., Herrera M. A., Perlaza B. L., Burki Y., Caspers P., Döbeli H., Rotmann D., Certa U. Immunization of Aotus monkeys with Plasmodium falciparum blood-stage recombinant proteins. Proc Natl Acad Sci U S A. 1990 May;87(10):4017–4021. doi: 10.1073/pnas.87.10.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R., Nicholls S. C. Immunization against Plasmodium falciparum with recombinant polypeptides produced in Escherichia coli. Parasite Immunol. 1988 Nov;10(6):607–617. doi: 10.1111/j.1365-3024.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984 Aug 1;160(2):624–629. doi: 10.1084/jem.160.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Sandhu J. S., Hillman Y., Davey L. S., Nicholls S. C., Cooper H., Lockyer M. J. Processing of the precursor to the major merozoite surface antigens of Plasmodium falciparum. Parasitology. 1987 Apr;94(Pt 2):199–208. doi: 10.1017/s0031182000053889. [DOI] [PubMed] [Google Scholar]
- Howard R. J., McBride J. S., Aley S. B., Marsh K. Antigenic diversity and size diversity of P. falciparum antigens in isolates from Gambian patients. II. the schizont surface glycoprotein of molecular weight approximately 200 000. Parasite Immunol. 1986 Jan;8(1):57–68. doi: 10.1111/j.1365-3024.1986.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Hui G. S., Hashimoto A., Chang S. P. Roles of conserved and allelic regions of the major merozoite surface protein (gp195) in immunity against Plasmodium falciparum. Infect Immun. 1992 Apr;60(4):1422–1433. doi: 10.1128/iai.60.4.1422-1433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui G. S., Palmer K. L., Siddiqui W. A. Use of human plasma for continuous in vitro cultivation of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1984;78(5):625–626. doi: 10.1016/0035-9203(84)90222-0. [DOI] [PubMed] [Google Scholar]
- Hui G. S., Siddiqui W. A. Serum from Pf195 protected Aotus monkeys inhibit Plasmodium falciparum growth in vitro. Exp Parasitol. 1987 Dec;64(3):519–522. doi: 10.1016/0014-4894(87)90068-3. [DOI] [PubMed] [Google Scholar]
- Kimura E., Mattei D., di Santi S. M., Scherf A. Genetic diversity in the major merozoite surface antigen of Plasmodium falciparum: high prevalence of a third polymorphic form detected in strains derived from malaria patients. Gene. 1990 Jul 2;91(1):57–62. doi: 10.1016/0378-1119(90)90162-k. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Lyon J. A., Geller R. H., Haynes J. D., Chulay J. D., Weber J. L. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2989–2993. doi: 10.1073/pnas.83.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon J. A., Haynes J. D., Diggs C. L., Chulay J. D., Haidaris C. G., Pratt-Rossiter J. Monoclonal antibody characterization of the 195-kilodalton major surface glycoprotein of Plasmodium falciparum malaria schizonts and merozoites: identification of additional processed products and a serotype-restricted repetitive epitope. J Immunol. 1987 Feb 1;138(3):895–901. [PubMed] [Google Scholar]
- Mackay M., Goman M., Bone N., Hyde J. E., Scaife J., Certa U., Stunnenberg H., Bujard H. Polymorphism of the precursor for the major surface antigens of Plasmodium falciparum merozoites: studies at the genetic level. EMBO J. 1985 Dec 30;4(13B):3823–3829. doi: 10.1002/j.1460-2075.1985.tb04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride J. S., Newbold C. I., Anand R. Polymorphism of a high molecular weight schizont antigen of the human malaria parasite Plasmodium falciparum. J Exp Med. 1985 Jan 1;161(1):160–180. doi: 10.1084/jem.161.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H., Roberts T., Shahabuddin M., McCutchan T. F. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1). Mol Biochem Parasitol. 1993 May;59(1):1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- Patarroyo M. E., Romero P., Torres M. L., Clavijo P., Moreno A., Martínez A., Rodríguez R., Guzman F., Cabezas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987 Aug 13;328(6131):629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Merkli B., Loche M., Chizzolini C., Smart J., Richle R. Antimalarial immunity in Saimiri monkeys. Immunization with surface components of asexual blood stages. J Exp Med. 1984 Aug 1;160(2):441–451. doi: 10.1084/jem.160.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. G., Coppel R. L., McIntyre P., Langford C. J., Woodrow G., Brown G. V., Anders R. F., Kemp D. J. Variation in the precursor to the major merozoite surface antigens of Plasmodium falciparum. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):291–301. doi: 10.1016/0166-6851(88)90049-7. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Coppel R. L., Moloney M. B., Kemp D. J. Third form of the precursor to the major merozoite surface antigens of Plasmodium falciparum. Mol Cell Biol. 1988 Jun;8(6):2664–2667. doi: 10.1128/mcb.8.6.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kramer K. J., Hui G. S., Case S. E., Yamaga K. M., Chang S. P., Chan E. B., Kan S. C. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1987 May;84(9):3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K., Mackay M., Goman M., Scaife J. G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987 May 20;195(2):273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Murakami K., Doi S. Plasmodium falciparum: dimorphism of the p190 alleles. Exp Parasitol. 1989 May;68(4):470–473. doi: 10.1016/0014-4894(89)90132-x. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]