Abstract
The threat of nuclear terrorism has led to growing worldwide concern about exposure to radiation. Acute radiation syndrome, or radiation sickness, develops after whole-body or a partial-body irradiation with a high dose of radiation. In the terrorist radiation exposure scenario, however, radiation victims likely suffer from additional injuries such as trauma, burns, wounds or sepsis. Thus, high-dose radiation injuries and appropriate therapeutic interventions must be studied. Despite advances in our understanding of the pathophysiology of radiation injury, very little information is available on the therapeutic approaches to radiation combined injury. In this review, we describe briefly the pathological consequences of ionizing radiation and provide an overview of the animal models of radiation combined injury. We highlight the combined radiation and sepsis model we recently established and suggest the use of ghrelin, a novel gastrointestinal hormone, as a potential therapy for radiation combined injury.
INTRODUCTION
The detonation of nuclear devices in Hiroshima and Nagasaki caused the death of >100,000 people (1). A mathematical model derived by the US Department of Defense using a 12.5-kiloton nuclear device, which can be delivered in a large suitcase, predicts tens of thousands of casualties with isolated trauma, radiation and combined-injury syndrome. Furthermore, it is also estimated that detonation of a 20-megaton warhead would result in 2 million deaths and a large number of casualties (2). Acute radiation syndrome (ARS) or radiation sickness develops after whole-body or partial-body irradiation with a high dose of radiation (3–6). Radiation injury is most often accompanied by trauma, burns, infection and sepsis and hence termed radiation combined injury (RCI).
RADIATION INJURY
The nuclear attacks in Hiroshima and Nagasaki provide the only direct information regarding the nature of expected injuries after a nuclear attack (1). Even though medical records of about 170 autopsies and clinical observations from 14,000 patients are available, clinical applicability of these reports is limited. In addition, because >100,000 people are believed to have perished in the attack, the limited number of autopsies are unlikely to reflect the pathological consequences of the affected population. Nonetheless, those people who were dying shortly after the attack had injuries to nearly every organ system, which included brain, cardiovascular, gastrointestinal tract, respiratory and renal—as well as hematologic/infectious complications (1). In addition to the nuclear attacks at Hiroshima and Nagasaki, a number of critical accidents, including the accident at Chernobyl in April 1986, have provided important information on the severity of injury caused by ionizing radiation ((3,7–13)). Although limited, these observations highlight the need to understand and to develop therapeutic measures to treat radiation-induced injuries.
RADIATION SICKNESS OR ARS
ARS, also known as radiation sickness, is defined as the signs and symptoms of exposure to radiation (3). These symptoms develop after total or partial body exposure to a high dose of radiation. These injuries are more severe in areas where cells have a high turnover rate, which include the skin, hematopoietic system, gut and cerebrovascular system (4,5). The severity of ARS depends on the area of irradiation, its dose, dose rate and particle type (alpha, beta or gamma particles or neutrons) and whether there are concomitant injuries such as trauma, burns and infection. Even at lower doses of radiation, the presence of concomitant injuries may lead to higher mortality than occurs with radiation alone.
ARS and the Gastrointestinal Tract
The earliest presentation of ARS is gastrointestinal (GI) toxicity (3). The GI tract is among the most radiosensitive organ systems in the body owing to the high rate of turnover of mucosal and intestinal cells. In addition to the intestinal epithelium crypt, radiation exposure damages supporting structures such as endocrine glands of the GI tract (14). The GI tract has been considered the largest endocrine organ because of its complement of endocrine cells, which produce a variety of peptides that are involved in GI motility, secretion, absorption, growth and development (15). Alterations in either hormone secretion or action can have deleterious effects on GI function.
To test whether GI hormone levels were altered due to radiation exposure in an experimental animal model, we exposed rats to whole body irradiation using a Gammacell 1000 irradiator (Atomic Energy of Canada Ltd, Mississauga, Ontario, Canada). The radioactive source used was cesium-137. The irradiator was set to deliver γ-irradiation at a dose rate of approximately 360 rad/min for 1.4 min, a total of 5 Gy per animal. Our study showed that whole body irradiation significantly decreased plasma cholecystokinin and secretin levels at 1–2 days after irradiation. The levels returned to normal at either 4 or 8 days after irradiation presumably due to the differences in radiosensitivity even in different regions of the GI tract (16).
ARS and the Systemic Inflammatory Response Syndrome
Although individual organ scores are useful, a more suitable view for ARS is the concept of multiple organ failure or multiple organ dysfunction syndrome caused by a systemic inflammatory response. This notion of multiple organ dysfunction syndrome became evident in the victims of the recent accidents in Nesvizh and Tokaimura, who showed a mixed pathology involving the liver and kidney rather than the classical target organs such as the hematopoietic tissues and the gut (17,18). Experimental data from a primate model supported the existence of a radiation-induced systemic inflammatory response. Proinflammatory cytokines such as interleukin (IL)-8 and IL-6 peaked in the blood of these primates during the initial 24 hours after whole body radiation of 6–8 Gy and returned to normal by day 2. Interestingly, the animals irradiated with sublethal dose of 6 Gy showed a second elevation of IL-8 and IL-6 several days after the irradiation (19). Those animals irradiated with 8 Gy showed significant elevation of these cytokines several days before their demise. Marked stimulation of IL-12 and IL-18, presumably by the activation of the Toll signaling pathway, has been shown in macrophages of animals exposed to whole body irradiation at a dose as low as 2 Gy (20). Various target organs are affected owing to the activation of the innate immune system, which results in the significant release of proinflammatory cytokines (19). The excessive release of these cytokines may lead to an uncontrolled inflammatory reaction with adverse metabolic and hemodynamic responses (21–23) and eventually multiple organ dysfunction.
In the classical view of ARS, the role of the central nervous system (CNS) has been underestimated. It was generally thought that the brain was highly radioresistant and is affected only at radiation doses as high as 20–30 Gy, at which a transient or permanent incapacitation of the CNS occurs, leading to coma and death (24,25). This idea is based on the knowledge that the CNS consists (to a great extent) of nonproliferating cells. In fact, the CNS is highly radiosensitive and its electrical activity and neurochemical metabolism can be damaged with doses as low as 1–2 Gy (26,27). Studies in mice also show early acute dose-dependent overexpression of tumor necrosis factor α (TNF-α), IL-1α and IL-1β 4 to 8 hours after midbrain x-ray irradiation with 7–25 Gy. Increases in IL-1α and IL-1β after 5-Gy whole body irradiation were also seen in the cortex of mice at 10 hours after irradiation. These data, though limited, support the hypotheses that the CNS is highly responsive to ionizing radiation, induction of cytokines is crucial in radiation-induced responses, and radiation causes local neuroimmune and inflammatory responses.
These studies raise the critical question of whether the inflammatory responses in the CNS are due to the consequences of systemic inflammatory responses after whole body irradiation or to a direct effect on the CNS. Peripheral proinflammatory cytokines are capable of affecting the CNS by direct entry or indirectly by vagal nerve stimulation (28–30). Recent studies demonstrated that electrical stimulation of the vagus nerve after lipopolysaccharide (LPS, endotoxin) administration in rats prevented the release of TNF-α from macrophages (31–33). These studies further indicated that action potentials from the vagus nerve traverse the subdiaphragmatic vagus nerve and reach macrophages present in the spleen and suppress TNF-α through molecular mechanisms that require signaling through the nicotinic acetlylcholine receptor subunit α7 (34). Therefore, it is conceivable that the CNS inflammatory response after whole body irradiation could be a consequence of the systemic inflammatory response.
ANIMAL MODELS OF RADIATION COMBINED INJURY
Only a limited amount of research has been done to study the consequences of ARS and owing to the nature of the studies they are limited solely to experiments in animals. Most of these studies have been performed to circumvent the pathological complications associated with radiation therapy. A number of reports show that radiation-induced production of proinflammatory cytokines contributes to radiotherapy-associated disorders in the blood, peripheral lymphoid tissues and lungs (35–37). In most cases, the inflammatory response is resolved by the release of endogenous antiinflammatory cytokines. The persistent accumulation and activation of immune cells, however, leads to inflammation and subsequent adverse effects. The current clinical approach for treatment is to inhibit the production of inflammatory mediators and suppress the initiation of the inflammatory response (38).
RADIATION AND THERMAL INJURY (BURNS) OR WOUNDS
Current animal models of RCI are limited to radiation exposure and thermal injury (burns) or wounds (39–43). Key features of radiation and burn are (a) shock, which occurs early and often becomes the main cause of death at early times after injury; (b) the dramatic suppression of hematopoiesis and the immune system; (c) extensive and severe gastrointestinal damage, which leads to dysfunction in absorption and secretion and increased risk of infection; and (d) delayed wound healing (44). Studies on radiation and burn injuries propose imbalances in the cytokine feedback as the underlying mechanism for RCI (45). Radiation-induced increases in IL-1 and transforming growth factor (TGF)β1 expressions are predictive of fibrovascular changes after high doses of radiation in several mouse strains and in human patients (46–48). These data suggest that therapies that reduce IL-1, IL-6 and/or TGFβ expression might change the fibrovascular effects of radiation exposure and thus enhance survival. Ran et al. showed that in the rat, serum levels of TNF-α and IL-6 are significantly increased after 12-Gy γ radiation followed by 30% body surface burn (49). Similarly, Budagov et al. demonstrated that serum levels of IL-6 increase at 6–24 hours after radiation and burn injury (50). Peritoneal macrophages from these animals exhibit increased production capacity of IL-6 and IL-1β in the RCI group (51). The authors suggest that endogenous infection and bacterial toxins are crucial to increased mortality after radiation and burn (52). Several studies using animal models of radiation injury and wounds have shown that radiation exposure delays wound healing (53–58). Thermal injury alone can lead to delayed wound healing, increased susceptibility to sepsis and multiorgan failure (59,60), and additional injuries can exacerbate these immunological complications. Logically, a significant factor involved in the delay is the severity of irradiation one receives at the time of a nuclear attack.
RADIATION AND SEPSIS
Regarding infection and sepsis after radiation injury, a study was reported that investigated radiation injury and endotoxemia (61). In that study, mice were exposed to 3-Gy radiation and 10 days later they were injected with LPS at a dose of 1 mg/kg body weight. LPS-induced thymic atrophy is worsened by radiation. Mice that received both radiation and LPS had lower red blood cell and platelet counts than those that received either insult alone. These results suggest that radiation has a significant influence on LPS-induced changes in several organs and cell populations such as leukocytes, red blood cells and platelets, which may increase severity of infection due to Gram-negative bacteria (61). In another study, mice irradiated with 7 Gy and infected with Klebsiella pneumoniae exhibited 0% survival whereas those subjected to radiation or infection alone showed 100% or 95% survival, respectively (45).
Recently, we have developed an animal model of RCI induced by radiation exposure, followed by sepsis induced by cecal ligation and puncture (CLP). Male Sprague-Dawley rats were exposed to whole body irradiation and 48 hours later, polymicrobial sepsis was induced by CLP (62). These studies showed that compared with sham-operated rats, serum levels of liver enzymes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in the CLP rats were increased by 3-fold and 5-fold in RCI, respectively. Likewise, lactate, creatinine and lactate dehydrogenase were increased by 15-fold, 6-fold and 4.8-fold, respectively. Similarly, serum levels of proinflammatory cytokines, TNF-α and IL-6 were also markedly increased after RCI. Myeloperoxidase activities, used as a measure for neutrophil infiltration to the injured sites, were also markedly elevated in the lungs, small intestine and kidneys in RCI rats (62). In a 10-day survival study, CLP after radiation resulted in survival rates of 69% at day 1 and 50% at days 2 and 3, which decreased to 38% at days 4–10 (62). These studies clearly provided a suitable RCI animal model that can be used to further pursue studies on potential therapies for such injuries.
THERAPEUTIC APPROACHES TO RCI
Despite our advances in the pathophysiology of RCI, very little information is available for treatment strategies. Nevertheless, a few investigators have reported treatments for such injuries. Studies have shown that the administration of anti–IL-6 monoclonal antibodies 1 hour prior and at 1, 2 and 3 day(s) after radiation combined burn injury improved survival rates from 0% to 60% in 30 days. Prolonged antibody treatment up to 8 days increased the survival rate to 90% (63,64). Intraperitoneal application of nerve growth factor (NGF) in a radiation and wound model in rats increased the survival rate, peripheral white blood cell counts and bone-marrow nucleated cells, suggesting NGF could be therapeutic for wound injuries caused by ionizing radiation (58). Pretreatment with curcumin in a radiation and wound model in mice significantly increased the rate of wound healing, increased collagen synthesis, and improved fibroblast and vascular densities in mice (65). In addition, ascorbic acid treatment improved survival and caused dose-dependent elevation of wound contraction in mice exposed to combined radiation and wound. The COX2 inhibitors pentoxifylline and esculentoside A are proposed to be beneficial in controlling inflammatory cytokines and protecting against radiation (66,67).
With such limited information in treatment strategies, we tested whether ghrelin, which has been shown to be beneficial in sepsis (68–70), could be a potential therapy for RCI. Ghrelin is a novel gastrointestinal hormone, first discovered as an endogenous ligand for the secretogogue receptor type 1a (GHSR-1a) (71). Ghrelin was originally reported to induce growth hormone release through pituitary GHSR-1a stimulation (72–74). However, a large body of evidence has indicated other physiological functions of ghrelin mediated by the central and peripheral ghrelin receptors (75). Ghrelin has been linked to the regulation of pituitary hormone secretion, feeding, energy homeostasis, gastrointestinal function and the cardiovascular and immune systems (76–78). Our recent studies have shown that circulating levels of ghrelin decreased significantly in a rat model of CLP, and ghrelin administration decreases inflammatory responses, improves organ blood flow, attenuates tissue injury and reduces mortality under such conditions ((69,70,78)).
To test the effect of ghrelin on RCI, we subjected rats to RCI and treated them with human ghrelin (62). Our studies showed that serum levels of ghrelin and its gene expression in the stomach were markedly decreased at 20 h after RCI. Administration of human ghrelin significantly reduced serum levels of AST and ALT by 54% and 41%, respectively, compared with the vehicle group. Similarly, serum lactate dehydrogenase (LDH), lactate and creatinine were decreased by 57%, 32% and 41% in the treatment group, respectively. Treatment with human ghrelin reduced serum TNF-α and TNF-α gene expression in the small intestine by 50% and 48% compared with the vehicle group, respectively. Likewise, serum IL-6 and IL-6 gene expression in the gut were decreased by 42% and 29%, respectively, compared with the vehicle group. Ghrelin treatment significantly attenuated myeloperoxidase activities in the lungs, gut and kidneys as well. Finally, treatment with ghrelin improved the survival rate to 69%, which was significantly higher than that in the vehicle-treated RCI rats. These results suggest that ghrelin is beneficial in preventing RCI in rats (62).
Mechanism of Action of Ghrelin in RCI
Studies from our lab (79) and Kovarik et al. (80) have shown that systemic levels of norepinephrine (NE) are increased significantly during polymicrobial sepsis or CLP. Enterectomy before CLP markedly reduces the circulating levels of NE (81). Interestingly, about 50% of the NE formed in the body is produced by the sympathetic nerve fibers in the gut (82,83). These studies demonstrated that gut is the major source of the increased circulating NE in sepsis. We have also shown that NE released from the gut during CLP is crucial in causing the upregulation of proinflammatory cytokines and that NE-induced hepatocellular dysfunction in early sepsis is mediated by the activation of α2A-adrenoceptors (84,85). In this regard, we examined whether circulating levels of NE are altered during RCI and determined whether ghrelin had any effect on NE release. These studies showed that plasma levels of NE were significantly increased in RCI rats compared with sham-operated rats. Interestingly, intravenous administration of ghrelin decreased these levels by 35% (62).
Previously we have demonstrated that ghrelin’s beneficial effect in sepsis was mediated by the activation of the vagus nerve (68). To further determine the mechanism by which ghrelin exerts its effect on RCI, sham and RCI rats subjected to vagotomy immediately before ghrelin treatment were examined for inflammatory responses and organ injury markers. These studies showed that at 20 hours after CLP, serum levels of AST, ALT, LDH, lactate and creatinine were significantly increased in both sham vagotomized and vagotomized RCI rats and were comparable to vagus nerve–intact RCI rats. Similarly, serum TNF-α, IL-6 and myeloperoxidase activities in the intestine, lungs and kidneys were increased in sham-vagotomized and vagotomized RCI rats. Interestingly, when sham-vagotomized rats were treated with ghrelin, the various organ injury parameters were markedly reduced. In contrast, vagotomized RCI rats treated with ghrelin exhibited increased levels of these markers, similarly to vehicle-treated sham vagotomized and vagotomized RCI rats (62). These data strongly supported the notion that ghrelin’s beneficial effect on RCI is mediated by the activation of the vagus nerve.
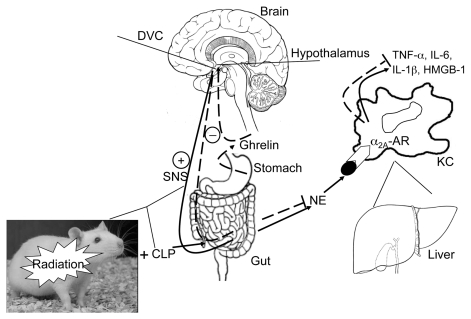
From these studies, we postulate the after-mechanism of ghrelin’s action in RCI (Figure 1). During RCI, the activation of the sympathetic nervous system causes the release of NE from the gut. The NE released from the gut travels through the portal vein, reaches the liver and binds to α2A-adrenoceptors on the surface of the Kupffer cells, and activates them to release proinflammatory cytokines such as TNF-α, IL-1β, IL-6 and high-mobility-group B1. Ghrelin treatment activates the vagus nerve, causing the inhibition of the sympathetic fibers, then leads to a decrease in NE release from the gut and thereby inhibits the proinflammatory cytokines.
Figure 1.
Whole body radiation (Radiation) combined with polymicrobial sepsis induced by cecal ligation and puncture (CLP) (RCI) activates the sympathetic nervous system (SNS) and causes the release of norepinephrine (NE) from the sympathetic fibers in the gut. The NE then travels through the portal vein into the liver. While in the liver, NE binds to the α2A-adrenoceptors (α2A-AR) and activates the signaling pathway(s) responsible for the production and release of proinflammatory cytokines, TNF-α, IL-6, IL-1β and HMGB-1, from Kupffer cells (KC). Ghrelin, a stomach-derived peptide, reaches the dorsal vagal complex (DVC) in the brain by crossing the blood-brain barrier, stimulates GHSR-1a receptors (ghrelin receptors), activates the vagus nerve and in turn, through the cholinergic pathways, downregulates TNF-α and other proinflammatory cytokines. While activating the cholinergic pathway, ghrelin can inhibit the SNS, decrease the release of the sympathetic neurotransmitter NE, and cause the downregulation of the proinflammatory cytokines as well. Therefore, ghrelin’s beneficial effect in RCI is caused by the rebalance of the dysregulated sympathetic/parasympathetic nervous systems. HMGB-1, high-mobility-group B1.
FUTURE STUDIES AND PERSPECTIVES
We provide herein a comprehensive review of the literature on radiation injury and the pathophysiological consequences of those injuries. We also provided a detailed review of the animal models of RCI and, although limited, the preclinical studies on the therapeutic approaches available for such injuries. It is important to recognize that no definite therapies are in use for patients suffering from radiation sickness or in the case of a nuclear attack. It is well accepted that radiation injury combined with trauma, burn and sepsis would be a most likely scenario after a nuclear attack. In such cases, a more suitable view of ARS is multiorgan dysfunction due to exacerbated innate immune responses.
The role of the CNS in ARS has been implicated and several studies support the notion that the CNS is involved in the systemic inflammatory response observed after radiation injury. Recently it has been shown that blocking the cervical sympathetic ganglion with diazepam or ketamine after radiation and burn produced significant decreases in mortality rates (from 90% to 55%) and lessened the increases in serum inflammatory factors TNF-α, IL-1 and IL-6, generally observed after radiation and burn injuries (44). In addition, these studies indicated that overactivation of the hypothalamic-pituitary-adrenal axis could be rebalanced to a near normal level after sympathetic ganglion block. Because one of the key features of radiation and burn is shock, which occurs early and becomes the main cause of death at early times after such injuries, it is plausible that the sympathetic ganglion block could also be beneficial in RCI. Future studies are warranted for such conclusions.
Our studies show that ghrelin treatment in RCI attenuates the inflammatory responses and organ injury and improves survival. These studies further indicated that ghrelin’s effect on RCI is mediated by the activation of the vagus nerve and subsequent inhibition of the sympathetic nervous system, leading to a rebalance of the dysregulated sympathetic/parasympathetic nervous system caused by RCI. These studies further support the role of the CNS in the systemic inflammatory response observed in RCI.
It is well recognized that ghrelin is predominantly secreted by the stomach and that it enters the circulation and also crosses the blood–brain barrier. We have previously shown that coadministration of ghrelin and LPS, a potent activator of cytokine release, did not decrease LPS-induced TNF-α from Kupffer cells, suggesting that ghrelin’s downregulatory effect on cytokine release during sepsis may not be mediated by ghrelin receptors on Kupffer cells (68). Others have shown that ghrelin and ghrelin receptor are expressed on immune cells (86). Ghrelin inhibits the production of proinflammatory cytokines by activated T cells, monocytes and endothelial cells (86,87). This suggests ghrelin functions in the immune cells to regulate local inflammatory response. Therefore, the targets of the exogenous ghrelin could also be immune cells. However, it is still unknown whether ghrelin released from the GI tract, which is sensitive to RCI, can regulate inflammatory responses locally. Further studies are required to investigate such findings.
It has been postulated that radiation followed by trauma, burn or wound would lead to multiorgan dysfunction due to uncontrolled systemic inflammatory responses. Thus, ghrelin could also be beneficial in radiation injuries associated with such complications. However, future studies are warranted. Nevertheless, our studies on ghrelin effects on RCI suggest that ghrelin can be developed as a potential therapy for RCI.
ACKNOWLEDGMENTS
This study was in part supported by the National Institutes of Health grants R21 AI 080536, R01 GM053008 and R01 AG028352 (PW).
Footnotes
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Online address: http://www.molmed.org
REFERENCES
- 1.Manthous CA, Jackson WL., Jr The 9–11 Commission’s invitation to imagine: a pathophysiology-based approach to critical care of nuclear explosion victims. Crit Care Med. 2007;35:716–23. doi: 10.1097/01.CCM.0000257328.31668.22. [DOI] [PubMed] [Google Scholar]
- 2.Ervin FR, et al. Human and ecologic effects in Massachusetts of an assumed thermonuclear attack on the United States. N Engl J Med. 1962;266:1127–37. doi: 10.1056/NEJM196205312662204. [DOI] [PubMed] [Google Scholar]
- 3.Chao NJ. Accidental or intentional exposure to ionizing radiation: biodosimetry and treatment options. Exp Hematol. 2007;35:24–7. doi: 10.1016/j.exphem.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Dainiak N, Ricks RC. The evolving role of haematopoietic cell transplantation in radiation injury: potentials and limitations. BJR Suppl. 2005;27:169–74. [Google Scholar]
- 5.Nagayama H, et al. Severe immune dysfunction after lethal neutron irradiation in a JCO nuclear facility accident victim. Int J Hematol. 2002;76:157–64. doi: 10.1007/BF02982579. [DOI] [PubMed] [Google Scholar]
- 6.Waselenko JK, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–51. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 7.Akashi M, et al. Initial symptoms of acute radiation syndrome in the JCO criticality accident in Tokai-mura. J Radiat Res (Tokyo) 2001;42(Suppl):S157–66. doi: 10.1269/jrr.42.s157. [DOI] [PubMed] [Google Scholar]
- 8.Hempelmann LH, Lisco H, Hoffman JG. The acute radiation syndrome: a study of nine cases and a review of the problem. Ann Intern Med. 1952;36:279–510. doi: 10.7326/0003-4819-36-2-279. [DOI] [PubMed] [Google Scholar]
- 9.Hirama T, et al. Initial medical management of patients severely irradiated in the Tokai-mura criticality accident. Br J Radiol. 2003;76:246–53. doi: 10.1259/bjr/82373369. [DOI] [PubMed] [Google Scholar]
- 10.Ishii T, et al. Brief note and evaluation of acute-radiation syndrome and treatment of a Tokai-mura criticality accident patient. J Radiat Res (Tokyo) 2001;42(Suppl):S167–82. doi: 10.1269/jrr.42.s167. [DOI] [PubMed] [Google Scholar]
- 11.Karas JS, Stanbury JB. Fatal radiation syndrome from an accidental nuclear excursion. N Engl J Med. 1965;272:755–61. doi: 10.1056/NEJM196504152721501. [DOI] [PubMed] [Google Scholar]
- 12.Williams ED. Chernobyl and thyroid cancer. J Surg Oncol. 2006;94:670–77. doi: 10.1002/jso.20699. [DOI] [PubMed] [Google Scholar]
- 13.Yamada M, Wong FL, Fujiwara S, Akahoshi M, Suzuki G. Noncancer disease incidence in atomic bomb survivors, 1958–1998. Radiat Res. 2004;161:622–32. doi: 10.1667/rr3183. [DOI] [PubMed] [Google Scholar]
- 14.Hauer-Jensen M, Wang J, Boerma M, Fu Q, Denham JW. Radiation damage to the gastrointestinal tract: mechanisms, diagnosis, and management. Curr Opin Support Palliat Care. 2007;1:23–9. doi: 10.1097/SPC.0b013e3281108014. [DOI] [PubMed] [Google Scholar]
- 15.Rozengurt E, Guha S, Sinnett-Smith J. Gastrointestinal peptide signalling in health and disease. Eur J Surg Suppl. 2002;587:23–38. [PubMed] [Google Scholar]
- 16.Katanyutanon S, Wu R, Wang P. The effect of whole-body radiation on blood levels of gastrointestinal peptides in the rat. Int J Clin Exp Med. 2008;1:332–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Baranov AE, Selidovkin GD, Butturini A, Gale RP. Hematopoietic recovery after 10-Gy acute total body radiation. Blood. 1994;83:596–9. [PubMed] [Google Scholar]
- 18.Nagayama H, et al. Transient hematopoietic stem cell rescue using umbilical cord blood for a lethally irradiated nuclear accident victim. Bone Marrow Transplant. 2002;29:197–204. doi: 10.1038/sj.bmt.1703356. [DOI] [PubMed] [Google Scholar]
- 19.Gourmelon P, Marquette C, Agay D, Mathieu J, Clarencon D. Involvement of the central nervous system in radiation-induced multi-organ dysfunction and/or failure. BJR Suppl. 2005;27:62–8. [Google Scholar]
- 20.Shan YX, Jin SZ, Liu XD, Liu Y, Liu SZ. Ionizing radiation stimulates secretion of proinflammatory cytokines: dose-response relationship, mechanisms and implications. Radiat Environ Biophys. 2007;46:21–9. doi: 10.1007/s00411-006-0076-x. [DOI] [PubMed] [Google Scholar]
- 21.Heaton T, et al. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet. 2005;365:142–9. doi: 10.1016/S0140-6736(05)17704-6. [DOI] [PubMed] [Google Scholar]
- 22.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 23.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 24.Anno GH, Baum SJ, Withers HR, Young RW. Symptomatology of acute radiation effects in humans after exposure to doses of 0.5–30 Gy. Health Phys. 1989;56:821–38. doi: 10.1097/00004032-198906000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Prasad KN. Radiation Damage of the Nervous System. 2nd ed. Chapter 10. Boca Raton (FL): CRC Press; 1995. Handbook of Radiobiology; pp. 161–170. [Google Scholar]
- 26.Gourmelon P, Lebaron-Jacob L, Marquette C, Clarencon D. Radiation-induced neurovas-cular injuries. In: Fliedner TM, Feinendegen LE, Hopewell JW, editors. Chronic Irradiation: Tolerance and Failure in Complex Biological Systems. BJR. Supplement 26 British Institute of Radiology; London (UK): 2002. [Google Scholar]
- 27.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153:357–70. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Goehler LE, Gaykema RP, Hammack SE, Maier SF, Watkins LR. Interleukin-1 induces c-Fos immunoreactivity in primary afferent neurons of the vagus nerve. Brain Res. 1998;804:306–10. doi: 10.1016/s0006-8993(98)00685-4. [DOI] [PubMed] [Google Scholar]
- 29.Goehler LE, et al. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357–64. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 30.Hansen MK, Taishi P, Chen Z, Krueger JM. Vagotomy blocks the induction of interleukin-1beta (IL-1beta) mRNA in the brain of rats in response to systemic IL-1beta. J Neurosci. 1998;18:2247–53. doi: 10.1523/JNEUROSCI.18-06-02247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 33.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 34.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–28. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holler E, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75:1011–6. [PubMed] [Google Scholar]
- 36.Hong JH, et al. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol. 1999;75:1421–7. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- 37.Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 38.Linard C, et al. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: effect of an NF-kappaB inhibitor. Int J Radiat Oncol Biol Phys. 2004;58:427–34. doi: 10.1016/j.ijrobp.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 39.Alpen EL, Sheline GE. The combined effects of thermal burns and whole body X irradiation on survival time and mortality. Ann Surg. 1954;140:113–8. doi: 10.1097/00000658-195407000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baxter H, Drummond JA, Stephens-Newsham LG, Randall RG. Studies on acute total body irradiation in animals; I: effect of streptomycin following exposure to a thermal burn and irradiation. Plast Reconstr Surg (1946) 1953;12:439–45. doi: 10.1097/00006534-195312000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Brooks JW, Evans EI, Ham WT, Jr, Reid JD. The influence of external body radiation on mortality from thermal burns. Ann Surg. 1952;136:533–45. doi: 10.1097/00000658-195209000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korlof B. Infection of burns. I. A bacteriological and clinical study of 99 cases. II. Animal experiments; burns and total body x-irradiation. Acta Chir Scand Suppl. 1956;209:1–144. [PubMed] [Google Scholar]
- 43.Valeriote FA, Baker DG. The combined effects of thermal trauma and x-irradiation on early mortality. Radiat Res. 1964;22:693–702. [PubMed] [Google Scholar]
- 44.Zou Z, Sun H, Su Y, Cheng T, Luo C. Progress in research on radiation combined injury in China. Radiat Res. 2008;169:722–9. doi: 10.1667/RR1284.1. [DOI] [PubMed] [Google Scholar]
- 45.DiCarlo AL, et al. Medical countermeasures for radiation combined injury: radiation with burn, blast, trauma and/or sepsis. report of an NIAID Workshop, March 26–27, 2007. Radiat Res. 2008;169:712–21. doi: 10.1667/RR1295.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anscher MS, et al. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1998;41:1029–35. doi: 10.1016/s0360-3016(98)00154-0. [DOI] [PubMed] [Google Scholar]
- 47.Liu W, et al. Interleukin 1beta (IL1B) signaling is a critical component of radiation-induced skin fibrosis. Radiat Res. 2006;165:181–91. doi: 10.1667/rr3478.1. [DOI] [PubMed] [Google Scholar]
- 48.Okunieff P, et al. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys. 2006;65:890–8. doi: 10.1016/j.ijrobp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 49.Ran XZ, et al. Effects of serum from rats with combined radiation-burn injury on the growth of hematopoietic progenitor cells. J Trauma. 2007;62:193–8. doi: 10.1097/01.ta.0000215434.24726.72. [DOI] [PubMed] [Google Scholar]
- 50.Budagov RS, Ul’ianova LP. Comparative analysis of proinflammatory cytokines in plasma of mice exposed to radiation or in combined radiation injury [in Russian] Radiats Biol Radioecol. 2000;40:188–91. [PubMed] [Google Scholar]
- 51.Budagov RS, Ul’ianova LP. Cytokine production by different population of macrophages following radiation or combined radiation injury [in Russian] Radiats Biol Radioecol. 2000;40:684–7. [PubMed] [Google Scholar]
- 52.Budagov RS, Ul’ianova LP. Some consequences of systemic inflammatory response in the pathogenesis of aggravation of outcomes of combined radiation and thermal injuries [in Russian] Radiats Biol Radioecol. 2005;45:191–5. [PubMed] [Google Scholar]
- 53.Gu Q, et al. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17:117–23. [PubMed] [Google Scholar]
- 54.Qu J, et al. Reduced presence of tissue-repairing cells in wounds combined with whole-body irradiation injury is associated with both suppression of proliferation and increased apoptosis. Med Sci Monit. 2003;9:BR370–7. [PubMed] [Google Scholar]
- 55.Dantzer D, et al. Effect of radiation and cell implantation on wound healing in a rat model. J Surg Oncol. 2003;83:185–90. doi: 10.1002/jso.10242. [DOI] [PubMed] [Google Scholar]
- 56.Schaffer M, et al. Differential expression of inflammatory mediators in radiation-impaired wound healing. J Surg Res. 2002;107:93–100. [PubMed] [Google Scholar]
- 57.Ran X, et al. The effects of total-body irradiation on the survival and skin wound healing of rats with combined radiation-wound injury. J Trauma. 2004;57:1087–93. doi: 10.1097/01.ta.0000141885.72033.c7. [DOI] [PubMed] [Google Scholar]
- 58.Shi CM, Qu JF, Cheng TM. Effects of the nerve growth factor on the survival and wound healing in mice with combined radiation and wound injury. J Radiat Res (Tokyo) 2003;44:223–228. doi: 10.1269/jrr.44.223. [DOI] [PubMed] [Google Scholar]
- 59.Daniel T, et al. Regulation of the postburn wound inflammatory response by gammadelta T-cells. Shock. 2007;28:278–83. doi: 10.1097/shk.0b013e318034264c. [DOI] [PubMed] [Google Scholar]
- 60.Schwacha MG, Schneider CP, Chaudry IH. Differential expression and tissue compartmentalization of the inflammatory response following thermal injury. Cytokine. 2002;17:266–74. doi: 10.1006/cyto.2001.1003. [DOI] [PubMed] [Google Scholar]
- 61.Gridley DS, Miller GM, Pecaut MJ. Radiation and primary response to lipopolysaccharide: bone marrow-derived cells and susceptible organs. In Vivo. 2007;21:453–61. [PubMed] [Google Scholar]
- 62.Shah KG, et al. Human ghrelin ameliorates organ injury and improves survival after radiation injury combined with severe sepsis. Mol Med. 2009;15:407–14. doi: 10.2119/molmed.2009.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Budagov RS, Ul’ianova LP. Role of interleukin-6 (IL-6) in the pathogenesis of combined radiation/thermal injuries [in Russian] Radiats Biol Radioecol. 2004;44:398–402. [PubMed] [Google Scholar]
- 64.Budagov RS, Ul’ianova LP. Effects of modulators of cytokine levels on mice and rats survival under combined radiation/thermal injuries [in Russian] Radiats Biol Radioecol. 2004;44:392–7. [PubMed] [Google Scholar]
- 65.Jagetia GC, Rajanikant GK. Role of curcumin, a naturally occurring phenolic compound of turmeric in accelerating the repair of excision wound, in mice whole-body exposed to various doses of gamma-radiation. J Surg Res. 2004;120:127–38. doi: 10.1016/j.jss.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Liang L, et al. Celecoxib reduces skin damage after radiation: selective reduction of chemokine and receptor mRNA expression in irradiated skin but not in irradiated mammary tumor. Am J Clin Oncol. 2003;26:S114–21. doi: 10.1097/01.COC.0000074149.95710.40. [DOI] [PubMed] [Google Scholar]
- 67.Okunieff P, et al. Pentoxifylline in the treatment of radiation-induced fibrosis. J Clin Oncol. 2004;22:2207–13. doi: 10.1200/JCO.2004.09.101. [DOI] [PubMed] [Google Scholar]
- 68.Wu R, et al. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg. 2007;245:480–6. doi: 10.1097/01.sla.0000251614.42290.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu R, et al. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin-1. Cardiovasc Res. 2005;68:318–326. doi: 10.1016/j.cardiores.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 70.Wu R, et al. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med. 2007;176:805–13. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 72.Arvat E, et al. Preliminary evidence that Ghrelin, the natural GH secretagogue (GHS)- receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest. 2000;23:493–5. doi: 10.1007/BF03343763. [DOI] [PubMed] [Google Scholar]
- 73.Date Y, et al. Central effects of a novel acylated peptide, ghrelin, on growth hormone release in rats. Biochem Biophys Res Commun. 2000;275:477–80. doi: 10.1006/bbrc.2000.3342. [DOI] [PubMed] [Google Scholar]
- 74.Nass R, et al. Intracerebroventricular administration of the rat growth hormone (GH) receptor antagonist G118R stimulates GH secretion: evidence for the existence of short loop negative feedback of GH. J Neuroendocrinol. 2000;12:1194–9. doi: 10.1046/j.1365-2826.2000.00586.x. [DOI] [PubMed] [Google Scholar]
- 75.Cowley MA, Grove KL. Ghrelin—satisfying a hunger for the mechanism. Endocrinology. 2004;145:2604–6. doi: 10.1210/en.2004-0346. [DOI] [PubMed] [Google Scholar]
- 76.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 77.Wang G, Lee HM, Englander E, Greeley GH., Jr Ghrelin—not just another stomach hormone. Regul Pept. 2002;105:75–81. doi: 10.1016/s0167-0115(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 78.Wu R, et al. Ghrelin inhibits sympathetic nervous activity in sepsis. Am J Physiol Endocrinol Metab. 2007;293:E1697–702. doi: 10.1152/ajpendo.00098.2007. [DOI] [PubMed] [Google Scholar]
- 79.Hahn PY, et al. Sustained elevation in circulating catecholamine levels during polymicrobial sepsis. Shock. 1995;4:269–73. doi: 10.1097/00024382-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Kovarik MF, Jones SB, Romano FD. Plasma catecholamines following cecal ligation and puncture in the rat. Circ Shock. 1987;22:281–90. [PubMed] [Google Scholar]
- 81.Yang S, Koo DJ, Zhou M, Chaudry IH, Wang P. Gut-derived norepinephrine plays a critical role in producing hepatocellular dysfunction during early sepsis. Am J Physiol Gastrointest Liver Physio. 2000;279:G1274–81. doi: 10.1152/ajpgi.2000.279.6.G1274. [DOI] [PubMed] [Google Scholar]
- 82.Eisenhofer G, et al. Production and metabolism of dopamine and norepinephrine in mesenteric organs and liver of swine. Am J Physiol. 1995;268:G641–9. doi: 10.1152/ajpgi.1995.268.4.G641. [DOI] [PubMed] [Google Scholar]
- 83.Eisenhofer G, Aneman A, Hooper D, Rundqvist B, Friberg P. Mesenteric organ production, hepatic metabolism, and renal elimination of norepinephrine and its metabolites in humans. J Neurochem. 1996;66:1565–73. doi: 10.1046/j.1471-4159.1996.66041565.x. [DOI] [PubMed] [Google Scholar]
- 84.Yang S, Zhou M, Chaudry IH, Wang P. Norepinephrine-induced hepatocellular dysfunction in early sepsis is mediated by activation of alpha2-adrenoceptors. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1014–21. doi: 10.1152/ajpgi.2001.281.4.G1014. [DOI] [PubMed] [Google Scholar]
- 85.Miksa M, et al. Pivotal role of the alpha(2A)-adrenoceptor in producing inflammation and organ injury in a rat model of sepsis. PLoS One. 2009;4:e5504. doi: 10.1371/journal.pone.0005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dixit VD, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li WG, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–6. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]