Abstract
The role of the Legionella pneumophila protease in the pathogenesis of Legionnaires' disease is unclear. In this study, we assessed the effect of purified protease preparations on human recombinant interleukin-2 (IL-2), the IL-2 receptor, and several additional human T-cell surface proteins to determine whether protease contributes to the virulence of L. pneumophila by interfering with human T-cell activation and function. IL-2-induced proliferation of CTLL-2 cells was inhibited by coincubation with protease (10 to 100 U/ml). Protease at concentrations of > or = 10 U/ml cleaved human recombinant IL-2 as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of reaction mixtures containing 125I-labeled IL-2 and protease. Protease treatment of activated human T cells did not inhibit binding of a monoclonal antibody directed against the alpha subunit of the IL-2 receptor and did not interfere with binding of IL-2 to IL-2 receptors on the lymphocytes. Treatment of blood mononuclear cells or activated T cells with protease (50 U/ml) inhibited the binding of a monoclonal antibody directed against CD4. In contrast, protease treatment did not inhibit the binding of antibodies against CD3, CD8, class II major histocompatibility complex, and the transferrin receptor. Heat inactivation (65 degrees C for 20 min) of the protease or treatment with the metal chelator EDTA ablated the inhibitory effect of the protease. The ability of the protease to degrade IL-2 and cleave CD4 on human T cells suggests that protease may contribute to the pathogenesis of Legionnaires' disease by impeding T-cell activation and immune function.
Full text
PDF

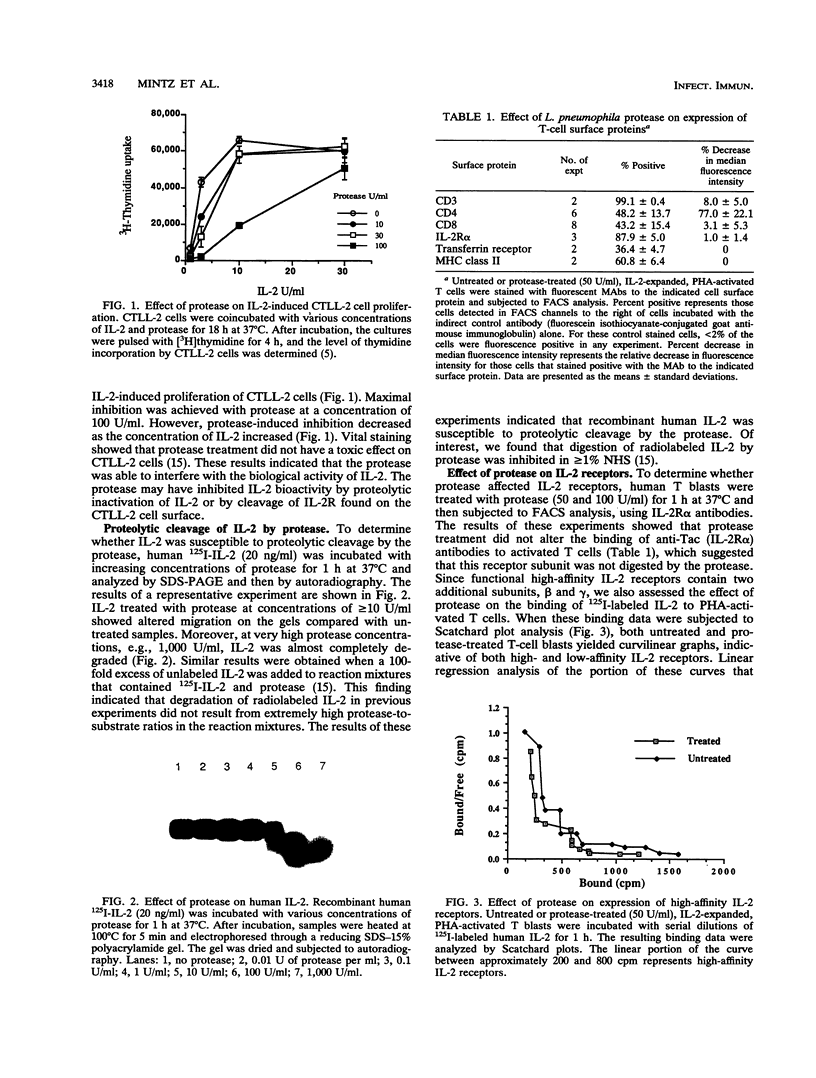
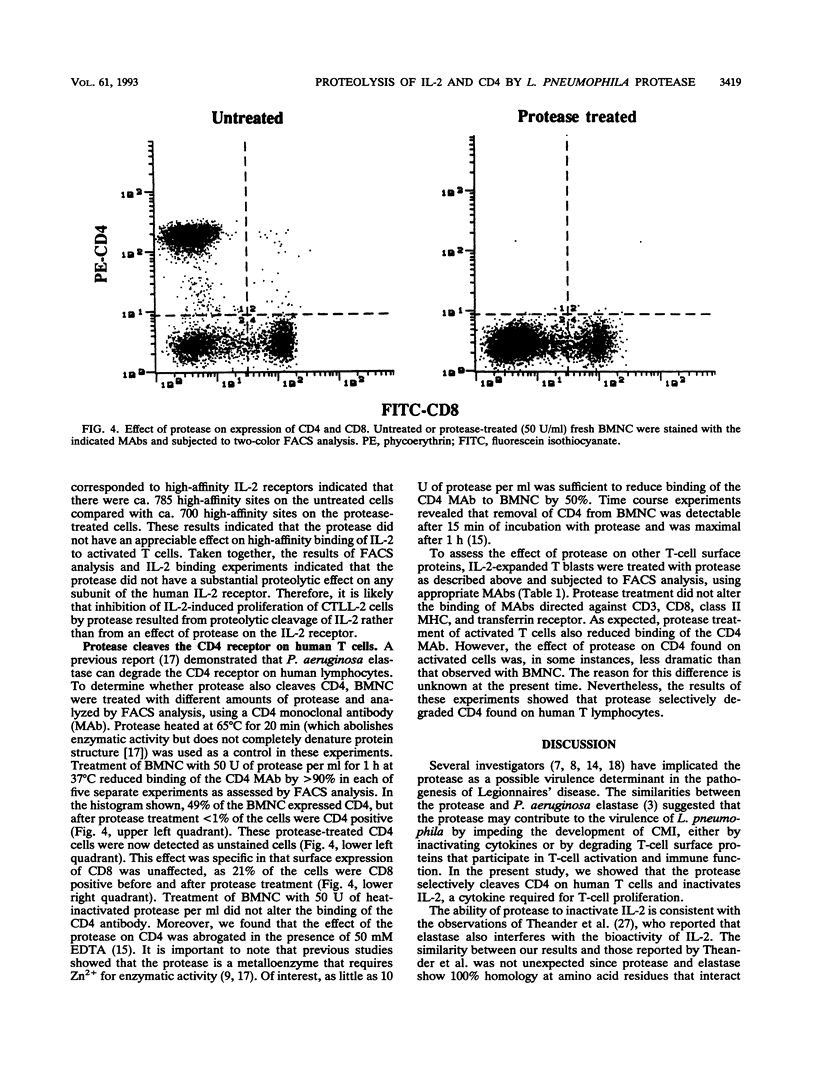


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell D. Y., Haseman J. A., Spock A., McLennan G., Hook G. E. Plasma proteins of the bronchoalveolar surface of the lungs of smokers and nonsmokers. Am Rev Respir Dis. 1981 Jul;124(1):72–79. doi: 10.1164/arrd.1981.124.1.72. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Sleckman B. P., Ratnofsky S. E., Burakoff S. J. The biologic roles of CD2, CD4, and CD8 in T-cell activation. Annu Rev Immunol. 1989;7:579–599. doi: 10.1146/annurev.iy.07.040189.003051. [DOI] [PubMed] [Google Scholar]
- Black W. J., Quinn F. D., Tompkins L. S. Legionella pneumophila zinc metalloprotease is structurally and functionally homologous to Pseudomonas aeruginosa elastase. J Bacteriol. 1990 May;172(5):2608–2613. doi: 10.1128/jb.172.5.2608-2613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander S. J., Horwitz M. A. Vaccination with the major secretory protein of Legionella pneumophila induces cell-mediated and protective immunity in a guinea pig model of Legionnaires' disease. J Exp Med. 1989 Mar 1;169(3):691–705. doi: 10.1084/jem.169.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander S. J., Szeto L., Shuman H. A., Horwitz M. A. An immunoprotective molecule, the major secretory protein of Legionella pneumophila, is not a virulence factor in a guinea pig model of Legionnaires' disease. J Clin Invest. 1990 Sep;86(3):817–824. doi: 10.1172/JCI114779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codias E. K., Rutter J. E., Fleming T. J., Malek T. R. Down-regulation of IL-2 production by activation of T cells through Ly-6A/E. J Immunol. 1990 Sep 1;145(5):1407–1414. [PubMed] [Google Scholar]
- Conlan J. W., Baskerville A., Ashworth L. A. Separation of Legionella pneumophila proteases and purification of a protease which produces lesions like those of Legionnaires' disease in guinea pig lung. J Gen Microbiol. 1986 Jun;132(6):1565–1574. doi: 10.1099/00221287-132-6-1565. [DOI] [PubMed] [Google Scholar]
- Conlan J. W., Williams A., Ashworth L. A. Inactivation of human alpha-1-antitrypsin by a tissue-destructive protease of Legionella pneumophila. J Gen Microbiol. 1988 Feb;134(2):481–487. doi: 10.1099/00221287-134-2-481. [DOI] [PubMed] [Google Scholar]
- Dreyfus L. A., Iglewski B. H. Purification and characterization of an extracellular protease of Legionella pneumophila. Infect Immun. 1986 Mar;51(3):736–743. doi: 10.1128/iai.51.3.736-743.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hatakeyama M., Minamoto S., Uchiyama T., Hardy R. R., Yamada G., Taniguchi T. Reconstitution of functional receptor for human interleukin-2 in mouse cells. Nature. 1985 Dec 5;318(6045):467–470. doi: 10.1038/318467a0. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Activated human monocytes inhibit the intracellular multiplication of Legionnaires' disease bacteria. J Exp Med. 1981 Nov 1;154(5):1618–1635. doi: 10.1084/jem.154.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen M. G., Hoffman P. S. Characterization of a Legionella pneumophila extracellular protease exhibiting hemolytic and cytotoxic activities. Infect Immun. 1989 Mar;57(3):732–738. doi: 10.1128/iai.57.3.732-738.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmely M., Gale A., Clabaugh M., Horvat R., Zhou W. W. Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect Immun. 1990 Sep;58(9):3009–3014. doi: 10.1128/iai.58.9.3009-3014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen B. K., Kharazmi A., Theander T. G., Odum N., Andersen V., Bendtzen K. Selective modulation of the CD4 molecular complex by Pseudomonas aeruginosa alkaline protease and elastase. Scand J Immunol. 1987 Jul;26(1):91–94. doi: 10.1111/j.1365-3083.1987.tb02239.x. [DOI] [PubMed] [Google Scholar]
- Quinn F. D., Keen M. G., Tompkins L. S. Genetic, immunological, and cytotoxic comparisons of Legionella proteolytic activities. Infect Immun. 1989 Sep;57(9):2719–2725. doi: 10.1128/iai.57.9.2719-2725.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn F. D., Tompkins L. S. Analysis of a cloned sequence of Legionella pneumophila encoding a 38 kD metalloprotease possessing haemolytic and cytotoxic activities. Mol Microbiol. 1989 Jun;3(6):797–805. doi: 10.1111/j.1365-2958.1989.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Rechnitzer C., Diamant M., Pedersen B. K. Inhibition of human natural killer cell activity by Legionella pneumophila protease. Eur J Clin Microbiol Infect Dis. 1989 Nov;8(11):989–992. doi: 10.1007/BF01967571. [DOI] [PubMed] [Google Scholar]
- Rechnitzer C., Kharazmi A. Effect of Legionella pneumophila cytotoxic protease on human neutrophil and monocyte function. Microb Pathog. 1992 Feb;12(2):115–125. doi: 10.1016/0882-4010(92)90114-4. [DOI] [PubMed] [Google Scholar]
- Rechnitzer C., Tvede M., Döring G. A rapid method for purification of homogeneous Legionella pneumophila cytotoxic protease using fast protein liquid chromatography. FEMS Microbiol Lett. 1989 May;50(1-2):39–44. doi: 10.1016/0378-1097(89)90455-2. [DOI] [PubMed] [Google Scholar]
- Sahney N. N., Lambe B. C., Summersgill J. T., Miller R. D. Inhibition of polymorphonuclear leukocyte function by Legionella pneumophila exoproducts. Microb Pathog. 1990 Aug;9(2):117–125. doi: 10.1016/0882-4010(90)90085-5. [DOI] [PubMed] [Google Scholar]
- Szeto L., Shuman H. A. The Legionella pneumophila major secretory protein, a protease, is not required for intracellular growth or cell killing. Infect Immun. 1990 Aug;58(8):2585–2592. doi: 10.1128/iai.58.8.2585-2592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theander T. G., Kharazmi A., Pedersen B. K., Christensen L. D., Tvede N., Poulsen L. K., Odum N., Svenson M., Bendtzen K. Inhibition of human lymphocyte proliferation and cleavage of interleukin-2 by Pseudomonas aeruginosa proteases. Infect Immun. 1988 Jul;56(7):1673–1677. doi: 10.1128/iai.56.7.1673-1677.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A., Baskerville A., Dowsett A. B., Conlan J. W. Immunocytochemical demonstration of the association between Legionella pneumophila, its tissue-destructive protease, and pulmonary lesions in experimental legionnaires' disease. J Pathol. 1987 Nov;153(3):257–264. doi: 10.1002/path.1711530310. [DOI] [PubMed] [Google Scholar]