Abstract
Background
We evaluated prevalence and correlates of elevated C-reactive protein (CRP) in a large population of blacks and whites, and the impact of CRP measurement on coronary heart disease (CHD) risk reclassification.
Methods
We studied 19,080 participants of the REasons for Geographic And Racial Differences in Stroke (REGARDS) study; age over 45, without vascular diagnoses, and living dispersed across the United States. 8,309 nondiabetic participants not using lipid-lowering medications were classified into four risk categories of Framingham Vascular Disease Risk Score. If CRP was <1 mg/L, participants were reclassified to the next lower risk group and if >3 mg/L, they were reclassified to the next higher risk group. Reclassification of risk by the Reynolds Vascular Risk Score, incorporating CRP and family history, was also assessed.
Results
Overall, 40% had CRP >3 mg/L. Those at highest risk for elevated CRP were blacks, women and obese people. Among nondiabetic women at 5–20% Framingham Vascular predicted risk, CRP reclassified 48% to a higher risk group and 19% to a lower risk group. For men, these percentages were 24% and 40%. Blacks were more often reclassified to a higher risk group than whites. The Reynolds Vascular Risk Score reclassified 85% of women and 67% of men, almost exclusively to a lower risk group than the Framingham Vascular Score.
Conclusion
In this national study, a majority, especially blacks and women, were reclassified to a different 10-year vascular risk level using CRP testing after risk assessment. The Reynolds Risk Score classified the population differently than the new Framingham Vascular Score with CRP testing.
Keywords: cardiovascular disease, risk factor, epidemiology
Elevated C-reactive protein (CRP) identifies patients at increased cardiovascular risk (1). A consensus group convened by the Centers for Disease Control and Prevention and the American Heart Association issued a statement in 2003 to guide physicians on measurement of CRP in clinical practice (1). The guideline suggested that patients at intermediate risk (10–20% 10-year predicted risk) for future cardiovascular events, who also have elevated CRP (>3 mg/L) be considered for more aggressive vascular disease prevention strategies, such as lipid-lowering therapy (2). Additional data have suggested that vascular risk is even greater with CRP >10 mg/L (3, 4) and that those with a low predicted risk of 5–10% also be considered for CRP testing (5). In addition, a new risk prediction score incorporating CRP (the Reynolds Risk Score) has been proposed for cardiovascular risk assessment in women (6) and men (7).
Stroke mortality in the U.S. is approximately 50% greater in the southeastern “stroke belt” states (8). Stroke mortality also differs in ethnic groups, with rates about 50% higher among blacks compared to whites (9, 10). Differences in prevalences of traditional risk factors for stroke may only partly explain these geographic and ethnic group differences in stroke risk (11, 12), so differences in newer risk factors such as CRP may play a role.
Due to possible ethnic variation in CRP distribution (13–18), and also ethnic and regional differences in vascular risk, there is a need for population-based information on ethnic and regional differences in CRP concentration to guide clinicians in risk assessment in these selected populations. We are not aware of studies reporting geographic variation of CRP in the U.S.
We evaluated the prevalence of elevated CRP by sex, ethnic group and U.S. region in a large national population-based observational study. We studied whether differences in adiposity or other risk factors explained observed differences in CRP by these factors. We further assessed how knowledge from CRP measurement would reclassify individuals’ 10-year predicted cardiovascular disease risk, based on the Framingham Vascular Disease Risk Score, and the Reynolds Risk Score, both of which are designed to predict total cardiovascular disease, and the Framingham Coronary Risk Score, designed to predict coronary events..
Materials and Methods
Subjects
The REasons for Geographic And Racial Differences in Stroke (REGARDS) study is a national population-based cohort study. Between February 2003 and September, 2007, 30,101 individuals over age 45 were enrolled, targeted for equal representation of whites and blacks, and men and women (19). Fifty-six percent resided in the stroke belt (NC, SC, GA, AL, MS, TN, AR, and LA), with the rest from the other 40 contiguous states. Individuals were recruited from a commercial list using mail and telephone contact. Demographic and medical history was obtained using a computer-assisted telephone interview. At an in-home examination written informed consent was obtained and blood pressure, anthropomorphic measures, blood samples, electrocardiogram, and medication inventory were assessed using standardized protocols. Questionnaires were left with the participant to assess family history of vascular disease. Participants are followed by telephone every six months for surveillance of medical events. Study methods were reviewed and approved by the Institutional Review Boards at each study institution.
Of 30,101 participants, we excluded 9074 (30%) reporting a vascular diagnosis. Vascular disease was considered present if participants reported being told by a health professional that they had myocardial infarction, stroke, peripheral arterial disease, transient ischemic attack, carotid endarterectomy, peripheral artery bypass surgery/angioplasty, leg amputation, coronary artery angioplasty or stenting, or if electrocardiogram showed myocardial infarction. Excluding 1189 (5%) participants with missing data for CRP, and 758 with missing covariates there were 19,080 participants included in most analyses. Main analyses of the Framingham and Reynolds Risk Scores only included non-diabetics since diabetes is a coronary disease risk equivalent (limiting the role for novel risk factors) (20). We also excluded 4,690 participants using lipid-lowering medications since this risk assessment is used to decide whether to prescribe this. For Reynolds Risk Score analyses, data on family history of heart disease was not yet coded for 6,081 participants at the time of analysis, leaving 8,309 participants in these analyses.
Laboratory Analysis
Phlebotomy was performed by trained personnel using standardized procedures after a 10–12 hour fast. Within 2 hours of collection (mean 97, SD 127 min), samples were centrifuged and serum or plasma separated and shipped overnight on ice packs to the University of Vermont. Of participants with samples, overnight shipping was achieved for 95%. On arrival, samples were centrifuged at 30,000 ×G and 4°C, and either analyzed (general chemistries) or stored at −80°C.
CRP was analyzed in batches by particle enhanced immunonephelometry using the BNII nephelometer (N High Sensitivity CRP; Dade Behring, Deerfield, IL) with interassay coefficients of variation of 2.1–5.7%. There was no difference in CRP distribution by number of sample shipping days. Cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides and glucose were measured by colorimetric reflectance spectrophotometry using the Ortho Vitros Clinical Chemistry System 950IRC instrument (Johnson & Johnson Clinical Diagnostics, Rochester, NY).
Definitions
Elevated CRP was defined as >3 mg/L (1). Self-reported education was categorized as shown in Table 1. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic pressure ≥90 mmHg, or self-reported high blood pressure with use of anti-hypertensive medications. Prehypertension was defined as systolic pressure 120–140 mm Hg or diastolic pressure 80–90 mm Hg. Obesity was a body-mass index >30 kg/m2, and overweight was 26–30 kg/m2. Region of residence was analyzed as the stroke belt or the remainder of the states. Diabetes was defined by self-reported physician diagnosis with use of anti-diabetic medications, fasting glucose >6.99 mmol/L or non-fasting glucose >11.1 mmool/L, and impaired fasting glucose as fasting glucose 6.10–6.99 mmol/L. Family history of heart disease was defined as parental history of myocardial infarction before age 60.
Table 1.
Baseline Characteristics by Gender
| Characteristic (mean (SD or frequency (%) | Men (n=8077) | Women (n=11003) |
|---|---|---|
| Age | 64.6 (9.1) | 63.8 (9.2) |
| Race, black | 2882 (36%) | 4965 (45%) |
| Region, belt | 4248 (53%) | 6376 (58%) |
| Education | ||
| < High School | 767 (10%) | 1239 (11%) |
| High School Graduate | 1781 (22%) | 2986 (27%) |
| Some College | 2074 (26%) | 3159 (29%) |
| Postmenopausal Hormone Therapy (women) | NA | 1599 (15%) |
| Current Smoking | 1144 (14%) | 1515 (14%) |
| Prehypertension / Hypertension | 6333 (78%) | 8283 (75%) |
| Impaired Fasting glucose / Diabetes | 1911 (24%) | 2475 (22%) |
| BMI, kg/m2 | 28.5 (5.1) | 29.9 (6.9) |
| Obesity | 2556 (32%) | 4723 (43%) |
| Cholesterol, mmol/L | 4.87 (0.96) | 5.21 (1.0) |
| HDL, mmol/L | 1.20 (0.36) | 1.49 (0.42) |
| Triglyceride, mmol/L | 1.52 (1.06) | 1.41 (0.85) |
| CRP, mg/L (median, IQR) | 1.6 (2.8) | 2.7 (4.9) |
| Median Framingham 10-year Predicted Coronary Risk, % | 10.9 | 4.3 |
| Median Framingham 10-year Predicted Vascular Risk, % | 21.6 | 8.6 |
| Median Reynolds 10-year Predicted Vascular Risk, % | 10.0 | 2.8 |
The Framingham 10-year Vascular and Coronary disease risk scores were calculated using age, total and HDL cholesterol, current smoking, systolic pressure, and antihypertensive medication use (21, 22). The Reynolds Risk Score, which also includes parental history of myocardial infarction and CRP, was calculated (6, 7).
Statistical Analysis
Prevalence of elevated CRP by sex, ethnicity, region and cardiovascular risk factors were compared among groups using Chi-squared tests. Multivariable logistic regression was used to determine whether sex, ethnicity and regional differences in prevalence of elevated CRP were explained by differences in risk factors related to CRP. Excluding diabetics and those on lipid-lowering medication, values for CRP were used to reclassify Framingham Risk Score groups (very low, low, intermediate, high): for CRP <1 mg/L, participants were reclassified to the next lower risk group; for CRP >3 mg/L they were reclassified to the next higher risk group.
Results
Table 1 shows participant characteristics by sex. Women were more likely to be black and obese and had higher cholesterol and CRP than men, but had a median 10-year predicted vascular disease risk about 60% lower than men, based on the Framingham and Reynolds scores.
Of 19,080 participants without vascular diagnoses, the median CRP was 2.2 mg/L with 25th and 75th percentile values of 0.93 mg/L and 4.9 mg/L, respectively. There were 7568 (40%) participants with elevated CRP defined as >3 mg/L, and 1697 (9%) with CRP >10 mg/L.
Table 2 shows the median CRP and prevalence of elevated CRP (>3 mg/L) by categories of cardiovascular risk factors and the two risk scores. Elevated CRP was more common among women than men and blacks than whites (both p <0.0001). Black women had the highest prevalence of elevated CRP and white men the lowest with these percentages: 61% of 4969 black women, 40% of 6043 white women, 36% of 2884 black men and 27% of 5195 white men.
Table 2.
Prevalence of Elevated C-reactive Protein Concentration by Baseline Characteristics.
| Characteristic (n) | Median (IQR) CRP, mg/L |
Number (%) with CRP >3 mg/L |
Number (%) with CRP >10 mg/L |
|---|---|---|---|
| Age, years | |||
| 45–55 (2853) | 2.1 (4.6) | 1138 (40%)b | 267 (9%)c |
| 55–65 (7869) | 2.3 (4.1) | 3252 (41%) | 735 (9%) |
| 65–96 (8369) | 2.1 (3.7) | 3196 (38%) | 695 (8%) |
| Sex | |||
| Men (8079) | 1.6 (2.8) | 2443 (30%)a | 461 (6%)a |
| Women (11012) | 2.7 (4.9) | 5143 (47%) | 1236 (11%) |
| Race | |||
| Black (7853) | 2.8 (5.1) | 3769 (48%)a | 990 (13%) a |
| White (11238) | 1.8 (3.2) | 3817 (34%) | 707 (6%) |
| Region | |||
| Stroke Belt (10629) | 2.3 (4.2) | 4402 (41%)a | 1004 (9%)b |
| Other States (8462) | 2.0 (3.7) | 3184 (38%) | 693 (8%) |
| Education | |||
| <High School (2006) | 3.1 (5.1) | 1021 (51%)a | 278 (14%)a |
| High School Graduate (4767) | 2.4 (4.5) | 2051 (43%) | 489 (10%) |
| Some College (5233) | 2.3 (4.1) | 2203 (42%) | 482 (9%) |
| College Graduate (7074) | 1.7 (3.2) | 2308 (33%) | 448 (6%) |
| Postmenopausal hormone therapy (women) | |||
| Yes (1599) | 3.3 (5.1) | 840 (53%)a | 197 (12%) |
| No (9413) | 2.6 (4.8) | 4303 (46%) | 1039 (11%) |
| Smoking Status | |||
| Never (9146) | 2.0 (3.8) | 3439 (38%)a | 765 (8%)a |
| Former (7283) | 2.1 (3.7) | 2804 (39%) | 610 (8%) |
| Current (2662) | 3.1 (5.1) | 1343 (50%) | 322 (12%) |
| Blood Pressure | |||
| Normal (<120/80) (7544) | 1.6 (3.1) | 2413 (32%)a | 971 (6%) |
| Prehypertension (120–139/80–90) (1465) | 2.1 (3.6) | 552 (38%) | 115 (8%) |
| Hypertension (≥140/90) (10082) | 2.7 (4.7) | 4621 (46%) | 1111 (11%) |
| Diabetes Status | |||
| None (14698) | 2.0 (3.6) | 5383 (37%)a | 1124 (8%)a |
| Impaired fasting glucose (1050) | 3.1 (5.1) | 534 (51%) | 131 (12%) |
| Diabetes (3343) | 3.0 (5.2) | 1669 (50%) | 442 (13%) |
| Obesity | |||
| None (6231) | 1.3 (2.5) | 1596 (26%)a | 324 (5%)a |
| Overweight (5577) | 1.9 (3.1) | 1898 (34%) | 317 (6%) |
| Obese (7283) | 3.6 (5.6) | 4092 (56%) | 1056 (15%) |
| Cholesterol | |||
| Normal (<5.18 mmol/L) (10836) | 2.0 (3.9) | 4156 (38%)a | 976 (9%) |
| Borderline (5.18–6.19 mmol/L) (5942) | 2.3 (4.0) | 2439 (41%) | 523 (9%) |
| High (≥6.20 mmol/L) (2313) | 2.5 (4.0) | 991 (43%) | 198 (9%) |
| HDL cholesterol | |||
| Normal (>1.04 mmol/L men, >1.30 mmol/L women) (11962) |
1.8 (3.5) | 4237 (35%)a | 889 (7%)a |
| Low (≤1.04 mmol/L men, ≤1.30 mmol/L women) (7129) |
2.7 (4.7) | 3349 (47%) | 808 (11%) |
| Triglycerides | |||
| Normal (<1.70 mmol/L) (13937) | 1.9 (3.8) | 5226 (38%)a | 1210 (9%) |
| Borderline (1.70–2.25 mmol/L) (2678) | 2.7 (4.2) | 1214 (45%) | 258 (10%) |
| High (≥2.26 mmol/L) (2476) | 2.7 (4.3) | 1146 (46%) | 229 (9%) |
| Framingham 10-Year Predicted Coronary Risk | |||
| <0–5% (6951) | 1. 9 (3.7) | 2533 (36%)a | 537 (8%) |
| 5-<10% (4416) | 2.0 (3.6) | 1592 (36%) | 346 (8%) |
| 10-<20% (3137) | 2.2 (3.7) | 1241 (40%) | 270 (9%) |
| >20% (1218) | 2.6 (3.7) | 536 (44%) | 98 (8%) |
| Framingham 10-Year Predicted Vascular Risk | |||
| <0–5% (2996) | 1. 7 (3.3) | 1009 (34%)a | 214 (7%) d |
| 5-<10% (4540) | 2.2 (4.3) | 1849 (41%) | 416 (9%) |
| 10-<20% (6305) | 2.3 (4.0) | 2479 (41%) | 587 (9%) |
| >20% (5239) | 2.3 (3.9) | 2146 (41%) | 480 (9%) |
| Reynolds 10-Year Predicted Vascular Risk | |||
| <0–5% (2453) | 2.1 (3.5) | 2253 (36%)a | 470 (7%) |
| 5-<10% (3172) | 2.0 (3.6) | 1173 (37%) | 263 (8%) |
| 10-<20% (6343) | 1.8 (3.6) | 947 (39%) | 200 (8%) |
| >20% (1031) | 2.7 (4.0) | 489 (47%) | 114 (11%) |
p <0.0001 by ANOVA
p =0.002 by ANOVA
p =0.04
p=0.003
This pattern was similar for prevalence of CRP >10 mg/L. The prevalence of elevated CRP was greater in the stroke belt than the remainder of the U.S. Elevated CRP was also more common in the presence of every cardiovascular risk factor examined. Among risk factors, the highest prevalence of CRP >3 mg/L, 56%, was seen with obesity; 50% of current smokers and 50% of diabetics had CRP >3mg/L. The prevalence of CRP >3 mg/L increased similarly across the four increasing categories of Framingham Scores. Most risk factor associations were similar for CRP >10 mg/L.
Table 3 shows the univariate and multivariable associations of demographic factors, region and risk factors with CRP >3 mg/L. The unadjusted odds of elevated CRP among women compared to men was two-fold increased, was minimally altered after adjustment for sex differences in other factors, and was lower after excluding women taking hormone replacement therapy (adjusted OR 1.7 (95% CI 1.6, 1.9). Blacks were 80% more likely than whites to have CRP >3 mg/L, and this was reduced to a 30% higher likelihood adjusting for other risk factors. There was no significant difference comparing men and women in the odds of elevated CRP by race (p for interaction 0.66). Stroke belt residents had a 10% greater adjusted odds of elevated CRP compared to those living elsewhere. Interpretation of results on differences in CRP by race, sex and region were not altered when model covariates were included as continuous variables; mean adjusted CRP concentration was 49% higher in women, 19% higher in blacks, and 9% higher in the stroke belt. Dividing states into 4 regions (south, midwest, northeast, west), age, sex and race-adjusted CRP was significantly higher in the south compared to each other region (p<0.0001, data not shown), with other pairwise differences not significant. In the multivariable model the largest association of risk factors with elevated CRP was for obesity. Some risk factor associations with elevated CRP differed between men and women. Associations with age, low education and smoking were larger among men and associations of triglycerides and in particular obesity, were larger among women. Among women, associations of risk factors with elevated CRP were similar excluding women using postmenopausal hormones. Associations of risk factors with CRP >10 mg/L were similar (data not shown), although odds ratios were lower for obesity (2.3, 95% CI 2.0–2.6), overweight (1.0, 95% CI 0.8–1.20) and triglyceride (1.0, 95% CI 0.8–1.1)).
Table 3.
Odds Ratios of Elevated CRP (>3 mg/L) by Risk Factors
| Risk Factor | Overall Odds of CRP >3 mg/L | Sex-specific Adjusted ORa of CRP >3 mg/L | |||
|---|---|---|---|---|---|
| Crude OR (95% CI) |
Adjusted ORa (95% CI) |
Men (n=8079) | Women (n=11003) |
Women not on Hormones (n=9413) |
|
| Age | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.2) | 1.2 (1.0, 1.4) | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.2) |
| 55–64 years | |||||
| >65 years | 0.9 (0.9, 1.0) | 1.1 (1.0, 1.2) | 1.5 (1.3, 1.8) | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.1) |
| Sex, women | 2.0 (1.9, 2.1) | 1.9 (1.8, 2.0) | NA | NA | NA |
| Race, black | 1.8 (1.7, 1.9) | 1.3 (1.2, 1.4) | 1.3 (1.2, 1.5) | 1.4 (1.2, 1.5) | 1.4 (1.3, 1.6) |
| Region, belt | 1.2 (1.1, 1.2) | 1.1 (1.1, 1.2) | 1.1 (0.95, 1.2) | 1.2 (1.1, 1.3) | 1.2 (1.1, 1.3) |
| Education | |||||
| < HS | 2.1 (1.9, 2.4) | 1.4 (1.3, 1.6) | 1.8 (1.5, 2.1) | 1.2 (1.1, 1.4) | 1.3 (1.1, 1.5) |
| HS Graduate | 1.6 (1.4, 1.7) | 1.2 (1.1, 1.3) | 1.3 (1.2, 1.5) | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.3) |
| Some College | 1.5 (1.4, 1.6) | 1.2 (1.1, 1.3) | 1.3 (1.2, 1.5) | 1.2 (1.1, 1.3) | 1.2 (1.1, 1.4) |
| Hormone Therapy | NA | NA | NA | 1.7 (1.5, 1.9) | NA |
| Current Smoking | 1.7 (1.5, 1.8) | 1.8 (1.6, 2.0) | 2.3 (2.0, 2.6) | 1.5 (1.3, 1.7) | 1.6 (1.4, 1.8) |
| Blood Pressure | |||||
| Prehypertension | 1.4 (1.3, 1.5) | 1.2 (1.1, 1.3) | 1.2 (1.0, 142) | 1.2 (1.1, 1.4) | 1.2 (1.1, 1.4) |
| Hypertension | 2.1 (1.9, 2.2) | 1.4 (1.3, 1.5) | 1.3 (1.1, 1.5) | 1.4 (1.3, 1.6) | 1.4 (1.3, 1.6) |
| Diabetes Status | |||||
| IFG | 1.8 (1.6, 2.0) | 1.3 (1.2, 1.5) | 1.2 (1.0, 1.5) | 1.4 (1.2, 1.7) | 1.5 (1.3, 1.9) |
| Diabetes | 1.7 (1.6 ,1.9) | 1.1 (1.0, 1.2) | 1.1 (0.9, 1.2) | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.3) |
| Weight | |||||
| Overweight | 1.5 (1.4, 1.6) | 1.4 (1.3, 1.6) | 1.2 (1.0, 1.3) | 1.7 (1.5, 1.9) | 1.6 (1.5, 1.9) |
| Obese | 3.7 (3.5, 4.0) | 3.0 (2.8, 3.3) | 2.4 (2.1, 2.7) | 3.7 (3.3, 4.1) | 3.8 (3.4, 4.3) |
| Cholesterol >6.20 mmol/L | 1.2 (1.1, 1.3) | 1.1 (1.0, 1.2) | 1.2 (1.0, 1.4) | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.3) |
| HDL ≤1.04 mmol/L men, ≤1.30 mmol/L women |
1.6 (1.5, 1.7) | 1.3 (1.2, 1.4) | 1.3 (1.2, 1.5) | 1.3 (1.2, 1.5) | 1.5 (1.3, 1.6) |
| Triglyceride ≥2.26 mmol/L | 1.4 (1.3, 1.5) | 1.2 (1.0, 1.3) | 01.0 (0.9, 1.1) | 1.3 (1.1, 1.5) | 1.2 (1.0, 1.4) |
adjusted models include all variables in the table
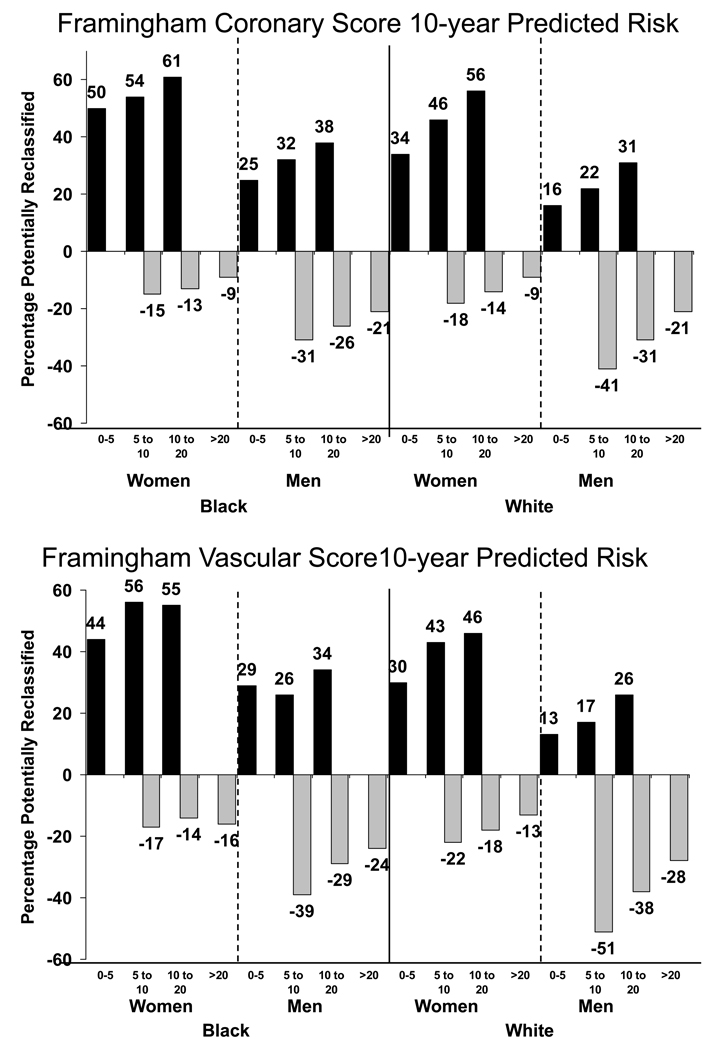
Figure 1 shows the percentage of non-diabetic participants in each Framingham score category that would be potentially reclassified into a different risk group if CRP was measured after Framingham risk assessment. Analyses excluded those using lipid lowering medication. Blacks and women were more likely than whites or men to be potentially reclassified to a higher risk category. For example, considering the Framingham Coronary Score, 61% of black women and 38% of black men at 10-<20% predicted risk would be potentially reclassified to higher risk based on CRP >3 mg/L, compared to 56% of white women and 31% of white men. For both races, men were more likely than women to be reclassified to a lower level of risk based on CRP <1 mg/L, and these sex differences were larger for the Vascular Risk Score than the Coronary Risk Score.
Figure 1.
Percentage with potential for risk reclassification based on CRP testing among non-diabetics not taking lipid-lowering medications. Black bars show those with CRP >3 mg/L (black bars) and gray bars, those with CRP <1 mg/L (gray bars). Data are shown for the Coronary (top panel) and Vascular (bottom panel) Framingham Scores. It was not considered possible to be reclassified to a lower level of predicted risk for those in the 0–5% category or to to a higher level of risk for those in the >20% category, so no gray and black bars are shown in these categories.
Table 4 shows the distributions of the two Framingham Risk Scores, the Reynolds Risk Score and the two CRP-reclassified Framingham Scores. Analyses excluded diabetics and those using lipid lowering medication. Among women the Reynolds Score resulted in a much higher proportion at 0-<5% risk and a lower proportion at 5-<10% or 10-<20% predicted risk than the two Framingham Scores or CRP reclassified scores; 92% of women had a 10-year risk <10% with the Reynolds Score. CRP reclassification of the Framingham Vascular Score resulted in far more women at higher levels of risk than the other scores, with 44% at >10% risk. Among men, the Reynolds Score categorized far fewer men at high risk than the Framingham Vascular Score, which placed 43% of men at >20% risk. CRP reclassification of the Framingham Vascular Score resulted in a fairly similar distribution of risk as the Framingham Vascular Score without CRP, with more men at 0-<5% risk. The CRP reclassified Framingham Coronary Score categorized roughly equal numbers of men into the four risk categories.
Table 4.
Ten-year Predicted Risk by Three Different Risk Scores among 8,309 Non-diabetics not Taking Lipid-Lowering Medication.
| 10-year Predicted Risk |
Framingham Coronary Risk Score |
CRP Reclassification of Framingham Coronary Risk Score |
Framingham Vascular Risk Score |
CRP Reclassification of Framingham Vascular Risk Score |
Reynolds Risk Score |
|---|---|---|---|---|---|
| Women (n=4767) | |||||
| 0 to <5% | 3151 (66%) | 2132 (48%) | 1565 (33%) | 1413 (30%) | 3562 (75%) |
| 5 to <10% | 1104 (23%) | 1640 (34%) | 1662 (35%) | 1272 (27%) | 798 (17%) |
| 10 to <20% | 412 (9%) | 669 (14%) | 1346 (28%) | 1223 (26%) | 318 (7%) |
| >20% | 100 (2%) | 326 (7%) | 194 (4%) | 838 (18%) | 89 (2%) |
| Men (n=3542) | |||||
| 0 to <5% | 551 (16%) | 902 (25%) | 89 (3%) | 372 (11%) | 662 (17%) |
| 5 to <10% | 1179 (33%) | 920 (26%) | 586 (17%) | 695 (20%) | 1132 (32%) |
| 10 to <20% | 1234 (35%) | 882 (25%) | 1357 (38%) | 1006 (28%) | 1165 (33%) |
| >20% | 578 (16%) | 838 (24%) | 1510 (43%) | 1469 (42%) | 583 (16%) |
Table 5 shows individual level reclassification of risk by the Reynolds Risk Score of the Framingham Vascular Score. Reclassification of the Framingham Vascular Risk Score by the Reynolds Score rarely reclassified a woman or man to a higher risk group (<2% of the time). Among women at 10–20% Framingham Vascular predicted risk the Reynolds score reclassified 82% downward and only 2% upward. Among women with a Framingham Vascular risk above 20%, 68% were reclassified downward. Overall, the Reynolds Score reclassified 2739/4767 (57%) of women to a different risk group than the Framingham Vascular Score, but reclassified 2736/3202 (85%) of women with Framingham Vascular predicted risk >5%. Among men with a Framingham Vascular predicted risk of 10–20%, 71% were reclassified downward and only 1% upward. Among high risk men 62% were reclassified downward. Overall, the Reynolds Score reclassified 2356/3542 (67%) of men to a different risk group than Framingham, all with a Framingham predicted risk >5%. For comparison, the Reynolds Score reclassification of the Framingham Coronary Score is shown in web table 1, where more individuals were moved to higher levels of risk than for the Vascular Score.
Table 5.
Reclassification of the Framingham Vascular Risk Score by the Reynolds Vascular Risk Score in 4,767 Non-Diabetic Women and 3,542 Nondiabetic Men not Taking Lipid Lowering Medication.
| Framingham Vascular Risk Score Category |
Reynolds Vascular Risk Score Category | |||
|---|---|---|---|---|
| Lower | Same | Higher | Total | |
| Women | ||||
| <5% | - | 1562 (>99%) | 3 (<1%) | 1565 |
| 5–10% | 1448 (87%) | 188 (11%) | 26 (2%) | 1662 |
| 10–20% | 1105 (82%) | 216 (16%) | 25 (2%) | 1346 |
| >20% | 132 (68%) | 62 (32%) | - | 194 |
| Men | ||||
| <5% | - | 89 (100%) | 0 | 89 |
| 5–10% | 426 (73%) | 152 (26%) | 8 (1%) | 586 |
| 10–20% | 963 (71%) | 378 (28%) | 16 (1%) | 1357 |
| >20% | 943 (62%) | 567 (38%) | - | 1510 |
Discussion
In this large study of geographically dispersed white and black men and women, elevated CRP was more common among women than men, blacks than whites, and in the stroke belt compared to the rest of the U.S. These differences were not accounted for by other factors strongly related to CRP. Reclassification of Framingham Coronary or Vascular predicted risk by CRP testing suggested that well over half of participants would have their risk reclassified with addition of CRP testing. Movement to higher risk categories was greatest among women and blacks. Using the Reynolds Risk Score to reclassify the Framingham Vascular Risk Score, reclassified less people, and almost exclusively reclassified men and women to a lower risk level.
In this study among over 19,000 participants, 48% of blacks and 34% of whites had CRP >3 mg/L. While some of this difference was accounted for by differences in vascular risk factors, blacks were 30% more likely to have elevated CRP after confounder adjustment. The data add to growing evidence on ethnic variation in CRP, and supports a hypothesis that higher inflammation might underlie differences in vascular risk by ethnicity. One study including 1,086 non-white women, reported higher CRP in blacks than whites and Hispanics (who had levels similar to whites), while Asian women had much lower levels (15). Some, but not all of the difference in CRP among these groups was explained by differences in prevalence of obesity. In a study of 1,250 Canadian adults, much but not all of the difference in CRP concentrations among ethnic groups was explained by other metabolic risk factors, with higher CRP in Aboriginals and South Asians, and lower CRP in Chinese than whites (16). Among 1940 men participating in the National Health and Nutrition Examination Survey (NHANES) 1999–2000, there was no difference in CRP by race among whites, blacks and Mexican-Americans (13). Among 1,912 women in NHANES 1999–2000, Mexican-Americans had higher CRP than whites, but blacks had similar values (14). In the 15,341 NHANES III 1988–1994 participants, utilizing a low-sensitivity CRP assay, CRP was higher in blacks but whether this difference was explained by differences in other CRP correlates was not studied (17). In 6,814 participants of the Multi-Ethnic Study of Atherosclerosis (MESA), CRP was lower in Chinese and higher in Hispanics and blacks compared to whites (18). In two reports from the Study of Women’s Health Across the Nation, among 2,834 premenopausal women, differences in CRP by ethnicity were partly explained by dietary factors, cardiac risk factors and physical activity level (23, 24).
In this study 47% of women had CRP >3 mg/L, and women had nearly twice the odds of elevated CRP than men. This was despite the much lower Framingham-predicted risk of heart disease in women than men. In NHANES 1999–2000, 31% of women had CRP 3–10 mg/L and accounting for other risk factors, women were 2.6-fold more likely than men to have CRP 3–10 mg/L (14). In NHANES III, women were also 2.1-fold more likely to have CRP >3 mg/L (using a low-sensitivity assay), independent of other factors (17). The MESA investigators also reported higher CRP among women after accounting for differences in other risk factor levels, with 45% of women and 25% of men having CRP >3 mg/L (18). Although higher CRP predicts vascular risk in population samples of women (25), results have been variable (4) and the high prevalence of elevated CRP in women suggests clinical utility may be lower in women than men (4) if CRP values are not taken in context of other risk factors by using a risk score (6). Further follow up in the large REGARDS cohort will help clarify these questions.
To our knowledge, this is the first report of CRP concentration in stroke belt residents. This regional difference was not explained by differences in traditional stroke risk factors including hypertension, diabetes, and smoking, which are more prevalent in the stroke belt (12). In a previous REGARDS report, geographic differences in stroke risk factors were predicted to explain less than ¼ of the increased stroke risk in that region (12). Along with the findings here, results suggest that the higher risk of death from stroke in this region may be associated, in part with non-traditional risk factors such as CRP.
CRP testing is being adopted in clinical practice for risk assessment for prescription of lipid-lowering treatment to prevent first cardiovascular events (26). Information used to develop CRP cutpoints for clinical practice was derived from selected populations (1). If CRP were used according to current guidelines (1) and the new Framingham Vascular Risk Score applied (to predict overall vascular risk), in non-diabetic REGARDS women with a Framingham Vascular predicted risk of 5–20%, 48% would be potentially reclassified as higher risk and 19% as lower risk. Among men, these percentages were 24% and 40%, respectively. Blacks would more often be reclassified to a higher risk category than whites. More people would move to a higher risk group considering the Framingham Coronary Score, perhaps because the Vascular Score classifies more people at high risk. While follow up cardiovascular events are needed to determine validity of these reclassifications in this population sample, the current data raise important questions about the generalizability of standard CRP cutpoints among ethnic and gender groups and across geographic region.
The Reynolds Risk Score includes assessment of traditional vascular risk factors, family history and CRP (6, 7), and like the new Framingham Vascular Score, predicts overall vascular outcomes. Applying the Reynolds Score in non-diabetic REGARDS women not using lipid lowering medication, 85% of 3,202 women with a 10-year predicted risk >5% by Framingham would be reclassified to a different level of predicted risk with the Reynolds Score, with <2% moving to a higher risk group. This pattern differs from the Women’s Health Study, the large clinical trial population where the Reynolds Score was developed and validated (6). In that study more women were reclassified to higher risk levels; the Reynolds Score was compared to standard risk scoring for coronary disease, and the sample size was smaller, which may explain the difference. Among REGARDS men, 67% of 3,542 men with a 10-year predicted risk >5% by Framingham were reclassified to a different level with the Reynolds Score, with only 24 men reclassified upward. In the Physicians Health Study II, where the Reynolds Score for men was developed and compared to standard coronary risk scoring, only 20% of 6,884 men with a traditional predicted risk 5–20% were reclassified, with 12% reclassified downward and 8% upward (7). It is important to note that the Reynolds model was validated to predict MI, stroke, coronary revascularization and cardiovascular mortality, while the Framingham Vascular Score included these outcomes and the partly overlapping endpoints of angina, coronary insufficiency, transient ischemic attack, peripheral artery disease, and heart failure. Thus, some observed reclassification here might relate to differing predicted risks based on this fact. Data for the Framingham coronary score, which predicts angina, recognized and unrecognized MI, coronary insufficiency and coronary death is shown in the web table 1. The implications of this risk reclassification in REGARDS, a cohort with different characteristics the Women’s Health Study and Physicians Health Study II (more ethnically diverse, lower socioeconomic status and not clinical trial participants), require further evaluation by assessing vascular outcomes in REGARDS.
Limitations of our study merit discussion. Importantly, we did not include prediction of vascular events. The different risk algorithms evaluated may not be comparable for reasons other than CRP inclusion because they predict different endpoints. Importantly, for the reclassification of risk based on Framingham Score, we were not able to incorporate CRP into a model that would allow re-weighting of all the risk factors since we did not assess outcomes. Data on family history of heart disease was not yet coded for all subjects at the time of this analysis so the Reynolds Score could not be calculated. These participants had a similar distribution of Framingham Score as those with complete data. Strengths of this analysis include generalizability and large sample size. We included 7,847 black participants and 11,003 women, while most previous data focused on men or primarily white populations. The cohort was geographically dispersed so that geography could be studied. This also improves confidence in the generalizability of our findings compared to field-center based studies on which most previous data are based.
In conclusion, in this large population-based sample of white and black men and women there was a higher prevalence of elevated CRP than has been observed in most other studies. Women, blacks and those living in the stroke belt had a higher prevalence of elevated CRP. All studied vascular risk factors and lower socioeconomic status were associated with elevated CRP, but these did not explain the observed differences by sex, race and region. If CRP testing was included in vascular risk prediction algorithms in this population to alter lipid prescription (26), larger numbers of people than previously reported, especially blacks and women, would be reclassified to a different level of predicted vascular risk. Follow up of this cohort for clinical outcomes will determine the significance of these findings.
Supplementary Material
Acknowledgments
Authors acknowledge participating investigators: University of Alabama at Birmingham: Libby Wagner, Virginia Wadley, Rodney Go, Monika Safford, Ella Temple, Margaret Stewart; University of Vermont: Rebekah Boyle; Wake Forest University: Ron Prineas; Alabama Neurological Institute: Camilo Gomez; University of Arkansas: LeaVonne Pulley; University of Cincinnati: Brett Kissela; Examination Management Services, Incorporated: Andra Graham; National Institute of Neurological Disorders and Stroke: Claudia Moy.
Research Funding
This project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, U.S. Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Nonstandard Abbreviations
- REGARDS
REasons for Geographic And Racial Differences in Stroke
- FRS
Framingham Risk Score
- NHANES
National Health and Nutrition Examination Survey
- MESA
Multi-Ethnic Study of Atherosclerosis.
Contributor Information
Mary Cushman, Departments of Medicine and Pathology, University of Vermont, Burlington, VT.
Leslie A. McClure, Department of Biostatistics, University of Alabama at Birmingham, Birmingham, AL.
Virginia J. Howard, Department of Epidemiology, University of Alabama at Birmingham, Birmingham, AL.
Nancy S. Jenny, Department of Pathology, University of Vermont.
Susan G. Lakoski, Department of Internal Medicine/Cardiology, University of Texas Southwestern Medical Center, Dallas, TX.
George Howard, Department of Biostatistics, University of Alabama at Birmingham, Birmingham, AL.
References
- 1.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. 2003;108:2292–2297. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109:1955–1959. doi: 10.1161/01.CIR.0000125690.80303.A8. [DOI] [PubMed] [Google Scholar]
- 4.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 5.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145:21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2251. doi: 10.1161/CIRCULATIONAHA.108.814251. 4p following 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard G. Why do we have a stroke belt in the southeastern United States? A review of unlikely and uninvestigated potential causes. Am J Med Sci. 1999;317:160–167. doi: 10.1097/00000441-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Howard G, Anderson R, Sorlie P, Andrews V, Backlund E, Burke GL. Ethnic differences in stroke mortality between non-Hispanic whites, Hispanic whites, and blacks. The National Longitudinal Mortality Study. Stroke. 1994;25:2120–2125. doi: 10.1161/01.str.25.11.2120. [DOI] [PubMed] [Google Scholar]
- 10.Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–421. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 11.Gillum RF, Ingram DD. Relation between residence in the southeast region of the United States and stroke incidence. The NHANES I Epidemiologic Followup Study. Am J Epidemiol. 1996;144:665–673. doi: 10.1093/oxfordjournals.aje.a008979. [DOI] [PubMed] [Google Scholar]
- 12.Cushman M, Cantrell RA, McClure LA, Howard G, Prineas RJ, Moy CS, et al. Estimated 10-year stroke risk by region and race in the United States: geographic and racial differences in stroke risk. Ann Neurol. 2008;64:507–513. doi: 10.1002/ana.21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford ES, Giles WH, Myers GL, Mannino DM. Population distribution of high-sensitivity C-reactive protein among US men: findings from National Health and Nutrition Examination Survey 1999–2000. Clin Chem. 2003;49:686–690. doi: 10.1373/49.4.686. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Giles WH, Mokdad AH, Myers GL. Distribution and correlates of C-reactive protein concentrations among adult US women. Clin Chem. 2004;50:574–581. doi: 10.1373/clinchem.2003.027359. [DOI] [PubMed] [Google Scholar]
- 15.Albert MA, Glynn RJ, Buring J, Ridker PM. C-Reactive protein levels among women of various ethnic groups living in the United States (from the Women's Health Study) Am J Cardiol. 2004;93:1238–1242. doi: 10.1016/j.amjcard.2004.01.067. [DOI] [PubMed] [Google Scholar]
- 16.Anand SS, Razak F, Yi Q, Davis B, Jacobs R, Vuksan V, et al. C-reactive protein as a screening test for cardiovascular risk in a multiethnic population. Arterioscler Thromb Vasc Biol. 2004;24:1509–1515. doi: 10.1161/01.ATV.0000135845.95890.4e. [DOI] [PubMed] [Google Scholar]
- 17.Miller M, Zhan M, Havas S. High attributable risk of elevated C-reactive protein level to conventional coronary heart disease risk factors: the third national health and nutrition examination survey. Arch Intern Med. 2005;165:2063–2068. doi: 10.1001/archinte.165.18.2063. [DOI] [PubMed] [Google Scholar]
- 18.Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D'Agostino RB, Jr, Herrington DM. Gender and C-reactive protein: Data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152:593–598. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The REasons for Geographic And Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 20.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 22.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 23.Matthews KA, Sowers MF, Derby CA, Stein E, Miracle-McMahill H, Crawford SL, Pasternak RC. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women's Health Across the Nation (SWAN) Am Heart J. 2005;149:1066–1073. doi: 10.1016/j.ahj.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, Matthews KA, Johnston J, Sowers MR, et al. Ethnic differences in C-reactive protein concentrations. Clin Chem. 2008;54:1027–1037. doi: 10.1373/clinchem.2007.098996. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.