Abstract
The discovery and characterization of activation-induced cytidine deaminase (AID) 10 years ago provided the basis for a mechanistic understanding of secondary antibody diversification and the subsequent generation and maintenance of cellular memory in B lymphocytes, which signified a major advance in the field of B cell immunology. Here we celebrate and review the triumphs in the mission to understand the mechanisms through which AID influences antibody diversification, as well as the implications of AID function on human physiology. We also take time to point out important ongoing controversies and outstanding questions in the field and highlight key experiments and techniques that hold the potential to elucidate the remaining mysteries surrounding this vital protein.
A fundamental question in biology is that of how an organism, or more simply a population of cells, is able to respond to an almost infinite and unknown array of environmental stimuli given only a limited genome. This problem arises in a variety of systems in biology. Neuronal connections generate a stable network that is able to maintain information but dynamic enough to learn new information; pathogens display an ever-changing pattern of coat proteins on their surface to evade recognition by host immune systems; and finally, the focus of this review, B lymphocytes have evolved mechanisms to produce a repertoire of antibodies diverse enough to respond to the vast number of possible foreign antigens.
Over 50 years ago Frank MacFarlane Burnet, with no experimental evidence, hypothesized the existence of a randomization process that would result in the alteration and variation of immunoglobulin molecules1. At that time the only biological precedent for such a process was Lederberg’s study on mutation in phage adaptation2. The first experimental evidence that such a process does indeed occur came with the demonstration that immunization alters the amino acid sequence of immunoglobulin-λ light chains by introducing single–amino acid changes3–5. Half a decade later, after the advent of recombinant DNA technology, it was shown that in addition to mutation, a somatic gene-rearrangement event assembles functional immunoglobulin genes from individual gene segments6. Together these two discoveries began the movement to characterize the molecular basis of this process, which corresponded closely with Burnet’s original hypothesis of randomization7.
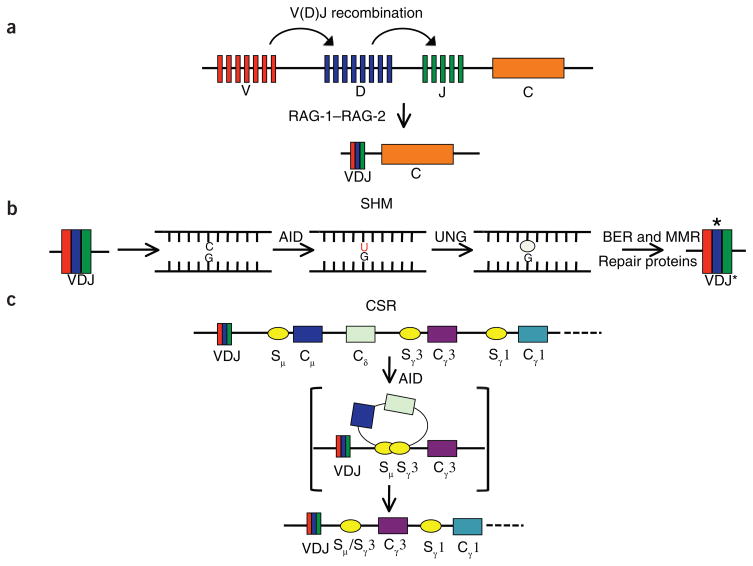
Today there is a far better understanding of the mechanisms involved in immunoglobulin gene diversification. Recombination of variable (V), diversity (D) and joining (J) gene segments generates the primary repertoire of antibodies in an antigen-independent manner8–10 (Fig. 1a). Later, the encounter of a B cell with its cognate antigen initiates secondary diversification processes at the immunoglobulin loci; these processes include somatic hypermutation (SHM; Fig. 1b), immunoglobulin gene conversion (GCV) and class-switch recombination (CSR; Fig. 1c). SHM and GCV increase the variability of the antigen-binding domain of the immunoglobulin, and CSR alters immunoglobulin effector function by switching the constant regions of the immunoglobulin heavy chain. As GCV is very similar to SHM in terms of the role of AID (and thus far has only been reported for birds and rabbits), we will mostly focus on SHM; however, almost all findings should be applicable to both processes.
Figure 1.
Antibody diversification. (a) A deletional recombination event between individual V, D and J segments creates the variable region of the immunoglobulin gene. This process is catalyzed by the RAG-1–RAG-2 recombinase complex and occurs in an antigen-independent way. C, constant. (b) SHM, the first of two secondary antibody-diversification processes, results in the accumulation of point mutations in the recombined variable region. AID initiates this process through the deamination of cytidine to uridine, followed by removal of the uracil base by UNG and repair by several base-excision repair (BER) and mismatch-repair (MMR) enzymes. The asterisk indicates the rearranged, mutated variable region. (c) CSR completes the secondary antibody diversification and results in the exchange of the default constant region, Cμ (IgM), for one of many downstream regions (Cγ3 (IgG3) is presented here). AID is thought to initiate this process through deamination of bases in the switch (S) region (yellow circles) upstream of each constant region, resulting in the formation of DSBs and recombination.
Because SHM and CSR are very different processes—SHM induces the accumulation of point mutations, whereas CSR induces double-strand breaks (DSBs) and genomic recombination—it was astonishing when AID was identified as the key participant in both reactions (Fig. 1b,c). Like the discovery of the RAG-1–RAG-2 recombinase complex8,9, the discovery of AID was the seminal finding that gave rise to all subsequent major advances toward understanding the molecular mechanisms involved in secondary immunoglobulin diversification. Although there is still much to learn, molecular immunologists have begun to rapidly uncover the molecular foundation that supports Burnet’s theory of immunoglobulin gene randomization. Here we focus on the advances that have been made in AID biology since its discovery 10 years ago. We will focus mainly on the AID protein itself and less on SHM and CSR. The latter have been reviewed elsewhere11–13.
Discovery and characterization of AID
The discovery of AID and the elucidation of its mechanism were greatly facilitated by the generation of the B lymphocyte cell line CH12F3, which was selected to inducibly undergo CSR at a high frequency. Theorizing that a specific recombinase was responsible for CSR, Muramatsu and Honjo applied a PCR-based ‘subtraction method’ to screen genes upregulated after stimulation of CH12F3 cells, ultimately identifying AID14. The subsequent generation of AID-deficient mice, which exhibit a striking inability to undergo CSR and SHM, confirmed that AID is a key participant in both processes15. At the same time, AID was identified as the molecule responsible for a subset of human hyper–immunoglobulin M (IgM) syndrome, which results in a complete loss of CSR (and thus an overproduction of the IgM isotype) and SHM16.
Initial investigations revealed that AID has a cytidine-deaminase domain with homology to a known RNA deaminase, APOBEC1 (apolipoprotein B mRNA–editing catalytic polypeptide 1)14. Thus, it was initially hypothesized that AID edits mRNA, a model now referred to as the ‘RNA-editing hypothesis’. To account for the involvement of AID in both SHM and CSR, it was imagined that AID targets either distinct mRNAs (a DNA mutator in the context of SHM and a DNA recombinase in the context of CSR) or a single mRNA that functions in both SHM and CSR, with the likely involvement of task-specific cofactors.
At the core of the alternative ‘DNA-editing hypothesis’ is the concept that AID mutates DNA directly. This model provides substance to earlier theories proposed by Peters and Storb17, Scharff and Edelmann18 and Neuberger19, who all postulated the existence of a mutating factor that is directly targeted to the immunoglobulin locus. A number of later studies demonstrated that ectopic expression of AID is able to induce mutations in the genome of a number of mammalian cell types (plasma cells, HEK293T cells and NIH3T3 cells)20,21, yeast22,23 and even bacteria24. As it is unlikely that AID would edit the same mRNA in prokaryotic and eukaryotic cells to generate a novel DNA mutator, the simplest interpretation of these data is that AID is a true DNA mutator.
Although it is straightforward, that interpretation is complicated by the fact that expression of APOBEC1, the prototypical mRNA editor, also gives rise to DNA mutations in bacteria25; by analogy, AID may act on a specific mRNA as well. Analysis of the enzymatic properties of purified AID has shown deaminase activity on DNA but not on RNA substrates26–30. However, AID can bind both DNA and RNA28,31 and, like APOBEC1, may require a (unknown) specific mRNA substrate. Overall, given that in vitro, AID ‘prefers’ to deaminate WRC motifs (where ‘W’ is adenosine or thymidine; ‘R’ is purine; and ‘C’ is cytidine)32,33, a ‘preference’ that coincides well with SHM ‘hot spots’ observed in vivo34, and that AID seems to be bound to immunoglobulin loci in vivo35,36, the present evidence is in favor of the DNA-editing model.
Robust experimental support for the DNA-editing hypothesis also emerged from studies focused on an important intermediate of the SHM reaction in this model: U-G mismatches. Mice deficient in uracil DNA glycosylase (UNG), the main glycosylase that removes uracil from DNA in the context of base-excision repair, mutate their immunoglobulin loci at rates identical to those of their wild-type littermates, but the spectra of mutations that accumulate are very different37. Mutations at G and C are strongly biased toward C-to-T and G-to-A mutations as a result of direct replication of such mismatches; however, mutations at A and T bases are similar in both Ung−/− and wild-type mice. In addition, CSR is substantially lower in the absence of UNG37–39.
More recently, variants of AID and UNG shown to be catalytically inactive in vitro have been found to display a surprising activity in vivo, restoring CSR in AID- and UNG-deficient activated B cells, respectively40,41. Although these data are intriguing, concerns have arisen regarding whether the mutants determined to be catalytically inactive in vitro were in fact inactive in vivo. Overall, although a number of reports question the DNA-editing hypothesis, there is still no direct evidence in support of the RNA-editing model, as no AID-edited mRNA transcript has been reported.
Transcription of AID
AID was originally described as a B cell–specific factor unique to activated germinal center B cells. In this setting, AID expression is induced by signals that induce CSR in naive B cells (lipopolysaccharide, interleukin 4, CD40 ligation, transforming growth factor-β and so on) and SHM in human lymphoma lines (IgM-CD19-CD21 crosslinking)42. Using large-scale noncoding sequence-homology predictions43 combined with histone H3–acetylation patterns44 and limited ‘promoter-bashing’ experiments45, a number of labs have identified four regions of the AID locus (Aicda; called ‘AID’ here) associated with transcriptional regulation. First, there is a 1.6-kilobase AID promoter43,45, which lacks a TATA box and includes sites for the transcription factors NF-κB, STAT6, HoxC4, Sp1 and Pax5 (refs. 43,45–47). In addition, there exists a putative regulatory region in the first intron that encompasses E-box motifs48, a 3′ enhancer element43,44 and a set of uncharacterized elements upstream of the locus43. How these cis-acting elements (and the trans-acting factors that bind them) operate remains to be elucidated by additional in vivo experiments.
Recently, AID expression has been reported to be sensitive to estrogen signaling, both in B cells and in estrogen-responsive tissues such as breast and ovary49. Because only mRNA was quantified, it remains to be seen whether AID protein is produced in response to estrogen. Furthermore, AID expression has been observed in a number of tissues under a variety of stimulation conditions associated with cellular transformation49,50. Even though in all of these cases AID expression is lower than it is in germinal center B cells, expression of AID is mutagenic and thus represents a threat to genomic stability.
Post-transcriptional regulation of AID expression
The microRNA miR-155, previously shown to have a role in the proper activation of B lymphocytes, directly regulates AID protein quantities in response to activating stimuli51,52. After being activated, B cells in animals with a disruption of the miR-155 target site in the 3′ untranslated region of AID express substantially more AID protein, which results in more CSR in vitro and in vivo and in temporally deregulated AID expression. Together these results suggest that miR-155 has a role in switching off AID expression in post–germinal center B cells51. Additionally, in the absence of miR-155-mediated control, excess AID protein leads to mutation on non-target genes, including the antiapoptotic gene Bcl6 (ref. 51); importantly, the occurrence of Myc-Igh translocations, a known side effect of aberrant AID activity, is 15-fold higher in the absence of miR-155 (ref. 52). Finally, miR-181 family members have also been reported to be differently modulated during CSR51,53 and to have an effect on AID abundance53. However, the in vivo importance of miR181 family members in AID expression remains to be determined. As the 3′ untranslated region of AID mRNA contains several AU-rich elements (S.D.F., unpublished data), mRNA-binding proteins that recognize such elements might also be important in the regulation of AID expression by influencing either the stability or translation of AID mRNA.
Post-translational modification of AID
Post-translational modifications of proteins are commonly involved in the regulation of protein activity and also act to provide diversity to protein function54. Thus far, much of the focus on AID regulation by post-translational modification has been in the realm of phosphorylation, specifically phosphorylation of serine 38 (Ser38), which is thought to be carried out by the cAMP-dependent kinase PKA (protein kinase A)55. ‘Knock-in’ mice expressing AID with an S38A substitution have 70% less CSR and SHM56,57. Evidence suggests that the specific localization of PKA to the immunoglobulin locus results in the phosphorylation of Ser38 of AID at the switch regions during CSR, a modification shown to be essential for the recruitment of the AID cofactor RPA (replication protein A)36. The formation of the complex of PKA, phosphorylated AID and RPA at the immunoglobulin locus has a positive effect on CSR, as inactivation of PKA hinders CSR34. However, the Ser38-phosphorylation event has also been observed after expression of AID in unstimulated 3T3 fibroblasts and 293T cells56,58, which raises the question of how specific this mode of regulation is for CSR and SHM in B cells.
Three additional phosphorylation sites have been documented on AID isolated from splenic B cells: Thr27, Thr140 and Tyr184. Thus far, the role of these phosphorylation sites is unclear. A T140A ‘knock-in’ mouse exhibits 30% less CSR and 50% less SHM56, but the mechanism by which Thr140 phosphorylation promotes CSR and SHM is unknown. It is possible that the phosphorylation of different residues, or of a different combination of residues, plays a part in distinguishing between AID activity during SHM and CSR. This will prove to be an interesting focus for future pursuits.
The study of post-translational modification of AID has expanded to ubiquitination. Nuclear AID has been shown to be polyubiquitinated after treatment of cells with a proteasome inhibitor59, which suggests that proteasomal degradation of nuclear AID limits its half-life in the nucleus. However, the pattern of ubiquitination observed in these studies is also consistent with multiple monoubiquitination events59. The description of a newly identified E3 ubiquitin ligase that can monoubiquitinate AID60 is interesting in this context. It is possible that both monoubiquitination and polyubiquitination can occur, as is the case with the tumor suppressor p53 and its regulator MDM2 (ref. 61), and it will be interesting to elucidate the effect of ubiquitination on AID activity and cellular function. The interdependence of phosphorylation events themselves, as well as phosphorylation events in combination with other post-translational modifications, will probably prove to be insightful.
AID cellular localization
There is an increasing body of evidence that suggests that the subcellular localization of AID is tightly controlled to limit the amount of enzyme in the nucleus. AID, although mainly cytosolic, has been shown to shuttle between the nucleus and cytoplasm. The mechanism by which AID is exported from the nucleus is fairly well characterized; export is sensitive to the export receptor CRM1 inhibitor leptomycin B62,63 and requires the final ten amino acids of AID (residues 188–198)62–64. Interestingly, substitution of the AID carboxy-terminal nuclear-export sequence also results in less CSR but maintains AID mutational activity62,63, a phenotype that can also be seen with common carboxy-terminal deletions found in human patients65. The potential function of the AID carboxyl terminus in CSR is discussed below.
Less is understood about how AID localizes to the nucleus for SHM and CSR. Ta et al. posited the existence of a weak nuclear-localization sequence encompassing the first 20 amino acids of AID66; however, others have observed that deletion of this sequence does not result in nuclear exclusion after expression of AID in 293T cells64. A subsequent report has suggested that nuclear import is active and is governed by a conformational nuclear-localization sequence dependent on the proper formation of multimers of AID67, which emphasizes the need for more work focused on understanding the structure and formation of oligomers of AID.
Finally, it is important to note that all investigations into the localization of AID have used cell lines with ectopic expression of AID. It is formally possible that the localization patterns observed thus far are partly due to overexpression of the protein in a heterologous system. Visualization of physiological expression of AID in activated B cells might yield a different picture.
Targeting AID to the immunoglobulin locus
One of the most interesting unanswered questions in the field of AID regulation is the question of how AID-mediated mutation is targeted specifically to the immunoglobulin locus. Despite its ability to deaminate any transcribed substrate in vitro27,28,32,68,69 and to act on selected highly transcribed genes when overexpressed20,70,71, AID targets the immunoglobulin loci almost exclusively in its physiological context. Non-immunoglobulin gene-mutation events are found only after numerous rounds of mutation in B cells from Peyer’s patches in vivo72.
In addition to being targeted to immunoglobulin loci, AID shows a distinct local distribution in the immunoglobulin locus. During SHM, AID acts solely at the variable region (composed of the VDJ or VJ exon), but during CSR its activity is restricted to the switch regions. Although this local distribution of mutation at immunoglobulin loci has been studied for decades, mechanistic insight is still lacking. Below, we discuss the prevailing hypotheses and their supporting evidence.
Local positioning
Even before the discovery of AID, mutations that occur during SHM were found to have a distinct pattern, with a very strong 5′ boundary at the promoter and a less abrupt 3′ boundary about 1 kilobase downstream of the promoter73. Similarly, CSR-associated mutations start to appear just downstream of the sterile intron-region (Iμ) promoter, as well as the downstream switch-region promoters11. This suggested that the mutation is associated with the onset of transcription at the immunoglobulin locus. Confirming that theory, experiments have shown that promoter deletion results in less mutation74; movement of the promoter upstream of its original position results in an identical shift in the distribution of the mutations75; duplication of the promoter involving the placement of a copy 5′ of the constant region ‘recruits’ mutation to this part of the locus17; and insertion of exogenous DNA 3′ of the promoter and 5′ of the remaining gene acts as a buffer that protects the gene from mutation76. These observations led to the hypothesis that the mutating factor (AID) is loaded on the transcription-initiation complex, follows RNA polymerase II as elongation proceeds, and dissociates stochastically to create the decaying pattern of mutation observed17,77. In fact, AID is able to bind RNA polymerase II (ref. 35) and has been shown to be intimately associated with the transcription complex29. In addition, observations of B cells activated to undergo CSR in vitro have shown that the density of RNA polymerase II complexes correlates with the frequency of mutation in the surrounding switch regions78.
Cis-acting targeting elements
Although the promoter is necessary for transcription and hence is required for SHM and CSR, the importance of distinct promoter elements for the recruitment of AID is not well understood. Early studies in mice suggested that any promoter is sufficient74,79, but subsequent studies in the DT40 chicken B cell line have found that replacement of the endogenous Igl promoter with the promoter of the human gene encoding elongation factor 1-α supports high transcription but results in less Igl mutation80. Therefore, it is likely that the nature of the promoter region is important to provide both a platform for loading AID onto the transcription complex and binding sites for AID-targeting factors. In addition, the promoter may provide cell cycle specificity, which is known to be important—for reasons that are not yet clear—for CSR81. Little is known about the role of cell cycle in SHM.
The search for cis-acting regulatory sequences in and near the immunoglobulin locus began with the use of mouse ‘passenger’ transgenes and, later, knockout animals, with a focus on known transcriptional control elements82–86. The consensus that emerged from these studies was that the known transcriptional enhancers are not essential for the recruitment of AID but are necessary for CSR and SHM, as they provide a high rate of transcription and promote efficient long-range genomic interactions87. Subsequently, two labs have identified additional locus-specific elements that seem to be both necessary and sufficient for AID recruitment. First, a systematic deletion study of the Igl locus in the DT40 chicken B cell line has identified a previously unknown regulatory element downstream of the known Igl enhancer (the 3′ regulatory region)88. The element contains a transcriptional enhancer, as its deletion abolished Igl expression; however, the addition of an SV40 enhancer was able to restore transcription but not SHM and GCV. Thus, it seems that this region also contains an element that ‘recruits’ AID-mediated mutation and whose function can be separated from a role in transcription. Similar observations were generated by the use of a constitutive promoter to overcome the effects of transcription in deletion of non-coding regions from the Igl locus in DT40 cells89. These experiments identified a targeting element identical to the 3′ regulatory region (the ‘diversification activator’ DIVAC), which was shown to promote SHM when integrated into a reporter transgene encoding green fluorescent protein independently of its genomic location.
Work still needs to be done to determine the minimal sequence responsible for the ‘recruitment’ of AID activity and the mechanism by which this recruitment works. For example, is AID directly recruited to this sequence or does it require another recruitment factor? How does the 3′ regulatory region act to support the loading of AID onto a promoter that is more than 6 kilobases upstream? Can this work in a chicken B cell line ‘translate’ into mammalian cells? As there is no clear sequence homology between the 3′ regulatory region and any mammalian immunoglobulin locus88, it is possible that the structure of this region is more important than its nucleotide sequence.
AID targeting factors
It has been hypothesized that AID is targeted to the immunoglobulin locus by a ‘targeting cofactor’ that acts as the bridge between transcription and AID-mediated mutation17. This cofactor complex must therefore be able to distinguish between the highly transcribed immunoglobulin locus and other highly transcribed regions, probably by three-dimensional interactions with immunoglobulin-specific cis-acting targeting elements. Despite great efforts, no cofactor able to target AID to the immunoglobulin locus has been identified thus far. There is, however, evidence that the presence of the basic helix-loop-helix transcription factor E2A, or similar factors that recognize E-box motifs, is correlated with AID-mediated mutation. Mouse transgenes containing multiple consensus E-box motifs have more SHM90, and non-immunoglobulin gene targets of AID frequently contain such motifs72. Similarly, inactivation of the gene encoding E2A in DT40 cells results in less SHM and GCV91. However, direct proof that E2A (or a complex containing E2A) recruits AID to immunoglobulin loci in vivo is lacking. Finally, it is important to remember that locus-specific elements may function not in recruiting AID itself but instead in targeting the immunoglobulin locus for error-prone repair72. Evidence suggests that AID induces widespread genomic breakage during CSR, and different repair mechanisms, rather than AID targeting, provide genomic specificity to the CSR reaction (unpublished data).
AID cofactors
Although not much is known about the factors that target AID to the locus, recent work has focused on a general search for AID-interacting proteins and their possible functions in SHM and CSR. The AID-interacting partner most studied, RPA, a ubiquitous general single-stranded DNA–binding protein, was originally thought to bind single-stranded DNA near SHM ‘hot spots’ in the transcription ‘bubble’, thereby facilitating the access of AID to double-stranded DNA92,93. On the basis of that hypothesis, RPA would be dispensable for CSR, as RNA-DNA hybrids (R loops) in the switch regions create a stable single-stranded DNA substrate for AID. Recently, however, experiments have shown that RPA is recruited to switch regions during CSR, which raises the question of what the function of RPA really is36. Because it has been observed that an AID mutant (S38A) unable to bind RPA is still recruited to switch regions during CSR, it does not seem that RPA promotes the binding of AID to DNA36. Thus, it is possible that RPA has a role in enhancing the activity of AID or is involved in events ‘downstream’ of the initial AID-mediated deamination event (such as the recruitment of repair factors). For example, in yeast, RPA accumulates at DSBs to recruit telomerase and form new telomeres when the necessary TG repeats are lacking94. It is also formally possible that the accumulation of RPA at the immunoglobulin locus facilitates relocalization of the immunoglobulin locus in the nucleus. At this point, we cannot rule out the possibility that RPA has different functions during SHM and CSR.
AID has also been shown to interact with the spliceosome-associated factor CTNNBL1 (ref. 95), which provided the first mechanistic connection between AID and the 10-year-old observation that splicing of the immunoglobulin transcript is necessary for CSR96. Disruption of the CTNNBL1-AID interaction by a specific mutation in AID results in less GCV in DT40 cells as well as less CSR in primary B cells stimulated in vitro. Furthermore, CTNNBL1-deficient DT40 cells show much less SHM and GCV; however, a specific role for CTNNBL1 in vivo awaits the generation of a B cell–specific knockout model. Emphasizing the role of splicing in AID-mediated reactions is recent work showing that depletion of the THO-TREX complex, in the context of AID expression in the yeast Saccharomyces cerevisiae, boosts mutation to near the rates in germinal center B cells97. THO-TREX functions at the interface among transcription, splicing and mRNA export in yeast97 and vertebrate cells98. Overall, these experiments hint at a central role for the coincident processes of transcription, splicing and mRNA export in CSR.
AID function in the context of the nucleus
AID is generally thought to be targeted to the immunoglobulin locus, but the reverse is equally valid and potentially revealing. It is possible that the immunoglobulin locus is targeted to a mutational and/or recombinational ‘factory’ that includes AID, error-prone polymerases and other necessary enzymes. Reports indicate that nuclear architecture and sub-compartment organization are important regulators of transcription99; moreover, observations in S. cerevisiae suggest that nuclear architecture is just as important for the repair of damaged DNA. Here, DSBs are sequestered to specific locations in the nuclear periphery and this movement is instrumental in preventing aberrant DNA repair.
In yeast, DSBs and telomeres localize to the nuclear periphery by a mechanism that is dependent on the inner nuclear membrane protein Mps3 (refs. 100–102). This mechanism confines ‘hard-to-repair’ breaks—which could potentially yield harmful translocations—to a restricted area, which allows alternative repair pathways to become active. The discovery of a Sumo-dependent ubiquitin ligase heterodimer of Slx5-Slx8 that localizes to nuclear pores and binds DSBs may help provide insight into the mechanism by which repair pathways are selected to repair these breaks103 (Fig. 2). A ‘hub’ can be envisioned in the nuclear periphery where the telomere-proximal, broken Igh locus, the transcription, splicing and mRNA-export machinery, and AID in complex with its interacting partners may be temporarily sequestered. Alternative repair pathways active in such a hub could contribute to the error-prone repair of AID-created uracil residues, with error-free repair occurring at different sites in the nucleus. There are hints that the AID carboxy-terminal nuclear-export sequence may be the signal for this sequestration, as the respective mutants are unable to undergo CSR. Furthermore, a heterologous nuclear-export sequence, although able to restore cellular trafficking of AID, is not sufficient to restore CSR104. Finally, elements of the locus (for example, the 3′ enhancers) are probably important for nuclear positioning and proper repair during CSR. In bacterial artificial chromosome–transgenic B cells, CSR occurs almost exclusively at the transgenic locus as long as the enhancers are present; however, in the absence of these elements, repair is distinctly aberrant105. At present this is a completely speculative model. However, high-resolution, high-sensitivity microscopy studies of the subnuclear, three-dimensional localization of AID and key regions of the immunoglobulin locus should help provide insight into the mechanisms that facilitate targeted mutation and recombination.
Figure 2.
Specific localization to the nuclear periphery may be important for DSB repair. Left, persistent DSBs in S. cerevisiae are tethered to the nuclear pore complex (NPC) and are sequestered from the rest of the genome. This localization may require the integral inner nuclear membrane protein Mps3 and is dependent on the kinases Mec1 and Tel1 (ATM and ATR in humans). These interactions are thought to then shuttle the DSBs to a complex containing the pore protein Nup84 and the SUMO ligase Slx5-Slx8 (RNF4 in humans). In yeast, these interactions are thought to facilitate an alternative pathway of repair that enhances gene conversion through template-switch recombination. Right, evidence regarding the importance of the AID carboxy-terminal nuclear-export signal (NES) in CSR suggests that similar sequestration to the nuclear pore may occur and should be studied. It is possible that this is promoted by binding of a cofactor to AID or even by the formation of an RNA-containing complex. This model is completely speculative but emphasizes the importance of a foray into high-resolution microscopy to understand the nuclear localization of AID and the immunoglobulin locus. An understanding of both could provide insight into the mechanism of CSR and also provide an assay for the identification of other proteins or cellular factors necessary for CSR.
Conclusions
It has been a decade since the discovery of AID and already the field has made great strides toward understanding its role in secondary antibody diversification. However, as in all young fields, there are pieces missing from the puzzle. Which signaling pathways are involved in controlling AID expression, and how these signals are integrated by the AID promoter and enhancers, are still not understood. Exposure of the layer of post-transcriptional regulation of AID is only just beginning, and better understanding of the influence of post-translational modification on AID function is needed. Finally, additional work is needed to understand the influence of dynamic relocalization of AID in the cytoplasm and nucleus. Over the next decade, progress should be made in all of these areas to further the understanding of the role of AID in antibody diversification.
Acknowledgments
Supported by the Department of Defense (R.K.D.), the US National Institutes of Health (CA098495 for AID-related work in the F.N.P. laboratory) and the Intramural Program of the National Institute on Aging of the National Institutes of Health (S.D.F.).
Footnotes
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Burnet FM. A modification of Jerne’s theory of antibody production using the concept of clonal selection. CA Cancer J Clin. 1976;26:119–121. doi: 10.3322/canjclin.26.2.119. [DOI] [PubMed] [Google Scholar]
- 2.Lederberg J. Genes and antibodies. Science. 1959;129:1649–1653. doi: 10.1126/science.129.3364.1649. [DOI] [PubMed] [Google Scholar]
- 3.Weigert MG, Cesari IM, Yonkovich SJ, Cohn M. Variability in the λ light chain sequences of mouse antibody. Nature. 1970;228:1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- 4.Crews S, Griffin J, Huang H, Calame K, Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981;25:59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- 5.Selsing E, Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981;25:47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- 6.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 7.Neuberger MS. Antibody diversification by somatic mutation: from Burnet onwards. Immunol Cell Biol. 2008;86:124–132. doi: 10.1038/sj.icb.7100160. [DOI] [PubMed] [Google Scholar]
- 8.Schatz DG, Oettinger MA, Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989;59:1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- 9.Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- 10.Gellert MV. (D)J recombination: RAG proteins, repair factors, and regulation. Annu Rev Biochem. 2002;71:101–132. doi: 10.1146/annurev.biochem.71.090501.150203. [DOI] [PubMed] [Google Scholar]
- 11.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Storb U, et al. Targeting of AID to immunoglobulin genes. Adv Exp Med Biol. 2007;596:83–91. doi: 10.1007/0-387-46530-8_8. [DOI] [PubMed] [Google Scholar]
- 13.Teng G, Papavasiliou FN. Immunoglobulin somatic hypermutation. Annu Rev Genet. 2007;41:107–120. doi: 10.1146/annurev.genet.41.110306.130340. [DOI] [PubMed] [Google Scholar]
- 14.Muramatsu M, et al. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J Biol Chem. 1999;274:18470–18476. doi: 10.1074/jbc.274.26.18470. Here, Honjo and colleagues report the cloning and initial characterization of AID. [DOI] [PubMed] [Google Scholar]
- 15.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. This report describes the astonishing finding that AID-deficient animals lack both CSR and SHM; thus, AID is central to both secondary antibody-diversification reactions. [DOI] [PubMed] [Google Scholar]
- 16.Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. Here, Durandy and colleagues demonstrate that a cohort of patients with hyper-IgM syndrome (who are deficient in CSR and SHM) have mutations in AID. Together with reference 13, this confirms the importance of AID in antibody diversification and disease. [DOI] [PubMed] [Google Scholar]
- 17.Peters A, Storb U. Somatic hypermutation of immunoglobulin genes is linked to transcription initiation. Immunity. 1996;4:57–65. doi: 10.1016/s1074-7613(00)80298-8. [DOI] [PubMed] [Google Scholar]
- 18.Wiesendanger M, Scharff MD, Edelmann W. Somatic hypermutation, transcription, and DNA mismatch repair. Cell. 1998;94:415–418. doi: 10.1016/s0092-8674(00)81581-0. [DOI] [PubMed] [Google Scholar]
- 19.Neuberger MS, et al. Monitoring and interpreting the intrinsic features of somatic hypermutation. Immunol Rev. 1998;162:107–116. doi: 10.1111/j.1600-065x.1998.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 20.Martin A, Scharff MD. Somatic hypermutation of the AID transgene in B and non-B cells. Proc Natl Acad Sci USA. 2002;99:12304–12308. doi: 10.1073/pnas.192442899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshikawa K, et al. AID enzyme-induced hypermutation in an actively transcribed gene in fibroblasts. Science. 2002;296:2033–2036. doi: 10.1126/science.1071556. [DOI] [PubMed] [Google Scholar]
- 22.Mayorov VI, et al. Expression of human AID in yeast induces mutations in context similar to the context of somatic hypermutation at G-C pairs in immunoglobulin genes. BMC Immunol. 2005;6:10. doi: 10.1186/1471-2172-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poltoratsky VP, Wilson SH, Kunkel TA, Pavlov YI. Recombinogenic phenotype of human activation-induced cytosine deaminase. J Immunol. 2004;172:4308–4313. doi: 10.4049/jimmunol.172.7.4308. [DOI] [PubMed] [Google Scholar]
- 24.Petersen-Mahrt SK, Harris RS, Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature. 2002;418:99–103. doi: 10.1038/nature00862. In this paper, Neuberger and colleagues demonstrate that ectopic overexpression of AID in bacteria increases the rate of mutation, which suggests that AID targets DNA. [DOI] [PubMed] [Google Scholar]
- 25.Harris RS, Petersen-Mahrt SK, Neuberger MS. RNA editing enzyme APOBEC1 and some of its homologs can act as DNA mutators. Mol Cell. 2002;10:1247–1253. doi: 10.1016/s1097-2765(02)00742-6. [DOI] [PubMed] [Google Scholar]
- 26.Bransteitter R, Pham P, Scharff MD, Goodman MF. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc Natl Acad Sci USA. 2003;100:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhuri J, et al. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 28.Dickerson SK, Market E, Besmer E, Papavasiliou FN. AID mediates hyper-mutation by deaminating single stranded DNA. J Exp Med. 2003;197:1291–1296. doi: 10.1084/jem.20030481. Together, references 26–28 show that AID is an active DNA deaminase that uses single-stranded DNA as its substrate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besmer E, Market E, Papavasiliou FN. The transcription elongation complex directs activation-induced cytidine deaminase-mediated DNA deamination. Mol Cell Biol. 2006;26:4378–4385. doi: 10.1128/MCB.02375-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen HM, et al. The activation-induced cytidine deaminase (AID) efficiently targets DNA in nucleosomes but only during transcription. J Exp Med. 2009;206:1057–1071. doi: 10.1084/jem.20082678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nonaka T, et al. Carboxy-terminal domain of AID required for its mRNA complex formation in vivo. Proc Natl Acad Sci USA. 2009;106:2747–2751. doi: 10.1073/pnas.0812957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pham P, Bransteitter R, Petruska J, Goodman MF. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature. 2003;424:103–107. doi: 10.1038/nature01760. [DOI] [PubMed] [Google Scholar]
- 33.Yu K, Huang FT, Lieber MR. DNA substrate length and surrounding sequence affect the activation-induced deaminase activity at cytidine. J Biol Chem. 2004;279:6496–6500. doi: 10.1074/jbc.M311616200. [DOI] [PubMed] [Google Scholar]
- 34.Rogozin IB, Diaz M. Cutting edge: DGYW/WRCH is a better predictor of mutability at G:C bases in Ig hypermutation than the widely accepted RGYW/WRCY motif and probably reflects a two-step activation-induced cytidine deaminase-triggered process. J Immunol. 2004;172:3382–3384. doi: 10.4049/jimmunol.172.6.3382. [DOI] [PubMed] [Google Scholar]
- 35.Nambu Y, et al. Transcription-coupled events associating with immunoglobulin switch region chromatin. Science. 2003;302:2137–2140. doi: 10.1126/science.1092481. [DOI] [PubMed] [Google Scholar]
- 36.Vuong B, et al. Specific recruitment of protein kinase A to the immunoglobulin locus regulates class-switch recombination. Nat Immunol. 2009;10:420–426. doi: 10.1038/ni.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rada C, et al. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr Biol. 2002;12:1748–1755. doi: 10.1016/s0960-9822(02)01215-0. [DOI] [PubMed] [Google Scholar]
- 38.Imai K, et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol. 2003;4:1023–1028. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 39.Durandy A, Revy P, Fischer A. Human models of inherited immunoglobulin class switch recombination and somatic hypermutation defects (hyper-IgM syndromes) Adv Immunol. 2004;82:295–330. doi: 10.1016/S0065-2776(04)82007-8. [DOI] [PubMed] [Google Scholar]
- 40.Begum NA, et al. Further evidence for involvement of a noncanonical function of uracil DNA glycosylase in class switch recombination. Proc Natl Acad Sci USA. 2009;106:2752–2757. doi: 10.1073/pnas.0813252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shivarov V, Shinkura R, Honjo T. Dissociation of in vitro DNA deamination activity and physiological functions of AID mutants. Proc Natl Acad Sci USA. 2008;105:15866–15871. doi: 10.1073/pnas.0806641105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faili A, et al. AID-dependent somatic hypermutation occurs as a DNA single-strand event in the BL2 cell line. Nat Immunol. 2002;3:815–821. doi: 10.1038/ni826. [DOI] [PubMed] [Google Scholar]
- 43.Yadav A, et al. Identification of a ubiquitously active promoter of the murine activation-induced cytidine deaminase (AICDA) gene. Mol Immunol. 2006;43:529–541. doi: 10.1016/j.molimm.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Crouch EE, et al. Regulation of AID expression in the immune response. J Exp Med. 2007;204:1145–1156. doi: 10.1084/jem.20061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonda H, et al. The balance between Pax5 and Id2 activities is the key to AID gene expression. J Exp Med. 2003;198:1427–1437. doi: 10.1084/jem.20030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dedeoglu F, Horwitz B, Chaudhuri J, Alt FW, Geha RS. Induction of activation-induced cytidine deaminase gene expression by IL-4 and CD40 ligation is dependent on STAT6 and NFκB. Int Immunol. 2004;16:395–404. doi: 10.1093/intimm/dxh042. [DOI] [PubMed] [Google Scholar]
- 47.Park S, et al. HoxC4 binds to the promoter of the cytidine deaminase AID gene to induce AID expression, class-switch DNA recombination and somatic hypermutation. Nat Immunol. 2009;10:540–550. doi: 10.1038/ni.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sayegh CE, Quong MW, Agata Y, Murre C. E-proteins directly regulate expression of activation-induced deaminase in mature B cells. Nat Immunol. 2003;4:586–593. doi: 10.1038/ni923. [DOI] [PubMed] [Google Scholar]
- 49.Pauklin S, Sernandez I, Bachmann G, Ramiro A, Petersen-Mahrt S. Estrogen directly activates AID transcription and function. J Exp Med. 2009;206:99–111. doi: 10.1084/jem.20080521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gourzi P, Leonova T, Papavasiliou FN. A role for activation-induced cytidine deaminase in the host response against a transforming retrovirus. Immunity. 2006;24:779–786. doi: 10.1016/j.immuni.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Teng G, et al. MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity. 2008;28:621–629. doi: 10.1016/j.immuni.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dorsett Y, et al. MicroRNA-155 suppresses activation-induced cytidine deaminase-mediated Myc-Igh translocation. Immunity. 2008;28:630–638. doi: 10.1016/j.immuni.2008.04.002. Together, references 51 and 52 show that a specific mutation of a miR-155 target site present in the 3′ untranslated region of AID leads to deregulation of AID protein in transgenic or gene-targeted mice; therefore, miR155 directly regulates the amount of AID protein in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Yebenes V, et al. miR-181b negatively regulates activation-induced cytidine deaminase in B cells. J Exp Med. 2008;205:2199–2206. doi: 10.1084/jem.20080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hunter T. The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol Cell. 2007;28:730–738. doi: 10.1016/j.molcel.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 55.Basu U, Chaudhuri J, Phan RT, Datta A, Alt FW. Regulation of activation induced deaminase via phosphorylation. Adv Exp Med Biol. 2007;596:129–137. doi: 10.1007/0-387-46530-8_11. [DOI] [PubMed] [Google Scholar]
- 56.Mcbride K, et al. Regulation of class switch recombination and somatic mutation by AID phosphorylation. J Exp Med. 2008;205:2585–2594. doi: 10.1084/jem.20081319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng H, et al. Integrity of the AID serine-38 phosphorylation site is critical for class switch recombination and somatic hypermutation in mice. Proc Natl Acad Sci USA. 2009;106:2717–2722. doi: 10.1073/pnas.0812304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mcbride K, et al. Regulation of hypermutation by activation-induced cytidine deaminase phosphorylation. Proc Natl Acad Sci USA. 2006;103:8798–8803. doi: 10.1073/pnas.0603272103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aoufouchi S, et al. Proteasomal degradation restricts the nuclear lifespan of AID. J Exp Med. 2008;205:1357–1368. doi: 10.1084/jem.20070950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delker R, Papavasiliou F. Elucidating the mechanism of specificity achieved by activation-induced cytidine deaminase. Keystone Meeting; Taos, NM. 2009. [Google Scholar]
- 61.Li M, et al. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science. 2003;302:1972–1975. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- 62.Ito S, et al. Activation-induced cytidine deaminase shuttles between nucleus and cytoplasm like apolipoprotein B mRNA editing catalytic polypeptide 1. Proc Natl Acad Sci USA. 2004;101:1975–1980. doi: 10.1073/pnas.0307335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mcbride K. Somatic hypermutation is limited by CRM1-dependent nuclear export of activation-induced deaminase. J Exp Med. 2004;199:1235–1244. doi: 10.1084/jem.20040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brar S, Watson M, Diaz M. Activation-induced cytosine deaminase (AID) is actively exported out of the nucleus but retained by the induction of DNA breaks. J Biol Chem. 2004;279:26395–26401. doi: 10.1074/jbc.M403503200. [DOI] [PubMed] [Google Scholar]
- 65.Durandy A, Revy P, Imai K, Fischer A. Hyper-immunoglobulin M syndromes caused by intrinsic B-lymphocyte defects. Immunol Rev. 2005;203:67–79. doi: 10.1111/j.0105-2896.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 66.Ta VT, et al. AID mutant analyses indicate requirement for class-switch-specific cofactors. Nat Immunol. 2003;4:843–848. doi: 10.1038/ni964. [DOI] [PubMed] [Google Scholar]
- 67.Patenaude A, et al. Active nuclear import and cytoplasmic retention of activation-induced deaminase. Nat Struct Mol Biol. 2009;16:517–527. doi: 10.1038/nsmb.1598. [DOI] [PubMed] [Google Scholar]
- 68.Ramiro AR, Stavropoulos P, Jankovic M, Nussenzweig MC. Transcription enhances AID-mediated cytidine deamination by exposing single-stranded DNA on the nontemplate strand. Nat Immunol. 2003;4:452–456. doi: 10.1038/ni920. [DOI] [PubMed] [Google Scholar]
- 69.Shen HM, Storb U. Activation-induced cytidine deaminase (AID) can target both DNA strands when the DNA is supercoiled. Proc Natl Acad Sci USA. 2004;101:12997–13002. doi: 10.1073/pnas.0404974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okazaki IM, et al. Constitutive expression of AID leads to tumorigenesis. J Exp Med. 2003;197:1173–1181. doi: 10.1084/jem.20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshikawa K. AID Enzyme-induced hypermutation in an actively transcribed gene in fibroblasts. Science. 2002;296:2033–2036. doi: 10.1126/science.1071556. [DOI] [PubMed] [Google Scholar]
- 72.Liu M, et al. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451:841–845. doi: 10.1038/nature06547. [DOI] [PubMed] [Google Scholar]
- 73.Lebecque SG, Gearhart PJ. Boundaries of somatic mutation in rearranged immunoglobulin genes: 5′ boundary is near the promoter, and 3′ boundary is approximately 1 kb from V(D)J gene. J Exp Med. 1990;172:1717–1727. doi: 10.1084/jem.172.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fukita Y, Jacobs H, Rajewsky K. Somatic hypermutation in the heavy chain locus correlates with transcription. Immunity. 1998;9:105–114. doi: 10.1016/s1074-7613(00)80592-0. [DOI] [PubMed] [Google Scholar]
- 75.Tumas-Brundage K, Manser T. The transcriptional promoter regulates hypermutation of the antibody heavy chain locus. J Exp Med. 1997;185:239–250. doi: 10.1084/jem.185.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winter DB, Sattar N, Mai JJ, Gearhart PJ. Insertion of 2 kb of bacteriophage DNA between an immunoglobulin promoter and leader exon stops somatic hypermutation in a kappa transgene. Mol Immunol. 1997;34:359–366. doi: 10.1016/s0161-5890(97)00073-4. [DOI] [PubMed] [Google Scholar]
- 77.Rada C, Milstein C. The intrinsic hypermutability of antibody heavy and light chain genes decays exponentially. EMBO J. 2001;20:4570–4576. doi: 10.1093/emboj/20.16.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rajagopal D, et al. Immunoglobulin switch mu sequence causes RNA polymerase II accumulation and reduces dA hypermutation. J Exp Med. 2009;206:1237–1244. doi: 10.1084/jem.20082514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shen HM, Peters A, Kao D, Storb U. The 3′ Igκ enhancer contains RNA polymerase II promoters: implications for endogenous and transgenic kappa gene expression. Int Immunol. 2001;13:665–674. doi: 10.1093/intimm/13.5.665. [DOI] [PubMed] [Google Scholar]
- 80.Yang S, Fugmann S, Schatz D. Control of gene conversion and somatic hyper-mutation by immunoglobulin promoter and enhancer sequences. J Exp Med. 2006;203:2919–2928. doi: 10.1084/jem.20061835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang S, Schatz D. Targeting of AID-mediated sequence diversification by cis-acting determinants. Adv Immunol. 2007;94:109–125. doi: 10.1016/S0065-2776(06)94004-8. [DOI] [PubMed] [Google Scholar]
- 82.Odegard VH, Schatz DG. Targeting of somatic hypermutation. Nat Rev Immunol. 2006;6:573–583. doi: 10.1038/nri1896. [DOI] [PubMed] [Google Scholar]
- 83.Goyenechea B, et al. Cells strongly expressing Igκ transgenes show clonal recruitment of hypermutation: a role for both MAR and the enhancers. EMBO J. 1997;16:3987–3994. doi: 10.1093/emboj/16.13.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Betz AG, et al. Elements regulating somatic hypermutation of an immunoglobulin κ gene: critical role for the intron enhancer/matrix attachment region. Cell. 1994;77:239–248. doi: 10.1016/0092-8674(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 85.Perlot T, Alt F, Bassing CH, Suh H, Pinaud E. Elucidation of IgH intronic enhancer functions via germ-line deletion. Proc Natl Acad Sci USA. 2005;102:14362–14367. doi: 10.1073/pnas.0507090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Inlay MA, et al. Roles of the Ig κ light chain intronic and 3′ enhancers in Igk somatic hypermutation. J Immunol. 2006;177:1146–1151. doi: 10.4049/jimmunol.177.2.1146. [DOI] [PubMed] [Google Scholar]
- 87.Wuerffel R, et al. S-S synapsis during class switch recombination is promoted by distantly located transcriptional elements and activation-induced deaminase. Immunity. 2007;27:711–722. doi: 10.1016/j.immuni.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kothapalli N, Norton DD, Fugmann SD. Cutting edge: a cis-acting DNA element targets AID-mediated sequence diversification to the chicken Ig light chain gene locus. J Immunol. 2008;180:2019–2023. doi: 10.4049/jimmunol.180.4.2019. [DOI] [PubMed] [Google Scholar]
- 89.Blagodatski A, et al. A cis-acting diversification activator both necessary and sufficient for AID-mediated hypermutation. PLoS Genet. 2009;5:e1000332. doi: 10.1371/journal.pgen.1000332. References 88 and 89 identify a mutational enhancer at the chicken Igl locus that is distinct from known enhancers and can confer mutability to heterologous genes; this is the first demonstration that an AID-catalyzed mutation is specifically ‘recruited’ to the immunoglobulin locus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Michael N, et al. The E box motif CAGGTG enhances somatic hypermutation without enhancing transcription. Immunity. 2003;19:235–242. doi: 10.1016/s1074-7613(03)00204-8. [DOI] [PubMed] [Google Scholar]
- 91.Schoetz U, Cervelli M, Wang YD, Fiedler P, Buerstedde JM. E2A expression stimulates Ig hypermutation. J Immunol. 2006;177:395–400. doi: 10.4049/jimmunol.177.1.395. [DOI] [PubMed] [Google Scholar]
- 92.Basu U, et al. The AID antibody diversification enzyme is regulated by protein kinase A phosphorylation. Nature. 2005;438:508–511. doi: 10.1038/nature04255. [DOI] [PubMed] [Google Scholar]
- 93.Chaudhuri J, Khuong C, Alt F. Replication protein A interacts with AID to promote deamination of somatic hypermutation targets. Nature. 2004;430:992–998. doi: 10.1038/nature02821. References 93 and 94 offer the first demonstration of a specific post-translational modification of AID (Ser38 phosphorylation), as well as the identification of the first putative AID cofactor (RPA) whose interaction depends on this modification. [DOI] [PubMed] [Google Scholar]
- 94.Schramke V, et al. RPA regulates telomerase action by providing Est1p access to chromosome ends. Nat Genet. 2004;36:46–54. doi: 10.1038/ng1284. [DOI] [PubMed] [Google Scholar]
- 95.Conticello S, et al. Interaction between antibody-diversification enzyme AID and spliceosome-associated factor CTNNBL1. Mol Cell. 2008;31:474–484. doi: 10.1016/j.molcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 96.Hein K, et al. Processing of switch transcripts is required for targeting of antibody class switch recombination. J Exp Med. 1998;188:2369–2374. doi: 10.1084/jem.188.12.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gomez-Gonzalez B, Aguilera A. Activation-induced cytidine deaminase action is strongly stimulated by mutations of the THO complex. Proc Natl Acad Sci USA. 2007;104:8409–8414. doi: 10.1073/pnas.0702836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X, Manley JL. Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell. 2005;122:365–378. doi: 10.1016/j.cell.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 99.Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- 100.Gartenberg MR. Life on the edge: telomeres and persistent DNA breaks converge at the nuclear periphery. Genes Dev. 2009;23:1027–1031. doi: 10.1101/gad.1805309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oza P, Jaspersen SL, Miele A, Dekker J, Peterson CL. Mechanisms that regulate localization of a DNA double-strand break to the nuclear periphery. Genes Dev. 2009;23:912–927. doi: 10.1101/gad.1782209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schober H, Ferreira H, Kalck V, Gehlen LR, Gasser SM. Yeast telomerase and the SUN domain protein Mps3 anchor telomeres and repress subtelomeric recombination. Genes Dev. 2009;23:928–938. doi: 10.1101/gad.1787509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nagai S, et al. Functional targeting of DNA damage to a nuclear pore-associated SUMO-dependent ubiquitin ligase. Science. 2008;322:597–602. doi: 10.1126/science.1162790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Geisberger R, Rada C, Neuberger M. The stability of AID and its function in class-switching are critically sensitive to the identity of its nuclear-export sequence. Proc Natl Acad Sci USA. 2009;106:6736–6741. doi: 10.1073/pnas.0810808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dunnick W, et al. Switch Recombination and somatic hypermutation are controlled by the heavy chain 3′ enhancer region. J Exp Med. doi: 10.1084/jem.20091280. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]