Summary
Studies have identified interferon-inducible Ifi202 gene as a lupus susceptibility gene (encoding p202 protein) in mouse models of lupus disease. However, signaling pathways that regulate the Ifi202 expression in cells remain to be elucidated. We found that steady-state levels of Ifi202 mRNA and protein were high in mouse embryonic fibroblasts (MEFs) from E2F1-knockout (E2F1-/-) and E2F1 and E2F2 double knockout (E2F1-/- E2F2-/-) mice than isogenic wild type MEFs. Moreover, overexpression of E2F1 in mouse fibroblasts decreased expression of p202. Furthermore, expression of E2F1, but not E2F4, transcription factor in mouse fibroblasts repressed the activity of 202-luc-reporter in promoter-reporter assays. Interestingly, the E2F1-mediated transcriptional repression of the 202-luc-reporter was independent of p53 and pRb expression. However, the repression was dependent on the ability of E2F1 to bind DNA. We have identified a potential E2F DNA-binding site in the 5′-regulatory region of the Ifi202 gene and mutations in this E2F DNA-binding site reduced the E2F1-mediated transcriptional repression of 202-luc-reporter. Because p202 inhibits the E2F1-mediated transcriptional activation of genes, we compared the expression of E2F1 and its target genes in splenic cells from lupus-prone B6.Nba2 congenic mice, which express increased levels of p202, with age-matched C57BL/6 mice. We found that increased expression of Ifi202 in the congenic mice was associated with inhibition of E2F1-mediated transcription and decreased expression of E2F1 and its target genes that encode pro-apoptotic proteins. Our observations support for the idea that increased Ifi202 expression in certain strain of mice contributes to lupus susceptibility in part by inhibiting E2F1-mediated functions.
Systemic lupus erythematosus (SLE) is an autoimmune disease that predominantly affects women of childbearing age (1-3). The disease is characterized by the production of pathogenic autoantibodies, particularly IgG antibodies, to nuclear antigens and development of lupus nephritis (1-3). Based on genetic studies in human SLE patients and in mouse models of SLE, there is considerable evidence that SLE is a polygenic disease (1, 4, 5). Interestingly, studies have revealed that peripheral blood mononuclear cells from lupus patients exhibit interferon (IFN) signature: expression levels of mRNAs that are encoded by the IFN-inducible genes are up-regulated (6). Consistent with a role for IFN-inducible genes in human lupus patients, generation of congenic mice, such as B6.Nba2 (7) and B10.Yaa.Bxs2/3 (8), coupled with gene expression analyses, has identified the IFN-inducible Ifi202 gene as a lupus susceptibility gene. Furthermore, a study has revealed that increased expression of Ifi202 in spleen and kidney cells of MRL/lpr mice is associated with development of lupus disease (9). Although, these studies have suggested that increased expression of Ifi202 in certain strains of mice is associated with the development of lupus or lupus-like disease, signaling pathways that regulate expression of the Ifi202 in immune cells remain to be elucidated. Moreover, it remains unclear how increased expression of Ifi202 in certain strains of mice contributes to lupus susceptibility.
The E2F family of transcription factors comprises at least six structurally-related E2Fs (E2F1-6) (10-12). Based on their ability to complex with the pocket family proteins (pRb, p107 and p130) and their expression patterns, these transcription factors have been grouped into three groups. The E2F1, 2, and 3 have been grouped together whereas E2F4 and 5 are grouped together. The E2F6 is grouped separately. These E2F factors heterodimerize with members of the DP family (DP1 and DP2) (10) of proteins to form an active transcription factor. The E2F and DP protein heterodimer is kept transcriptionally silent and acts as a repressor by binding to a pocket protein. Cyclin/Cdk-mediated phosphorylation of pocket proteins results in the release of “free” E2F/DP dimer, allowing transcriptional activation of E2F target genes (10-12). Transcriptional activation of genes by E2F in response to mitogenic stimulation and the identification of E2F DNA-binding sites in a number of genes critical to the regulation of DNA synthesis implicate E2F regulation as an important step in mammalian cell cycle progression (10-12). Consistent with this idea, the E2F-family of transcription factors contribute to the regulation of G1-to S-phase transition of T and B cells in response to mitogenic stimulus (11, 12). Importantly, cell growth-inhibitory cytokines, such as interferons (IFNs), inhibit cell cycle progression, in part, by inhibiting the E2F-mediated transcription of genes (13, 14).
Interestingly, the E2F1 transcription factor auto regulates expression of the E2F1 gene (15) and the lack of E2F1 expression is associated with defects apoptosis of cells (16). The E2F1 transcriptional target genes encode proteins with functions in cell cycle progression and apoptosis (10-12). The E2F1 target genes encode proteins, such as cyclin E, DHFR, and E2F1 that function in the G1 to S-phase transition during the cell cycle progression (10, 11, 15). Moreover, E2F1 target genes also encode proteins, such as p73, PUMA, Noxa, and Bim that are known to have pro-apoptotic functions in immune cells (12, 17).
Interferon-inducible Ifi202 gene encodes a 52-kDa phosphoprotein p202 (18-21). Overexpression of p202 protein in a variety of mouse fibroblasts retards cell proliferation and increases cell survival, in part, by inhibiting E2F-mediated transcription (20, 21). Furthermore, increased expression of p202 in splenic cells of the B6.Nba2 congenic mice (congenic for Nba2 locus derived from NZB lupus-prone mice) is associated with increased production of IgG antibodies, splenomegaly, and defects in apoptosis of B cells in vitro (7, 21). Importantly, generation of B6.Nba2 mice that are deficient for the IFN-α/β receptor resulted in more than two-fold reduction in Ifi202 mRNA levels in splenic cells (22). Consistent with this observation, others have reported (23) that basal levels of Ifi202 mRNA are 3-4-fold higher in NZB/W mice than BALB/c or MRL/lpr mice. Although, these observations are consistent with the regulation of Ifi202 gene by interferons (IFN-α or IFN-β) in immune cells, however, our studies (24) revealed that type-I interferon receptor deficiency reduced lupus-like disease in NZB mice, but did not result in decreased levels of p202 protein.
Our previous studies (25-28) revealed that expression of Ifi202 is also regulated by interferon-independent signaling pathways. For example, we found that IL-6 up-regulates expression of the Ifi202 gene in mouse fibroblasts and B6.Nba2 splenocytes through activation of transcription factor STAT3 (28). Furthermore, we noted that serum growth factors, such as platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), and tumor growth factor-β (TGF-β), negatively regulate the expression of Ifi202 (25). However, the molecular mechanisms remain unclear.
Because the E2F-family of transcription factors contribute to transition from G1-to-S phase of cell cycle in response to serum growth factors (10, 11) and certain E2F-family members, such as E2F1 and E2F2, are implicated in the regulation of autoimmunity (29, 30), we investigated whether the E2F family members regulate Ifi202 expression. Here we report that the E2F family members differentially regulate expression of the Ifi202 gene.
Materials and Methods
Cells, cell culture, and reagents
Mouse embryonic fibroblasts (MEFs) from p53-null (p53-/-) and isogenic wild type mice (31) were generously provided by Dr. Donehower (Baylor College of Medicine, Houston, Texas). Rb-null (Rb-/-) and isogenic wild type (Rb+/+) MEFs (32) were generously provided by Dr. Ed Harlow (Harvard Medical School, Boston, MA). MEFs from E2F1-null (E2F1-/-) and wild type (E2F1+/+) mice (16) were generously provided by Dr. Yamasaki (Columbia University, New York). MEFs and splenocytes from E2F1 and E2F2 double null (E2F1-/- E2F2-/-) and wild type (E2F1+/+ E2F2+/+) age-matched mice (30) were generously provided by Dr. DeGregori (University of Colorado, Denver, Colorado). MEFs and splenocytes from age-matched C57BL/6 (B6) and B6.Nba2 congenic mice (7) were generously provided by Drs. Rozzo and Kotzin (University of Colorado Health Sciences Center, Denver, CO). NIH3T3 fibroblasts stably over-expressing E2F1 (3T3/E2F1 cl3, ref. 33) were generously provided by Dr. H. Tanaka (Osaka University, Osaka, Japan). Mouse NIH3T3 fibroblasts were purchased from the American Type Culture Collection. MEFs (between passage 3-5) and NIH3T3 fibroblasts were maintained at low density in Dulbecco's modified Eagle's medium containing high glucose, supplemented with 10% calf serum and antibiotics in an incubator with 5% CO2. Antiserum to p202 has been described previously (19).
Plasmids and expression vectors
Dr. Peggy Farnham (University of California-Davis, Davis, CA) generously provided pCMV-mE2F1 expression vector. Dr. J. Nevins (Duke University, Durham, NC) generously provided plasmids encoding various mutants of E2F1 [E2F1 (E132), E2F1 Δ206-220, E2F1 Δ1-88, and E2F1 (411/421)]. The 202-luc-reporter plasmid (202-luc), containing the 5′-regulatory sequence (∼0.8 kb) of the Ifi202 gene (derived from the EAT cells), has been described previously (28). Mutations in the E2F1 DNA-binding site (indicated as 202-E2F-BS) in the 202-luc-reporter plasmid were introduced using the QuikChange site-directed mutagenesis kit (from Stratagene, La Jolla, CA) as suggested by the supplier. The sequencing of the mutant reporter plasmid confirmed that the nucleotide sequence in the 202-luc-reporter plasmid was changed from AAAGGGCGCGAAA to AAAGGTTTCGAAA.
Reporter assays
For reporter assays, sub-confluent cultures of cells (in 6-well plates) were transfected with the reporter plasmids 202-luc (2.5 μg) and pRL-TK (0.5 μg), using calcium phosphate transfection kit (from Invitrogen, Rockville, MD), as suggested by the supplier. Unless otherwise indicated, cells were harvested between 43 and 48 h after transfections. Cells were lysed, and the firefly and Renilla luciferase activities were determined as described previously (28).
Isolation of RNA from splenocytes and RT-PCR
Total single cell splenocytes were isolated from female B6 or B6.Nba2 mice (8-10 weeks of age). After lysis of red blood cells, splenocytes were re-suspended in RPMI 1640 with 10% fetal bovine serum, 1% penicillin/streptomycin/glutamate, and 1× minimal essential medium, non-essential amino acids/sodium pyruvate. Splenocytes (5 × 106 cells) were used to isolate total RNA using TRIzol (Invitrogen). Total RNA was digested with DNase I (to remove any DNA in the preparation), and 0.5 μg of RNA was used for RT-PCR reaction using a pair of the Ifi202-specific primers (forward primer: 5′-ggtcatctaccaactcagaat-3′; reverse primer: 5′-ctctaggatg ccactgctgttg-3′). For RT-PCR, we used the Superscript one-step RT-PCR system from Invitrogen (Grand Island, NY). Primers for other genes were following: mE2F1 (118 bp), Forward: 5′-GATCGAAGCTTTAATGGAGCG-3′, Backward: 5′-CCCTTGCTTCA GAGAACAG-3′; PUMA (211 bp), Forward: 5′-AGCACTTAGAGT CGCCCGT-3′, Backward: 5′-GAGGAGTCCCATGAAGAGATTG-3′; Bim (244 bp), Forward: 5′-TAAGTTCTGAG TGTGACAGAGAAGG-3′, Backward: 5′-CAGTTGTA AGATAACCATTTGA GGGTGG-3′.
Immunoblotting
Splenocytes, MEFs, or NIH3T3 cells were collected in PBS and resuspended in a modified radio-immune precipitation assay (RIPA) lysis buffer (50 mM Tris-HCl, pH 8.0, 250 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS), supplemented with protease inhibitors (leupeptin, 50 μg/ml; pepstatin-A, 50 μg/ml; and phenylmethylsulfonyl fluoride, 1 mM) and incubated at 4° C for 30 min. Cell lysates were sonicated briefly before centrifugation at 14,000 rpm in a microcentrifuge for 10 min at 4 °C. The supernatants were collected, and the protein concentration was measured by Bio-Rad protein assay kit. Equal amounts of protein were processed for immunoblotting as described previously (28).
Electrophoretic mobility shift assays
Nuclear extract was prepared from mouse fibroblasts as described previously (34). The protein concentration was measured with the Bio-Rad protein assay reagents. An oligonucleotide (25 nucleotides long) containing the potential E2F DNA-binding site (202-E2F-BS) present in the 5′-regulatory region of Ifi202, or containing an E2F DNA-binding site consensus sequence (purchased from Santa Cruz Biotech., Santa Cruz, CA), was end-labeled with [γ-32P] ATP and polynucleotide kinase. Gel-purified labeled oligonucleotide (probe, 1 ng/assay) was incubated with nuclear extracts containing equal amounts of protein in binding buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1 mM EDTA, 10% glycerol, 0.1% Nonidet P-40, 1 mM dithiothreitol, and 100 μg of BSA) for 20 min at room temperature in the presence of 0.5 μg of poly(dI·dC). Nuclear extracts were treated with detergent sodium deoxycholate as described previously (34). In the competition assays, the nuclear extracts were pre-incubated with excess unlabeled oligonucleotides at room temperature for 15 min. Samples were analyzed on a 5% native gel in 1× TBE (90 mM Tris-borate, 2 mM EDTA, pH 8.0) buffer. Gel was dried and exposed to X-ray film at -80° C.
Chromatin immunoprecipitation assays
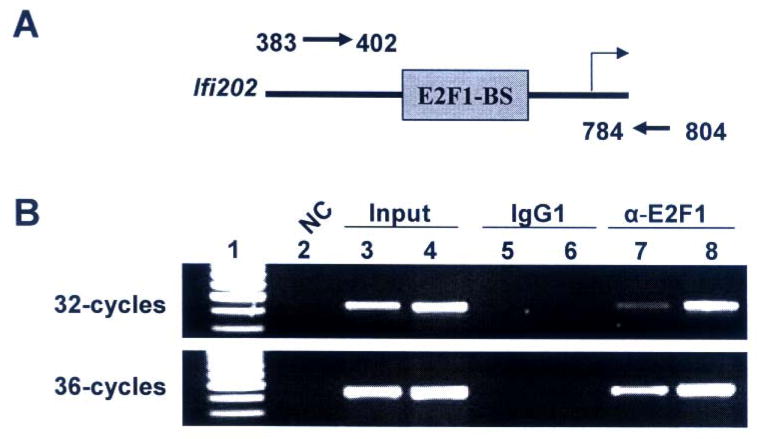
NIH 3T3 or NIH3T3-E2F1 cells (8 × 106) were cross-linked for 10 min at room temperature by directly adding 1/10th medium volume of cross-linking reagent (11% formaldehyde, 100 mM NaCl, 0.5 mM EGTA, 50 mM HEPES, pH 8.0) to the plate. The cross-linking reaction was quenched by adding 1/10th volume of 1.25 M Glycine and incubating cells at room temperature for 5 min. Cells were centrifuged and washed (twice) with PBS. Cells were re-suspended in 500 μl of lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.0, supplemented with the protease inhibitors) and tubes were incubated on ice for 10 min with occasional mixing. The cell lysates were sonicated (2 times, 15 seconds each) to break chromatin and processed for the reversal of cross-linking. The cell lysates were diluted to a final volume of 1 ml with a mixture of 9-parts of the dilution buffer (1% Triton X-100, 150 mM NaCl, 2 mM EDTA, 20 mM Tris-HCl, pH8.0, plus protease inhibitors) and 1 part of cell lysis buffer. The diluted cell lysates were first pre-cleared by incubating with protein A/G beads (Pierce) for 30 min at 4°C. After pre-clearing, the cell lysates (containing equal amounts of proteins) were divided into two fractions: one fraction was subjected to immunoprecipitation using mouse monoclonal antibody to E2F1 and another fraction was immunoprecipitated with the same amount of mouse normal IgG antibody as a control. The immunoprecipitates were collected using protein A/G beads. Beads were centrifuged, washed (5-times) in 1 ml of washing buffer (1% Triton X-100, 0.1% SDS, 150 mM NaCl, 2 mM EDTA, 20 mM Tris-HCl, pH8.0). The final wash was with a buffer containing 1% Triton X-100, 0.1 % SDS, 500 mM NaCl, 2 mM EDTA, 20 mM Tris-HCl (pH 8.0). Immune complexes were eluted from beads by adding 450 μl of elution buffer containing proteinase-K and RNase A (500 μg/ml each), and by incubating tubes at 37°C for 30 min. Tubes were centrifuged and the supernatants containing DNA were collected. The DNA was purified, precipitated, washed, and dissolved in water. Equal volumes of DNA were used to perform PCR to detect the promoter region of the Ifi202 gene. The following primers were used: region 383 to 402 forward primer (5′-gtgtctagtggccagtgtac-3′) and region 804 to 784 reverse primer (5′-tctgcagtgatgtacagatcc-3′) (see Fig. 5; ref. 18). The PCR amplification conditions were: incubation at 94°C for 5 min, followed by 94° C for 30 seconds, 45° C for 30 seconds, 72° C for 45 seconds. The amount of amplified DNA was compared after 32 or 36 amplification cycles by agarose gel electrophoresis to determine whether the amplification of the input DNA was in the linear range.
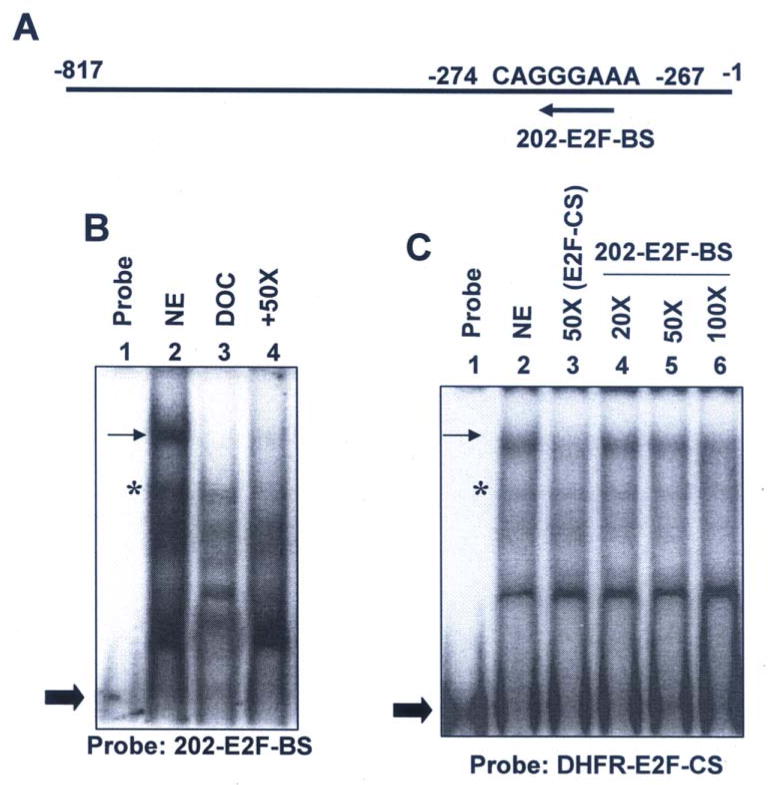
FIGURE 5. E2F1 binds to potential E2F DNA-binding site in the 5′-regulatory region of Ifi202 gene in chromatin immunoprecipitation assays.
(A) Schematic representation of the 5′-regulatory region of Ifi202 gene containing the 202-E2F-BS and relative location of PCR primers that were used to amplify the immunoprecipitated chromatin.
(B) Soluble chromatin was prepared from NIH3T3 (lanes 3, 5, and 7) or NIH3T3-E2F1 (lanes 4, 6, and 8) cells. Chromatin was incubated with antibodies to E2F1 (lanes 7 and 8) or, as a negative control, with isotype IgG1 antibodies (lanes 5 and 6). DNA was extracted from immunoprecipitates and PCR amplified (upper panel, 32 cycles; lower panel, 36 cycles) using a pair of primers that covered the E2F1 DNA-binding site in the 5′-regulatory region of the Ifi202 gene. As a positive control for PCR, we also amplified the input chromatin DNA from NIH3T3 (lane 3) and NIH3T3-E2F1 (lane 4) cells. As a negative control for PCR, we did not include any template DNA in PCR reaction (lane 2).
Results
Transcription factor E2F1 negatively regulates expression of Ifi202
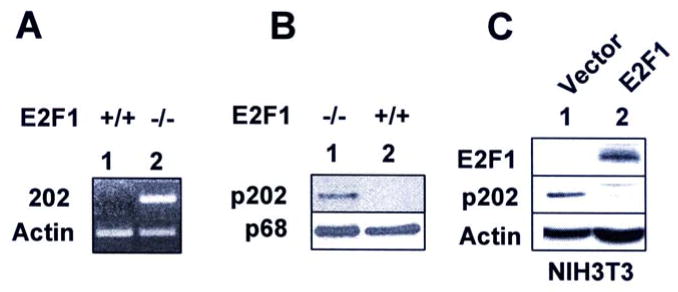
Our previous studies (25) revealed that serum growth factors, such as PDGF, bFGF, or TGF-β negatively regulate expression of Ifi202 in mouse fibroblasts. Moreover, we found (35) that levels of Ifi202 mRNA and protein decrease in mouse fibroblasts when cells exit from quiescence (the G0/G1 phase of cell cycle) to enter into S-phase of cell cycle after serum-stimulation. Because serum growth factors stimulate transition of cells from G0/G1 phase of cell cycle to S-phase, in part, by activating the E2F1-mediated transcription of S-phase genes (10, 11), we explored whether E2F1 could negatively regulate expression of the Ifi202. For this purpose, we compared basal levels of Ifi202 mRNA and protein between E2F1-null (E2F1-/-) and isogenic wild type (E2F+/+) MEFs. As shown in Fig. 1A, basal levels of Ifi202 mRNA were not detectable in the wild type MEFs. However, the mRNA levels were readily detectable in E2F-null MEFs. Consistent with detectable mRNA levels in E2F1-null MEFs, p202 protein levels were detectable in E2F1-null MEFs, but not in isogenic wild type cells (Fig. 1B). Furthermore, overexpression of E2F1 in NIH3T3 cells resulted in reduced levels of p202 protein (Fig. 1C). Together, these observations suggested that expression of E2F1 in MEFs negatively regulated expression of the Ifi202.
FIGURE 1. E2F1 negatively regulates expression of Ifi202.
(A) Total RNA was isolated from sub-confluent cultures of the wild type (E2F1+/+, lane 1) or E2F1-null (E2F1-/-, lane 2) MEFs and steady state levels Ifi202 and actin mRNA were analyzed by semi-quantitative RT-PCR.
(B) Total cell extracts were prepared from sub-confluent cultures of E2F1-null (lane 1) or wild type (lane 2) MEFs and steady state levels p202 and p68 protein (a protein detected by antiserum to p202 and serves as an internal control for protein amounts, ref. 19) were analyzed by immunoblotting.
(C) Total cell extracts were prepared from NIH3T3 cells stably transfected with control vector (lane 1) or plasmid expressing E2F1 (lane 2). The extracts were analyzed by immunoblotting using antibodies specific to the indicated proteins.
E2F1-mediated transcriptional repression of Ifi202 is p53 and pRb-independent
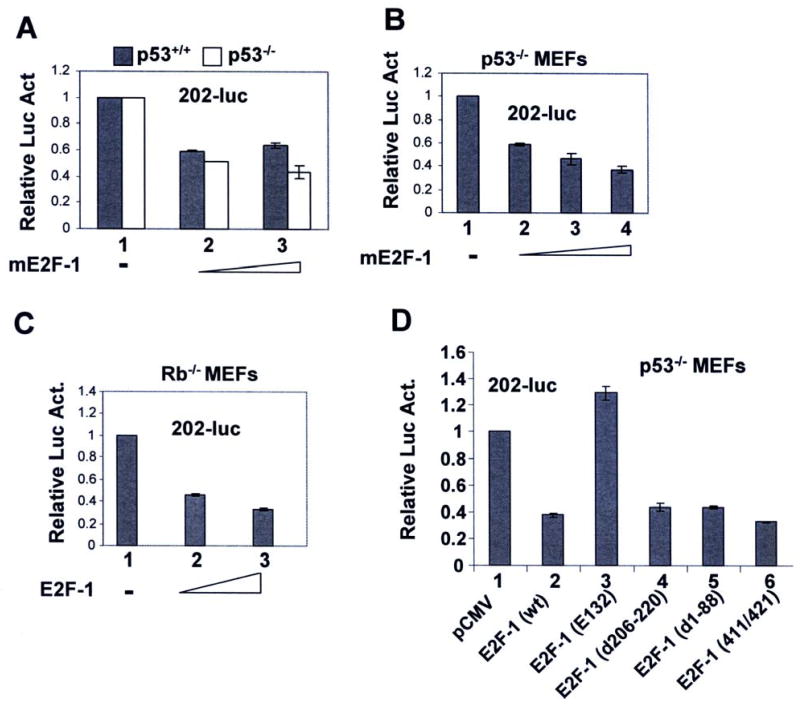
Our previous studies revealed that p53 transcriptionally represses expression of Ifi202 (26). This observation made it conceivable that E2F1 represses the expression of Ifi202 through stabilization of p53 (through transcriptional activation of p19ARF; see 36). Therefore, we tested whether E2F1-mediated negative regulation of Ifi202 expression is p53-dependent. For this purpose, we performed promoter-reporter assays. As shown in Fig. 2A, expression of mouse E2F1 (mE2F1) in p53-null (p53-/-) or wild type (p53+/+) MEFs reproducibly decreased the activity of the 202-luc-reporter about 50-60% in three independent experiments. This observation suggested that the E2F1-mediated transcriptional repression of 202-luc-reporter activity was independent of p53 expression. Furthermore, we noted that transfection of p53-null MEFs with increasing amounts of mE2F1 encoding plasmid decreased the activity of the 202-luc-reporter in dose-dependent manner and the decrease in the activity of the reporter was about 70% (Fig. 2B).
FIGURE 2. E2F1-mediated transcriptional repression of Ifi202 is independent of p53 and pRb expression, but dependent on DNA-binding domain of E2F1.
(A) Sub-confluent cultures of wild type or p53-null MEFs were transfected with 202-luc-reporter plasmid (2.5 μg) and pRL-TK plasmid (0.5 μg) along with pCMV (2.5 μg, column 1) or increasing amounts (1 μg or 2 μg, columns 2 and 3, respectively) of pCMV-mE2F1 plasmid using a calcium phosphate transfection kit as described in methods. Cells were harvested after 44 h to assays for the firefly and Renilla luciferase activities as described in methods. Normalized relative luciferase activity is shown. Results from a representative experiment are shown.
(B) Sub-confluent cultures of p53-null MEFs were transfected with 202-luc-reporter plasmid (2.5 μg) and pRL-TK plasmid (0.5 μg) along with pCMV (2.5 μg, column 1) or increasing amounts (1 μg, 2 μg, or 3 μg, columns 2, 3, and 4, respectively) of pCMV-mE2F1 plasmid using a calcium phosphate transfection kit. Cells were harvested after 44 h to assays dual luciferase activities as described in (A) above. Normalized relative luciferase activity is shown. Results from a representative experiment are shown.
(C) Sub-confluent cultures of Rb-null MEFs were transfected with 202-luc-reporter plasmid (2.5 μg) and pRL-TK plasmid (0.5 μg) along with pCMV (2.5 μg, column 1) or increasing amounts (1 μg or 2 μg columns 2 and 3, respectively) of pCMV-mE2F1 plasmid using a calcium phosphate transfection kit. Cells were harvested after 44 h to assays dual luciferase activities as described in (A) above. Normalized relative luciferase activity is shown. Results from a representative experiment are shown.
(D) Sub-confluent cultures of p53-null MEFs were transfected with 202-luc-reporter plasmid (2.5 μg) and pRL-TK plasmid (0.5 μg) along with pCMV plasmid (2.5 μg, column 1) or plasmid (pCMV-mE2F1; 2.5 μg, column 2) encoding wild type mE2F1, or plasmid (2.5 μg) encoding various mutants of E2F1 [E2F1 (E132), column 3; E2F1 Δ206-220, column 4; E2F1 Δ1-88, column 5; and E2F1 (411/421) column 6] using a calcium phosphate transfection kit. Cells were harvested after 46 h to assays dual luciferase activities as described in (A) above. Normalized relative luciferase activity is shown. Results from a representative experiment are shown.
Because E2F transcription factors in complex with the pRb (or other pocket proteins, such as p107 and p130), repress transcription of its target genes (10, 11), we also tested whether E2F1-mediated transcriptional repression of Ifi202 gene is pRb-dependent. Our promoter-reporter assays using pRb-null MEFs revealed that E2F1 repressed the activity of 202-luc-reporter in pRb-null MEFs (Fig. 2C). These observations suggested that E2F1-mediated transcriptional repression of Ifi202 is pRb-independent.
E2F1 DNA-binding activity is required for transcriptional repression of Ifi202
The E2F1-mediated transcriptional repression of genes is known to require the DNA-binding activity (10, 11). Therefore, we tested whether E2F1-mediated transcriptional repression of Ifi202 gene requires the DNA-binding domain in E2F1. As shown in Fig. 2D, expression of the wild type E2F1 in p53-null cells decreased the activity of 202-luc-reporter (compare column 2 with 1). Interestingly, transfection of cells with a deletion mutant of E2F1 (E132) in which the DNA-binding domain is deleted stimulated the activity of the reporter (compare column 3 with 2). However, expression of deletion mutants of E2F1 in which either the N-terminus (Δ1-88 amino acids) or the leucine zipper (Δ206-220) region was deleted repressed the activity of 202-luc-reporter. Moreover, expression of the E2F1 double point mutant 411/421, which has previously been shown (37) to selectively defective in overcoming p107, while maintaining wild-type pRb repressor function, inhibited the activity of 202-luc, suggesting that the p107 repressor function is not required for E2F1-medaited repression of Ifi202. Together, these observations suggested that the DNA-binding domain in E2F1 is required for transcriptional repression of Ifi202.
Differential regulation of Ifi202 expression by the E2F-family members
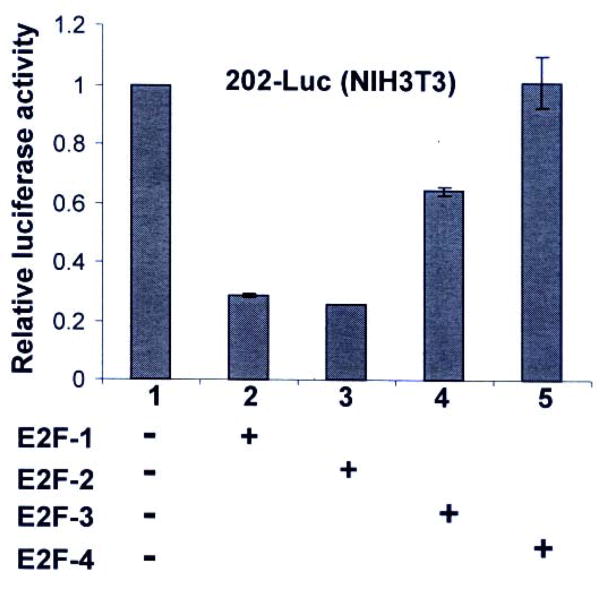
Because the E2F family of transcription factors, such as E2F1, E2F2, or E2F3, but not E2F4, are thought to participate in the G1-to-S phase transition of cells (10, 11), we compared the ability of these transcription factors to repress transcription of Ifi202 gene. As shown in Fig. 3, transfection of NIH3T3 cells with a plasmid encoding either E2F1 or E2F2 transcription factor repressed the activity of the 202-luc-reporter to a similar extent. Interestingly, transfection of plasmid that encodes the E2F3 transcription factor only partially (40%) inhibited the activity of the reporter. However, expression of the E2F4 transcription factor did not repress transcription of 202-luc-reporter in several independent experiments.
FIGURE 3. E2Fs differentially regulate expression of Ifi202.
Sub-confluent cultures of NIH3T3 cells were transfected with 202-luc-reporter plasmid (2.5 μg) and pRL-TK plasmid (0.5 μg) along with equal amounts (2.5 μg) of pCMV (column 1), pCMV-E2F1 (column 2), pCMV-E2F2 (column 3), pCMV-E2F3 (column 4), or pCMV-E2F4 (column 5) using a calcium phosphate transfection kit as described in methods. Cells were harvested after 44 h to assays for the firefly and Renilla luciferase activities as described in methods. Normalized relative luciferase activity is shown.
Furthermore, consistent with our above observations that both E2F1 and E2F2 repressed transcription of the Ifi202 gene, we found that basal levels of p202 protein were detectable in splenocytes from E2F1-/-E2F2-/- double knockout mice, but not in age-matched wild type (E2F1+/+E2F2+/+) mice (data not shown). Moreover, the basal activity of 202-luc-reporter was consistently ∼2-fold higher in MEFs from the double knockout mice than the wild-type mice in two independent experiments (data not shown). These observations suggested that structurally-related E2Fs, such as E2F1, E2F2, and E2F3 repress the transcription of the Ifi202 gene albeit to different extents.
E2Fs bind to a potential DNA-binding site in the 5′-regulatory region of the Ifi202 gene
Because E2F-mediated transcriptional repression of 202-luc-reporter was dependent on the ability of E2F to bind DNA (Fig. 2D), we analyzed the 5′-regulatory region of Ifi202 gene for the presence of potential E2F DNA-binding sites using the Matinspector 2.0 software program. The sequence analyses revealed a potential E2F DNA-binding site [5′-TTTCCCTG-3′; 202-E2F-BS]. A comparison of the 202-E2F-BS in the Ifi202 5′-regulatory region with other E2F DNA-binding consensus sites [5′-TTTCCCGC-3′; E2F-CS), which are bound by most (90%) E2F1/DP1 complexes (38), revealed at least two variations or mismatches between the 202-E2F-BS and E2F-CS sequence (see Table I).
Table-I.
A comparison of potential E2F1/DP1 DNA-binding in the Ifi202 gene with E2F DNA-binding consensus sequence.
| E2F1/DP1 DNA-binding consensus sequence: T T T C C C G C |
| 202-E2F1-BS: T T T C C C T G |
| 202-E2F-BS mutant: T T T C C T T T |
Boldface letters indicate nucleotides that appear in more than 90% of recovered E2F1/DP1 DNA-binding sites (38). Underlined letters indicate variations in the nucleotides from the E2F DNA-binding consensus sequence.
Next, we performed gel-mobility shift assays to determine whether 202-E2F-BS (Fig. 4A) in Ifi202 gene can bind to E2F transcription factors. As shown in Fig. 4B, in nuclear extracts from NIH 3T3 cells, we could detect binding of proteins to an oligonucleotide (probe) containing the 202-E2F-BS (compare lane 2 with 1). Importantly, treatment of nuclear extracts with detergent deoxycholate, which has been previously known to dissociate E2F proteins from the pocket family proteins in NIH3T3 cells (34), resulted in “free” E2F (a dimmer between E2F and DP proteins). Moreover, competition with 50-fold excess of unlabeled oligonucleotide containing the E2F-CS greatly reduced the binding of proteins to the oligonucleotide with the 202-E2F-BS sequence (compare lane 4 with lanes 2 or 3).
FIGURE 4. Identification of a potential E2F DNA-binding site in the 5′-regulatory region of Ifi202 gene in gel-mobility shift assays.
(A) Schematic representation of a potential E2F DNA-binding site (202-E2F-BS) in the 5′-regulatory region of Ifi202 gene.
(B) Nuclear extracts from NIH 3T3 cells were subjected to gel-mobility shift assays using radio-labeled oligonucleotide containing the 202-E2F DNA-binding sequence (probe) as described in methods. The nuclear extract without any treatment (lane 2), after treatment with deoxycholate (lane 3), or after competition with 50-fold excess of cold oligonucleotide containing the E2F DNA-binding consensus sequence (purchased from Santa Cruz Biotech.). As a control, we also analyzed the probe without incubation with the extracts (lane 1).
(C) Nuclear extracts from NIH3T3 cells were subjected to gel-mobility shift assays using radio-labeled oligonucleotide containing the E2F DNA-binding consensus sequence from the DHFR gene (probe) as described in methods. The nuclear extract without any treatment (lane 2), after competition with 50-fold excess of cold oligonucleotide containing the E2F-CS (lane 3), after competition with 20-fold (lane 4), 50-fold (lane 5), or 100-fold (lane 6) excess of cold oligonucleotide containing the 202-E2F-BS. As a control, we also analyzed the probe without incubation with the extracts (lane 1). The arrow indicates the location of E2F transcription factors in complex with the pocket family of proteins and the star indicates the location of “free” E2F transcription factors.
Because the 202-E2F-BS sequence contains at least two variations (or mismatches) in nucleotides from the E2F-CS (see Table I), we sought to compare the relative affinity of 202-E2F-BS with E2F-CS. For this purpose, we performed gel-mobility shift assays using nuclear extracts from NIH3T3 cells and labeled oligonucleotide with E2F-CS from the DHFR, a well known E2F target gene (10). As shown in Fig. 4C, proteins in nuclear extracts bound to E2F-CS and a competition with 50-fold excess of cold E2F-CS reduced the binding of proteins to the probe (compare lane 3 with 2). Furthermore, as expected from the presence of two variations in the 202-E2F-BS, competition with 20 or 50-fold excess of cold oligonucleotide with 202-E2F-BS did not reduce binding of labeled E2F-CS with proteins (compare lane 4 or 5 with lane 2). However, competition with 100-fold excess cold oligonucleotide containing the 202-E2F-BS sequence reduced the binding of the probe more than 50%. These observations suggested that potential E2F-BS in the 5′-regulator region of Ifi202 gene can bind to E2F protein complexes in gel-mobility shift assays with relatively low affinity than the E2F DNA-binding sites found in most E2F-responsive genes.
To confirm binding of E2F1 to 202-E2F-BS in vivo, we performed chromatin immunoprecipitation assays. As shown in Fig. 5B, some binding of E2F1 to Ifi202 regulatory region was detected in asynchronous cultures of NIH3T3 cells (compare lane 7 with lane 5). However, overexpression of E2F1 in NIH3T3 cells, which decreased the expression of p202 (see Fig. 1C), increased binding of E2F1 to the regulatory region of the Ifi202 (compare lane 8 with lane 7). This observation is consistent with our observation that the E2F1 DNA-binding activity is required to repress transcription of 202-luc-reporter (Fig. 2D) and E2Fs can bind to 202-E2F-BS sequence in gel-mobility shift assays (Fig. 4).
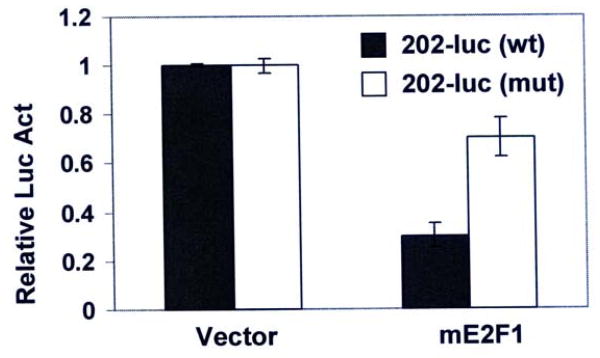
E2Fs represses transcription of Ifi202 gene through the E2F-BS in the regulatory region
Our observations that E2F1 expression repressed expression of the Ifi202 and E2F1 bound to a potential E2F DNA-binding site in the 5′-regulatory region of Ifi202 gene prompted us to determine whether this E2F DNA-binding site is needed for E2F1-mediated transcriptional repression. Therefore, we mutated the 202-E2F-BS in the 202-luc-reporter plasmid by changing a critical nucleotide from C-to-T (see Table-I) by site-directed mutagenesis and compared the activity of the wild type reporter with the mutant reporter. As shown in Fig. 6, expression of mE2F1 repressed the activity of wild type 202-luc-reporter. Interestingly, introduction of a point mutation in the 202-E2F biding sequence resulted in a 60% decrease in the E2F1-mediated transcriptional repression in two independent experiments. These observations suggested that E2F1 represses the expression of Ifi202 in part through the 202-E2F-BS in the 5′-regulatory region of the Ifi202 gene.
FIGURE 6.
The E2F1 transcriptionally represses Ifi202 expression through the E2F1 DNA-binding site. Sub-confluent cultures of p53-null MEFs were transfected with wild type or mutant 202-luc-reporter plasmid (2.5 μg) and pRL-TK plasmid (0.5 μg) along with pCMV (2.5 μg, column 1) or pCMV-mE2F1 (2.5 μg, column 2) plasmid using a calcium phosphate transfection kit as described in methods. Cells were harvested after 44 h to assays for the firefly and Renilla luciferase activities as described in methods. Normalized relative luciferase activity is shown. Results from a representative experiment are shown.
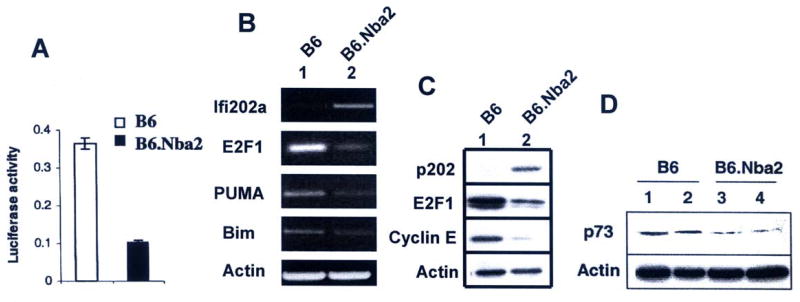
Increased expression of p202 in B6.Nba2 mice is associated with inhibition of E2F1 expression and down-regulation of the E2F1 target genes
Our previous studies (34, 39) revealed that increased levels of p202 in cells inhibit E2F1 and E2F4-medaited transcription of target genes. These observations raised the possibility that increased expression of p202 in B6.Nba2 mice contributes to lupus susceptibility, in part, by down-regulating the expression of E2F1 and its target genes. Therefore, we tested whether increased expression of p202 in MEFs derived from the B6.Nba2 mice affects the transcriptional activity of E2F. For this purpose, we compared the activity of a well-known E2F-responsive reporter (E2F-luc) in MEFs derived from B6 and B6.Nba2 mice. As shown in Fig. 7A, basal activity of the E2F-responsive reporter was 70% lower in unsynchronized B6.Nba2 than B6 MEFs. This observation prompted us to compare the expression of E2F1 mRNA and protein between B6 and B6.Nba2 splenocytes in vivo. As shown in Fig. 7B, levels of E2F1 mRNA were ∼80% lower in B6.Nba2 splenocytes than B6 splenocytes. Additionally, we found that mRNA levels of known E2F1 target genes, such as PUMA and Bim were also lower in splenocytes derived from the B6.Nba2 mice. We also compared the protein levels of E2F1 and its transcriptional target, cyclin E, between B6 and B6.Nba2 splenocytes (Fig. 7C). We noted that levels of both E2F1 and cyclin E were lower in B6.Nba2 splenocytes than B6. Additionally, we also compared the expression levels of p73, a transcriptional target of the E2F1, between age-matched B6 and B6.Nba2 female mice. As shown in Fig. 7D, levels of p73 were lower in splenocytes derived from two B6.Nba2 female mice than B6 female mice. These observations provide support to the idea that increased levels of p202 in B6.Nba2 mice contribute to lupus susceptibility by decreasing the expression of E2F1 transcription factor and inhibition of E2F1-mediated transcription of target genes.
FIGURE 7.
Increased expression of Ifi202 in B6.Nba2 mice is associated with reduced expression levels of E2F1 and inhibition of E2F1-mediated transcription of target genes.
(A) Sub-confluent cultures of B6 or B6.Nba2 MEFs were transfected with E2F-luc-reporter plasmid (2.5 μg) and pRL-TK plasmid (0.5 μg) using a calcium phosphate transfection kit as described in methods. Cells were harvested after 44 h to assays for the firefly and Renilla luciferase activities as described in methods. Normalized relative luciferase activity is shown.
(B) Total RNA was isolated from splenocytes from age-matched (10 weeks) B6 (lane 1) or B6.Nba2 (lane 2) female mice and steady state levels of mRNAs were analyzed by semi-quantitative RT-PCR using a pair of primers specific for the indicated individual genes.
(C) Total cell extracts were prepared from splenocytes isolated from age-matched (10 weeks) B6 (lane 1) or B6.Nba2 (lane 2) female mice and steady state levels of proteins were analyzed by immunoblotting using antibodies specific for the indicated proteins.
(D) Total cell extracts were prepared from splenocytes isolated from age-matched (10 weeks) B6 (lanes 1 and 2) or B6.Nba2 (lanes 3 and 4) female mice and steady state levels p73 and actin proteins were analyzed by immunoblotting using specific antibodies.
Discussion
Studies involving SLE patients (6) and mouse models of lupus disease (9, 23) have revealed a role for IFN-inducible genes in the development of lupus disease. Because IFN-inducible proteins that regulate cell proliferation and survival are likely to contribute to the development of autoimmunity, identification of such IFN-inducible proteins will allow us to elucidate their role in immune regulation. Furthermore, it is important to understand how cellular levels of IFN-inducible proteins are regulated by other signaling pathways in immune cells.
Through regulation of a set of genes that regulate transition from G1-to-S phase of cell cycle, the transcription factor E2F1 regulates cell proliferation, differentiation, and apoptosis of B and T cells (29, 30, 36, 40-47). Moreover, defects in cell cycle progression and apoptosis of immune cells that result from the lack of E2F1 or E2F1 and E2F2 function contribute to the development of lupus-like disease (29, 30). Consistent with this idea, the transcription factor E2F1 is important for activation-induced cell death in T cells (44). Furthermore, E2F1 and E2F2 transcription factors determine threshold for antigen-induced T-cell proliferation (30) and the activity of E2F2 is needed for suppression of T cell proliferation and immunologic self-tolerance (29). Consequently, disruption of the gene for the E2F1 transcription factor causes significant increases in T-cell number and splenomegaly (16). Similarly, mice that are null for E2F2 transcription factor develop a lupus-like disease (29). Importantly, the E2F2 transcription factor represses the transcription of the E2F1 gene whose activity is required in cells for normal S-phase entry during cell cycle progression (29). Because serum growth factors down-regulate the expression of Ifi202 (25) and p202 inhibits the E2F (E2F1 and E2F4)-mediated transcription (34, 39), we tested whether E2F transcription factors could regulate expression of the Ifi202.
Our experiments revealed that: (i) steady-sate levels of Ifi202 mRNA and protein were higher in E2F-null than wild type MEFs and overexpression of E2F1 decreased p202 levels (Fig. 1); (ii) splenic cells from E2F1 and E2F2 double knockout mice expressed high levels of p202 than isogenic wild type cells (Fig. 3); (iii) in promoter-reporter assays, expression of E2F1, 2 and 3, but not E2F4, repressed transcription of the 202-luc-reporter (Fig. 3); (iv) the 5′-regulatry region of the Ifi202 gene contains a potential E2F DNA-binding site (202-E2F-BS) that bound to E2F protein complexes in gel-mobility shift assays (Fig. 4) and in chromatin immunoprecipitation assays (Fig. 5), and a point mutation in this E2F DNA-binding site decreased the E2F-mediated transcriptional repression of 202-luc-reporter (Fig. 6); and (v) increased expression of Ifi202 in splenic cells from B6.Nba2 congenic mice was associated with reduced expression of E2F1 and its target genes (Fig. 7). Together, these observations demonstrated that: (i) E2Fs differentially regulate expression of the Ifi202 gene; and (ii) increased expression of p202 in splenic cells from B6.Nba2 mice negatively regulates E2F-medaited transcription.
The 202-luc reporter construct that is used in our promoter-reporter assays (Fig. 2 and 3) contains only ∼800 bp from the 5′-regulatory region of the Ifi202 gene. Therefore, it is conceivable that E2F-responsive elements upstream to this 800 bp region in Ifi202 gene also contribute to the regulation of the gene. This could account for moderate decreases in the activity of the 202-luc-reporter in our assays after expression of E2F (E2F1, E2F2, or E2F3) transcription factors and significantly increased steady-sate levels of Ifi202 mRNA and protein in E2F1-null cells.
We found that E2F-mediated transcriptional repression of Ifi202 gene was independent of p53 or pRb expression (Fig. 2). Because the p53 protein family includes p63 and p73 proteins (48) and the pRb protein family includes p107 and p130 proteins (11), our observations do not rule out the possibility that E2F1-mediated transcriptional repression of Ifi202 gene depends on other p53 or pRb-family proteins. Further work will be needed to test these possibilities.
The interferon-inducible p202 protein binds to E2F1 and inhibits E2F1-mediated transcription of target genes (34). Binding of p202 to E2F1 results in inhibition of specific DNA-binding activity of E2F complexes in gel-mobility shift assays (34). Moreover, p202 inhibits E2F1-mediated apoptosis (49). Together, these observations support the idea that increased levels of p202 in immune cells (B and T cells) increase the threshold for apoptosis by inhibiting E2F-mediated transcription of its target genes, which encode pro-apoptotic proteins. Consistent with the above idea we found that increased expression of Ifi202 in B6.Nba2 was associated with increases in the numbers of T and B cells and splenomegaly (7).
Stimulation of the T-cell receptor (TCR) on cycling peripheral T cells causes their apoptosis by TCR-activation-induced cell death (TCR-AICD; ref. 44). Furthermore, E2F1-null or p73-null primary T cells do not undergo TCR-mediated apoptosis (44). Similarly, E2F1-mediated expression of pro-apoptotic proteins, such as Bim and Puma, contributes to apoptosis of cells (50). Therefore, our observations that increased expression of p202 in B6.Nba2 splenic cells is associated with reduced expression of E2F1, Bim, and p73 provide support for the idea that inhibition of TCR-AICD by p202 contributes to accumulation of T cells.
Studies have suggested that E2F1 plays a role in the induction of ARF, p53, and apoptosis during thymic negative selection (36). Because increased expression of p202 in cells inhibits p53 and E2F1-mediated transcription (20, 21), our observations are consistent with the idea that increased levels of p202 in B6.Nba2 mice contribute to defects in apoptosis during thymic negative selection. Moreover, our observations suggest that transcriptional repression of Ifi202 gene by both p53 (26) and E2F1 (this study) may be required for normal thymic negative selection.
Increased expression of the p202 in cell lines inhibits the transcriptional activity of factors, such as p53, NF- κB, and AP-1 (20, 21). These transcription factors, by modulating transcription of target genes, are known to regulate cell proliferation and survival (20, 21). Consistent with inhibition of the transcriptional activity of the above factors by p202, the increased expression of p202 in cell lines, depending on the cell context, either sensitizes cells to apoptosis or decrease the susceptibility to apoptosis (21). Importantly, we found that increased expression of p202 in splenic B6.Nba2 cells was associated with inhibition of p53-mediated transcription of pro-apoptotic genes and defects in apoptosis of cells (51). Therefore, our observations that certain E2Fs, such as E2F1 and E2F2, repress the transcription of the Ifi202 gene are consistent with our above observations.
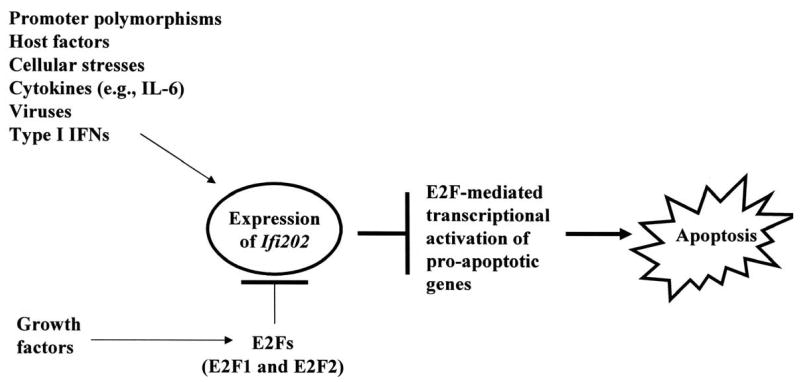
In summary, our observations provide support for our model (Fig. 8). The model predicts that mitogenic signaling pathways that activate the E2F-1-mediated transcription negatively regulate Ifi202 expression. In contrast, signaling pathways (and host factors) that contribute to increased expression of Ifi202 inhibit E2F1-mediated transcription of pro-apoptotic genes in cells. Therefore, defects in mutual regulation of Ifi202 and E2F1 expression and functions are likely to contribute to defects in cell proliferation and/or apoptosis, resulting in autoimmunity. Our observations will serve basis to identify signaling pathways and molecules that contribute to the development of autoimmune diseases.
FIGURE 8.
Regulation of Ifi202gene by E2F1 transcription factor and the role of p202 in lupus susceptibility through inhibition of E2F-mediated transcription of target genes.
Acknowledgments
We thank Drs. L. Donehower, Ed Harlow, L. Yamasaki, J. DeGregori, S. Rozzo, B. L. Kotzin, H. Tanaka, P. Farnham, and J. Nevins for generously providing cells and reagents. We also thank Drs. P. Lengyel and B. L. Kotzin for thoughtful suggestions.
This work was supported by a grant (AI066261) to D.C.
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Tsokos GC, Kammer GM. Molecular aberrations in human systemic lupus erythematosus. Mol Med Today. 2000;6:418–424. doi: 10.1016/s1357-4310(00)01798-6. [DOI] [PubMed] [Google Scholar]
- 3.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferon (α/β) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen TN, Gubbels MR, Kotzin BL. New insights into disease pathogenesis from mouse lupus genetics. Curr Opin Immunol. 2004;16:787–793. doi: 10.1016/j.coi.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Mohan C. SLE 1, 2, 3…genetic dissection of lupus. Adv Exp Med Biol. 2007;601:85–95. doi: 10.1007/978-0-387-72005-0_9. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Rozzo SJ, Allard JD, Choubey D, Vyse TJ, Izui S, Peltz G, Kotzin BL. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 2001;15:435–443. doi: 10.1016/s1074-7613(01)00196-0. [DOI] [PubMed] [Google Scholar]
- 8.Haywood MEK, Rose SJ, Horswell S, Lees MJ, Fu G, Walport MJ, Morley BJ. Overlapping BXSB congenic intervals, in combination with microarray gene expression, reveal novel lupus candidate genes. Gene Immun. 2006;7:250–263. doi: 10.1038/sj.gene.6364294. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Karypis G, Vegoe AL, Ruiz P, Gilkeson GS, Behrens TW. Genomic view of systemic autoimmunity in MRL/lpr mice. Genes Immun. 2006;7:156–168. doi: 10.1038/sj.gene.6364286. [DOI] [PubMed] [Google Scholar]
- 10.Slansky JE, Farnham PJ. Introduction to the E2F family: protein structure and gene regulation. Curr Top Microbiol Immunol. 1996;208:1–30. doi: 10.1007/978-3-642-79910-5_1. [DOI] [PubMed] [Google Scholar]
- 11.Nevins JR. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- 12.DeGregori J. The genetics of the E2F family of transcription factors: shared functions and unique roles. Biochem Biophys Acta. 2002;1602:131–150. doi: 10.1016/s0304-419x(02)00051-3. [DOI] [PubMed] [Google Scholar]
- 13.Iwase S, Furukawa Y, Kikuchi J, Nagai M, Terui Y, Nakamura M, Yamada H. Modulation of E2F activity is linked to interferon-induced growth suppression of hematopoietic cells. J Biol Chem. 1997;272:12406–12414. doi: 10.1074/jbc.272.19.12406. [DOI] [PubMed] [Google Scholar]
- 14.Thomas NS, Pizzey AR, Tiwari S, Williams CD, Yang J. p130, p107, and pRb are differentially regulated in proliferating cells and during cell cycle arrest by α-interferon. J Biol Chem. 1998;273:23659–23667. doi: 10.1074/jbc.273.37.23659. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DG, Ohtani K, Nevins JR. Auto-regulatory control of E2F1 expression in response to positive and negative regulators of cell cycle progression. Genes Dev. 1994;8:1514–1525. doi: 10.1101/gad.8.13.1514. [DOI] [PubMed] [Google Scholar]
- 16.Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson NJ. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell. 1996;85:537–548. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- 17.Phillips AC, Vousden KH. E2F-1 and apoptosis. Apoptosis. 2001;6:173–182. doi: 10.1023/a:1011332625740. [DOI] [PubMed] [Google Scholar]
- 18.Choubey D, Snoddy J, Chaturvedi V, Toniato E, Opdenakker G, Thakur A, Samanta H, Engel DA, Lengyel P. Interferons as gene activators. Indications for repeated gene duplication during the evolution of a cluster of interferon-activatable genes on murine chromosome 1. J Biol Chem. 1989;264:17182–17189. [PubMed] [Google Scholar]
- 19.Choubey D, Lengyel P. Interferon action: cytoplasmic and nuclear localization of the interferon-inducible 52-kD protein that is encoded by the Ifi202 gene from the gene 200 cluster. J Interferon Res. 1993;13:43–52. doi: 10.1089/jir.1993.13.43. [DOI] [PubMed] [Google Scholar]
- 20.Choubey D. p202: an interferon-inducible negative regulator of cell growth. J Biol Regul Homeost Agents. 2000;14:187–192. [PubMed] [Google Scholar]
- 21.Choubey D, Kotzin BL. Interferon-inducible p202 in the susceptibility to systemic lupus. Front Biosc. 2002;7:e252–262. doi: 10.2741/A921. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensesn TN, Roper E, Thurman JM, Marrack P, Kotzin BL. Type I interferon signaling is involved in the spontaneous development of lupus-like disease in B6.Nba2 and (B6.Nba2 × NZW) F1 mice. Genes Immun. 2007;8:653–662. doi: 10.1038/sj.gene.6364430. [DOI] [PubMed] [Google Scholar]
- 23.Lu Q, Shen N, Li XM, Chen SL. Genomic view of IFN-alpha response in pre-autoimmune NZB/W and MRL/lpr mice. Gene Immun. 2007;8:590–603. doi: 10.1038/sj.gene.6364421. [DOI] [PubMed] [Google Scholar]
- 24.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geng Y, D'Souza S, Xin H, Walter S, Choubey D. p202 levels are negatively regulated by serum growth factors. Cell Growth Deffer. 2000;11:475–483. [PubMed] [Google Scholar]
- 26.D'Souza S, Xin H, Walter S, Choubey D. The gene encoding p202, an interferon-inducible negative regulator of the p53 tumor suppressor, is a target of p53-mediated transcriptional repression. J Biol Chem. 2001;276:298–305. doi: 10.1074/jbc.M007155200. [DOI] [PubMed] [Google Scholar]
- 27.Xin H, Geng Y, Pramanik R, Choubey D. Induction of p202, a modulator of apoptosis, during oncogenic transformation of NIH 3T3 cells by activated H-Ras (Q61L) contributes to cell survival. J Cell Biochem. 2003;88:191–204. doi: 10.1002/jcb.10372. [DOI] [PubMed] [Google Scholar]
- 28.Pramanik R, Jorgensen TN, Xin H, Kotzin BL, Choubey D. Interleukin-6 induces expression of Ifi202, an interferon-inducible candidate gene for lupus susceptibility. J Biol Chem. 2004;279:16121–16127. doi: 10.1074/jbc.M313140200. [DOI] [PubMed] [Google Scholar]
- 29.Murga M, Fernandez-Capetillo O, Field SJ, Moreno B, Borlado LR, Fujiwara Y, Balomenos D, Vicario A, Carrera AC, Orkin SH, Greenberg ME, Zubiaga AM. Mutation of E2F2 in mice causes enhanced T lymphocyte proliferation, leading to the development of autoimmunity. Immunity. 2001;15:959–970. doi: 10.1016/s1074-7613(01)00254-0. [DOI] [PubMed] [Google Scholar]
- 30.Zhu JW, Field SJ, Gore L, Thomson M, Yang H, Fujiwara Y, Cardiff RD, Greenberg M, Orkin SH, DeGregori J. E2F1 and E2F2 determine thresholds for antigen-induced T-cell proliferation and suppress tumorigenesis. Mol Cell Biol. 2001;21:8547–8564. doi: 10.1128/MCB.21.24.8547-8564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 32.Classon M, Salama S, Gorka C, Mulloy R, Braun P, Harlow E. Combinatorial roles for pRB, p107, and p130 in E2F-mediated cell cycle control. Proc Natl Acad Sci USA. 2000;97:10820–10825. doi: 10.1073/pnas.190343497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka H, Matsumura I, Ezoe S, Satoh Y, Sakamaki T, Albanese C, Machii T, Pestell RG, Kanakura Y. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Mol Cell. 2002;9:1017–1029. doi: 10.1016/s1097-2765(02)00522-1. [DOI] [PubMed] [Google Scholar]
- 34.Choubey D, Li SJ, Datta B, Gutterman JU, Lengyel P. Inhibition of E2F-mediated transcription by p202. EMBO J. 1996;15:5688–5678. [PMC free article] [PubMed] [Google Scholar]
- 35.Xin H, Pramanik R, Choubey D. Retinoblastoma (Rb) protein up-regulates expression of the Ifi202 gene encoding an interferon-inducible negative regulator of cell growth. Oncogene. 2003;22:4775–4785. doi: 10.1038/sj.onc.1206780. [DOI] [PubMed] [Google Scholar]
- 36.Zhu JW, DeRyckere D, Li FX, Wan YY, DeGregori J. A role for E2F1 in the induction of ARF, p53, and apoptosis during thymic negative selection. Cell Growth Differ. 1999;10:829–838. [PubMed] [Google Scholar]
- 37.Cress WD, Johnson DG, Nevins JR. A genetic analysis of the E2F1 gene distinguishes regulation by Rb, p107, and adenovirus E4. Mol Cell Biol. 1993;13:6314–6325. doi: 10.1128/mcb.13.10.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao Y, Kassatly RF, Cress WD, Horowitz JM. Subunit composition determines E2F DNA-binding site specificity. Mol Cell Biol. 1997;17:6994–7007. doi: 10.1128/mcb.17.12.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choubey D, Gutterman JU. Inhibition of E2F-4/DP-1-stimulated transcription by p202. Oncogene. 1997;15:291–301. doi: 10.1038/sj.onc.1201184. [DOI] [PubMed] [Google Scholar]
- 40.Brennam P, Babbage JW, Burgering BM, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 41.Lam EW, Glassford J, van der Sman J, Banerji L, Pizzey AR, Shaun N, Thomas B, Klaus GG. Modulation of E2F activity in primary mouse B cells following stimulation via surface IgM and CD40 receptors. Eur J Immunol. 1999;29:3380–3389. doi: 10.1002/(SICI)1521-4141(199910)29:10<3380::AID-IMMU3380>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 42.van der Sman J, Thomas NS, Lam EW. Modulation of E2F complexes during G0 to S phase transition in human primary B-lymphocytes. J Biol Chem. 1999;274:12009–12016. doi: 10.1074/jbc.274.17.12009. [DOI] [PubMed] [Google Scholar]
- 43.Lam EW, Choi MS, van der Sman J, Burbidge SA, Klaus GG. Modulation of E2F activity via signaling through surface IgM and CD40 receptors in WEHI-231 B lymphoma cells. J Biol Chem. 1998;273:10051–10057. doi: 10.1074/jbc.273.16.10051. [DOI] [PubMed] [Google Scholar]
- 44.Lissy NA, Davis PK, Irwin M, Kaelin WG, Dowdy SF. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature. 2000;407:642–645. doi: 10.1038/35036608. [DOI] [PubMed] [Google Scholar]
- 45.Garcia I, Murga M, Vicario A, Field SJ, Zubiaga AM. A role for E2F1 in the induction of apoptosis during thymic negative selection. Cell Growth Differ. 2000;11:91–98. [PubMed] [Google Scholar]
- 46.Cao Q, Xia Y, Azadniv M, Crispe IN. The E2F-1 transcription factor promotes caspase-8 and bid expression, and enhances Fas signaling in T cells. J Immunol. 2004;173:1111–1117. doi: 10.4049/jimmunol.173.2.1111. [DOI] [PubMed] [Google Scholar]
- 47.DeRyckere D, DeGregori J. E2F1 and E2F2 are differentially required for homeostasis-driven and antigen-induced T cell proliferation in vivo. J Immunol. 2005;175:647–655. doi: 10.4049/jimmunol.175.2.647. [DOI] [PubMed] [Google Scholar]
- 48.Urist M, Prives C. p53 leans on its siblings. Cancer Cell. 2002;1:311–313. doi: 10.1016/s1535-6108(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 49.Yan DH, Abramina A, Li Z, Ding Y, Wen Y, Liu TJ, Hunt K. P202, an interferon-inducible protein, inhibits E2F1-mediated apoptosis in prostate cancer cells. Biochem Biophys Res Commun. 2003;303:219–222. doi: 10.1016/s0006-291x(03)00320-6. [DOI] [PubMed] [Google Scholar]
- 50.Hershko T, Ginsberg D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J Biol Chem. 2003;279:8627–8634. doi: 10.1074/jbc.M312866200. [DOI] [PubMed] [Google Scholar]
- 51.Xin H, D'Souza S, Jorgensen TN, Vaughan AT, Lengyel P, Kotzin BL, Choubey D. Increased expression of Ifi202, an IFN-activatable gene, in B6.Nba2 lupus susceptible mice inhibits p53-mediated apoptosis. J Immunol. 2006;176:5863–5870. doi: 10.4049/jimmunol.176.10.5863. [DOI] [PubMed] [Google Scholar]