Abstract
This study provides new evidence of deficient auditory cortical processing of speech in noise in autism spectrum disorders (ASD). Speech-evoked responses (~100–300 ms) in quiet and background noise were evaluated in typically-developing (TD) children and children with ASD. ASD responses showed delayed timing (both conditions) and reduced amplitudes (quiet) compared to TD responses. As expected, TD responses in noise were delayed and reduced compared to quiet responses. However, minimal quiet-to-noise response differences were found in children with ASD, presumably because quiet responses were already severely degraded. Moreover, ASD quiet responses resembled TD noise responses, implying that children with ASD process speech in quiet only as well as TD children do in background noise.
Keywords: Autism, Cortical encoding, Speech, Background noise, Children, Evoked potentials
Introduction
Autism spectrum disorders (ASD) are developmental disorders that include autism, Asperger’s disorder, and pervasive developmental disorder—not otherwise specified (PDD–NOS). ASD are defined by a triad of deficits: (1) language impairment in social communication, (2) repetitive or stereotyped behaviors or interests, and (3) impaired social interactions (Rapin and Dunn 2003; Siegal and Blades 2003; Tager-Flusberg and Caronna 2007). The language impairment spans perceptual, productive, and physiological domains (Boucher 2003; Herbert and Kenet 2007; Kuhl et al. 2005; Saalasti et al. 2008; Shriberg et al. 2001; Siegal and Blades 2003). One of the leading contributors to this language impairment is abnormal auditory processing (Rapin and Dunn 2003; Siegal and Blades 2003), similar to that shown in children with other language-based learning problems (Banai et al. 2009, 2005; Cunningham et al. 2001; King et al. 2002; Kraus et al. 1996; Warrier et al. 2004; Wible et al. 2002, 2005). Russo and colleagues have also reported subcortical auditory processing deficits specifically in children with ASD (Russo et al. 2008, 2009). Finally, recent findings by Salmond et al. (2007) suggested that children with ASD demonstrate different cognitive correlations with cortical processing.
In one of the only known studies of speech perception in noise in children with ASD, Alcantara and colleagues (2004) demonstrated elevated speech perception thresholds, poor temporal resolution and poor frequency selectivity. Alcantara and colleagues used five different noise conditions (single talker, speech shaped noise, speech shaped noise with temporal dips, speech shaped noise with spectral dips, and speech shaped noise with both temporal and spectral dips) to show that individuals with ASD have difficulty with behavioral recognition of speech in background noise compared to controls. Particularly difficult for these individuals were conditions with a single talker or those involving temporal dips in the background noise. Deficits were ascribed to poor temporal resolution or poor top-down processing (inability to use contextual cues). However, this investigation did not measure neural processing in background noise as an underlying explanation for the speech perception deficit.
Cortical Abnormalities in ASD
Many studies reported reversed or absent asymmetry in the inferior frontal (Broca’s area) and posterior superior temporal regions (Boddaert et al. 2004; Bruneau et al. 2003, 1999; Herbert et al. 2002; Jansson-Verkasalo et al. 2003), including the planum temporale (i.e., Wernicke’s area) (Rojas et al. 2002, 2005) and higher-order association cortices (Herbert et al. 2005). Data also suggested reduced inter- and excessive intra-connectivity of the frontal cortex (Courchesne and Pierce 2005; Just et al. 2004; Minshew and Williams 2007; Wickelgren 2005) and increased thickness in the temporal and parietal lobes (Hardan et al. 2004).
Aside from characterization of structural abnormalities, evaluation of cortical response timing and magnitude can provide valuable insight into the auditory processing deficits in ASD. Long latency auditory evoked potentials (P1, N1, P2, and N2), exogenous cortical responses generated in primary or secondary auditory cortices (e.g., superior temporal cortex, planum temporale), are elicited by the presence and physical features of sound (Ceponiene et al. 2001; Hall 1992; Naatanen and Picton 1987). Many early studies of cortical processing in ASD focused on simple stimuli, such as tones (Bruneau et al. 2003, 1999; Ferri et al. 2003; Gage et al. 2003a, b; Lincoln et al. 1995; Oades et al. 1988; Seri et al. 1999) and investigated hemispheric differences or differential encoding stimulus features such as frequency, duration, or intensity. Results are mixed, though there is some indication of reversed asymmetries and immature response patterns. Further, much of the data on exogenous responses have been extracted from studies focusing on endogenous responses involving oddball paradigms (mismatch-negativity, P3) or semantic processing (N4).
Due to the extensive literature on relationships between neural processing and speech perception in other populations (Cunningham et al. 2001, 2000; King et al. 2002; Kraus et al. 1996; Warrier et al. 2004; Wible et al. 2002, 2005), as well as the influence of disrupted cortical organization and connectivity on the language impairment in ASD (Boddaert et al. 2004; Bruneau et al. 2003, 1999; Hardan et al. 2006; Herbert et al. 2002, 2005; Jansson-Verkasalo et al. 2003; Just et al. 2004; Muller et al. 1999; Rojas et al. 2002, 2005), considerable research is now focused on the cortical processing of speech. There is some evidence for sensory processing deficits from studies focusing on speech-evoked cortical potentials in children with ASD. Ceponiene and colleagues (2003) found reduced P1 amplitude in response to vowels in children with ASD compared to typical controls, whereas Jansson-Verkasalo and colleagues (2003) identified reduced N2 amplitude in response to consonant-vowel (CV) syllables in children with Asperger’s disorder. In 2005, Lepisto and colleagues only reported significant reductions in the P1 amplitude in response to vowels in children with autism and, then in 2006, Lepisto and colleagues reported no differences in children with Asperger’s disorder. Recently, Whitehouse and Bishop (2008) reported disparate cortical processing of speech (vowels) versus nonspeech (tones) sounds. White-house and Bishop (2008) evaluated responses in both a passive listening condition and in a selective attention condition. In the passive listening condition, high-functioning children with ASD showed reduced amplitude of obligatory P1 and N2 responses in the vowel condition, but no differences in response to tones, suggesting that faulty efferent pathways may be involved in the deficient sensory processing of speech.
Cortical Processing of Speech in Noise
Although as yet uninvestigated in ASD, analysis of cortical evoked responses to speech sounds in background noise has proven useful for investigating language impairment in other populations, such as children with language-based learning and reading problems (LP) (Cunningham et al. 2001; King et al. 2002; Warrier et al. 2004; Wible et al. 2002, 2005). These data stem from the literature indicating that background noise has a deleterious effect on both audibility and cortical processing of speech (Martin et al. 1997; Whiting et al. 1998). Cunningham and colleagues (2001) reported excessive peak-to-trough amplitude reduction in children with LP than controls in cortical responses to the CV syllable “da” presented in a background noise condition. In a study investigating effects of repetition and noise on cortical encoding of speech, Wible and colleagues (2002) found that a subset of children with LP exhibited impaired response timing in noise that affected the correlation of responses under stresses of repetition. Using the same stimulus, several additional studies corroborated earlier results, including quiet-to-noise response correlation differences and latency differences in background noise in responses of children with LP compared to normal learning controls (King et al. 2002; Warrier et al. 2004; Wible et al. 2005). Moreover, auditory training has been shown to improve cortical responses to speech in background noise in children with LP (Hayes et al. 2003; Warrier et al. 2004).
Hypotheses
Given the known abnormalities in speech-evoked subcortical and cortical responses in quiet in ASD and recent behavioral findings of impaired speech perception in noise in ASD, we hypothesized that children with ASD would demonstrate prolonged timing and reduced magnitudes in response to speech-like stimuli presented in quiet and background noise compared with typically-developing (TD) children and that the group difference would be exacerbated in noise. We further predicted that the pattern of relationship between cognitive and language abilities and cortical responses would differ between groups. To test these hypotheses, we evaluated the effects of background noise on the cortical processing of the syllable “da” in children with ASD and TD controls. Additionally, correlations between cognitive and language abilities and neurophysiological measures were assessed.
Methods
The Institutional Review Board of Northwestern University approved all research and consent and assent were obtained both from the parent(s) or legal guardian(s) and the child.
Children were acclimated to the testing location and equipment prior to data collection. They were allowed to visit the laboratory and interact with the tester on multiple occasions. Some children brought electrodes home with them to help familiarize themselves with the neurophysiological procedure.
Participants
Participants included 16 verbal children with ASD (N = 14 boys, 2 girls) and 11 typically-developing children (TD, N = 7 boys, 4 girls). Age range was 7–13 years old and mean age did not differ between groups [TD, M (SD) = 9.82 (2.228) vs. ASD, M (SD) = 9.81 (1.682); independent two-tailed t-test; t(25) = 0.008, p = 0.99]. Study participants were recruited from community and internet-based organizations for families of children with ASD. Children in the diagnostic group were required to have a formal diagnosis of one form of ASD made by at least one examining professional (child neurologist or psychologist) and to be actively followed by their physicians and school professionals at regular intervals, such that the diagnostic impressions were consistent throughout their childhood and current at the time of study.
Although the Autism Diagnostic Observation Schedule [ADOS; (Lord et al. 2000, 1989)] and Autism Diagnostic Interview-Revised [ADI-R; (Le Couteur et al. 1989; Lord et al. 1994)] are the current research and academic standard for diagnosing ASD, many participants were diagnosed prior to the regular use of these instruments. The impact of this study may be strengthened by administration of the ADI-R and ADOS to confirm that the children met research diagnoses of ASD. Nevertheless, expert clinician diagnosis is thought to be a better predictor of later diagnosis than the ADOS (Chawarska et al. 2007; Lord and Richler 2006). Parents were asked to supply the names of the examining professionals, their credentials, office location, date of initial evaluation and the specific diagnosis made. Diagnostic information was supplemented by an internal questionnaire that provided developmental history (including diagnosis details, onset of symptoms, language development, other potential confounding diagnoses, etc.), a description of current status (including present symptoms, past and current treatments, medications, etc.) and functional level at time of entry into the study. Further inclusion criteria for both TD and ASD groups were (1) the absence of a confounding neurological diagnosis (e.g., active seizure disorder, cerebral palsy), (2) normal peripheral hearing as measured by air threshold pure-tone audiogram and click-evoked auditory brainstem responses [wave V latency from 5.15 to 5.79 ms, consistent with the previously reported normal range (Gorga et al. 1985; Hood 1998; Jacobson 1985)], and (3) a full-scale mental ability score ≥80.
Procedure
Hearing Screening
The collection of cortical responses was part of a larger experimental protocol in which children returned for several sessions. Therefore, at the child’s first test session, s/he underwent a hearing threshold audiogram for bilateral peripheral hearing (≤20 dB HL) for octaves between 250 and 8,000 Hz via an air conduction threshold audiogram on a Grason Stadler model GSI 61. Children wore insert earphones in each ear and were instructed to press a response button every time they heard a beep. If cortical data were not collected at the first session, a subsequent hearing screening at 20 dB HL was conducted on the same day as the cortical data collection to confirm that the child’s hearing had not changed.
Cognitive and Language Testing
Cognitive and language testing took place in a quiet office with the child seated across a table from the test administrator. Full-scale mental ability was assessed by four subtests of the Wechsler Abbreviated Scale of Intelligence [WASI; (Woerner and Overstreet 1999)]. The WASI also provided scores of performance and verbal mental ability which were not part of inclusion criteria (Table 1, mean and standard deviations; Table 2, individual scores). The clinical evaluation of language fundamentals-4 (Semel et al. 2003) was administered to provide indices of core, expressive, and receptive language abilities (Table 1).
Table 1.
Mental and language ability scores
| Test battery | Subtest | TD mean (SD) | ASD mean (SD) |
|---|---|---|---|
| WASI (mental ability) | Full scale | 116.09 (11.193) | 110.25 (12.322) |
| Verbal | 113.73 (13.016) | 104.94 (15.097) | |
| Performance | 112.00 (10.835) | 113.19 (12.475) | |
| Discrepancy* | −1.73 (10.627) | 8.25 (11.767) | |
| Core* | 115.73 (7.309) | 103.94 (15.062) | |
| CELF (language ability) | Expressive | 115.91 (10.348) | 108.00 (17.944) |
| Receptive* | 112.36 (8.477) | 100.19 (15.276) |
Mean (SD) of cognitive and language scores are reported for both typically-developing children (TD; n = 11) and children with autism spectrum disorders (ASD; n = 16). Full scale, verbal, and performance mental ability, along with the performance minus verbal ability discrepancy score were measured by the Wechsler Abbreviated Scale of Intelligence; core, expressive, and receptive abilities were assessed using the Clinical Evaluation of Language Fundamentals (CELF, 4th Edition). Children with ASD differed significantly from TD children on the discrepancy score and core and receptive language abilities (* p ≤ 0.032). However, they demonstrated similar ability on measures of full scale, verbal, and performance mental ability and expressive language ability
Table 2.
Individual performance and verbal mental ability and discrepancy scores
| Group | Score | Participant |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| TD | Performance | 96 | 97 | 99 | 107 | 112 | 115 | 118 | 118 | 119 | 124 | 127 | n/a | n/a | n/a | n/a | n/a |
| Verbal | 101 | 106 | 98 | 119 | 95 | 114 | 139 | 122 | 117 | 115 | 125 | n/a | n/a | n/a | n/a | n/a | |
| Discrepancy score | −5 | −9 | 1 | −12 | 17 | 1 | −21 | −4 | 2 | 9 | 2 | n/a | n/a | n/a | n/a | n/a | |
| ASD | Performance | 93 | 98 | 9 | 103 | 105 | 109 | 110 | 112 | 116 | 116 | 116 | 117 | 121 | 129 | 129 | 139 |
| Verbal | 81 | 103 | 103 | 105 | 87 | 103 | 105 | 85 | 116 | 109 | 84 | 121 | 109 | 134 | 109 | 125 | |
| Discrepancy score | 12 | −5 | −5 | −2 | 18 | 6 | 5 | 27 | 0 | 7 | 32 | −4 | 12 | −5 | 20 | 14 | |
Scores are reported for both TD children (n = 11) and children with ASD (n = 16) as measured by the Wechsler Abbreviated Scale of Intelligence. Children with ASD demonstrated lower verbal scores as would have been predicted by their overall intelligence. The ASD group also had higher discrepancy scores (performance minus verbal mental ability), which is characteristic of children with ASD
Stimuli and Data Collection
All neurophysiological recordings took place in a sound attenuated chamber in a single session. During testing, the child sat comfortably in a recliner chair and watched a movie of his or her choice. To encourage compliance, stimuli were presented monaurally which is a common procedure for recording cortical evoked responses (Connolly 1993; Cunningham et al. 2000; Hoormann et al. 2000; Ponton et al. 2002; Wible et al. 2002). Using this method, the children were able to listen to the movie soundtrack via the unoccluded, non-test ear. Although the video soundtrack is playing during the testing, we refer to this condition as “quiet” because masking of the sound-track is incomplete and is presented at <40 dB, a level known not to affect responses (Lavoie et al. 2008; McArthur et al. 2003). To further enhance compliance, children were accompanied by their parent(s) in the chamber and were permitted breaks during testing as needed.
All auditory stimuli were presented to the right ear through an insert earphone (ER-3, Etymotic Research, Elk Grove Village, IL, USA). Responses were recorded via Ag–AgCl electrodes, with contact impedance of ≤5 kΩ. For cortical responses, trials with artifacts exceeding 100 μV were rejected online.
Speech-Evoked Cortical Responses
Auditory evoked potentials were recorded in response to a 40 ms consonant-vowel syllable “da” synthesized in Klatt (1980). This stimulus length was chosen because it is long enough to elicit cortical responses and was brief enough to collect over 1,000 epochs (Bishop et al. 2007; Courchesne et al. 1989; Cunningham et al. 2000; Ferri et al. 2003; Groen et al. 2008; Johnstone et al. 1996; Whitehouse and Bishop 2008; Wible et al. 2002). Within the “da” syllable, the voicing begins at 5 ms and the first 10 ms are bursted. The frequency components are: F0: 103–125 Hz, F1: 220–720 Hz, F2: 1,700–1,240 Hz, F3: 2,580–2,500 Hz, F4: 3,600 (constant), F5: 4,500 (constant); frequencies spanning the F2–F5 range comprise what is referred to here as high frequency information. The “da” stimuli were presented with alternating polarity. The “da”-evoked responses were collected in two different conditions, at a conversational speech level in quiet (80 dB SPL) and embedded in background noise presented at 75 dB SPL.
Electrodes were placed on the vertex (Cz), the contra-lateral earlobe (reference), the forehead (ground), and the superior canthus of the left eye (to monitor eye blinks). Stimuli were presented (Neuroscan Sound) with an inter-stimulus interval of 631 ms. Continuous white Gaussian noise was generated by a Biologic Navigator system and mixed with the “da” stimulus in a Studiomaster mixer board to produce a signal to noise ratio of 5 dB. Responses were recorded in Neuroscan 4.2 Acquire continuous mode with a sampling rate of 2,000 Hz and a bandpass filter of 0.5–100 Hz (12 dB/octave), with a notch filter at 60 Hz, to isolate the frequencies that are most robustly encoded at the level of the cortex. An online average was recorded simultaneously to monitor when approximately 1,000 acceptable sweeps had been collected.
Data Analyses
Eye blink artifacts were removed from the continuous EEG recording using a spatial filtering method implemented in Neuroscan 4.3 Edit. The eye-blink free file was bandpass filtered from 1 to 40 Hz, and epoched using a 625 ms window (125 ms pre-stimulus period). An artifact rejection criterion of ±65 μV was applied to the epoched file to remove sweeps containing large myogenic noise. The 100–300 ms time range of each of the remaining sweeps was correlated with the corresponding time window of the ad hoc average of all artifact-free sweeps. Next, the sweeps were ranked according to how well they correlated with this average and the best 70% of correlated sweeps were used to create the final response average. Before performing statistical analyses, these final averages were pre-stimulus baseline corrected to remove the DC drift. (Unless indicated otherwise, all data reduction was performed in Matlab 7.4.)
Based on a resemblance to responses in a previous study using subjects of similar age and a similar stimulus (Cunningham et al. 2001) and analysis of the grand average quiet response, the largest positive deflection (occurring approximately between 100 and 200 ms) was defined as the P1′ response and the following negative trough (between 150 and 300 ms) was defined as N1′. For the background noise condition, individual responses were overlaid with the quiet responses and corresponding waveform morphology guided the choice of peak. An experienced peak picker manually picked all peaks and two additional peak pickers then confirmed these marks. All peak pickers were blind to subject diagnosis. Response measures included positive and negative peak latencies, peak-to-trough duration, amplitude and slope, signal-to-noise ratio, and quiet-to-noise response correlations. Peak-to-trough duration was the difference between the P1′ and N1′ latencies. Peak-to-trough amplitude was defined as the difference between P1′ and N1′ amplitudes. Peak-to-trough slope is the slope of the line between connecting P1′ and N1′ response peaks. Signal-to-noise ratio was the ratio of the RMS amplitudes of the stimulus-related activity to non-stimulus related (pre-stimulus) activity. Quiet-to-noise response correlations are cross-correlations between the quiet response and the noise response.
Independent Student’s t-tests (two-tailed) were used to evaluate group differences in mental and language abilities; the two-tailed result is reported because no differences were expected since all children met our inclusion criterion. Measures of cortical neurophysiology were first evaluated via a mixed design repeated measures analysis of variance (RMANOVA) to test the hypothesis that sensory encoding of speech in quiet and noise is disrupted in the cortex of children with ASD. Dependent variables included the cortical response measures listed above; the between-subjects factor was diagnosis; the within-subjects factor was condition (quiet versus noise). Based on prior data showing group differences in cortical encoding of speech, our statistics were hypothesis-driven and thus, when appropriate, post-hoc analyses were conducted with one-tailed independent Student’s t-tests. To control for Type 1 errors during post hoc analyses, an adjusted alpha level of p ≤ 0.017 was required for establishing significance. Additionally, paired t-tests within groups were conducted to evaluate whether children with ASD show the same effect of background noise as TD children. Levene’s Test for Equality of Variances was applied to each statistical analysis and, when relevant, the reported p-values reflect corrections based on unequal variances. In order to discern any behavioral significance of cortical deficits, relationships between cortical response measures that differed between groups and cognitive and language abilities were evaluated via Pearson’s correlations. Significant relationships were defined as r-values ≥ 0.35 and p-values ≤ 0.05. For all statistical analyses involving quiet-to-noise response correlations, r-values were converted first to Fisher z′-scores.
Age and Sex Considerations
Age was considered a variable in preliminary statistical analyses. There were no correlations between age and any of the dependent variables (Pearson’s r ≤ 0.21; p ≥ 0.191, in all cases). Due to the greater incidence of ASD in males versus females in the general population, the ASD group in this study included a majority of male participants. However, sex is not thought to affect cortical responses (Cunningham et al. 2000). Further, inclusion of age and sex as covariates indicated that they were not statistically significant, and as a result, they were not considered in subsequent analyses. Additional statistical analyses conducted on smaller age-matched groups and sex-matched groups showed similar results. Effect sizes (Cohen’s d) for all significant comparisons within both the larger and smaller groups were greater than 0.89, suggesting that these effects are robust despite age range. Therefore, to increase power, data reported were with respect to the larger groups.
Results
Cognitive and Academic Testing
Children with ASD did not differ significantly from TD children on measures of full-scale [t(25) = 1.13; p = 0.268], verbal [t(25) = 1.57; p = 0.129] or performance [t(25) = −0.26; p = 0.800] mental ability, or expressive language ability [t(25) = 1.31; p = 0.201], but did differ from TD children on measures of core [t(25) = 2.60; p = 0.015] and receptive [t(25) = 2.39; p = 0.025] language abilities (see Table 1 for means and standard deviations). Comparison of the performance minus verbal intelligence discrepancy score revealed a significant difference [t(25) = 2.28; p = 0.032]. The TD group discrepancy score was −1.73 (indicating overall better verbal ability), whereas the ASD group score is 8.25 (overall better performance ability). Individual participants’ discrepancy scores (performance minus verbal mental ability) are provided in Table 2. This pattern of results is consistent with what is commonly reported in children on the autism spectrum (Tsatsanis 2005). Although as a group, the children with ASD did not meet the clinical standard of an 11 point discrepancy score, 7 of the 16 children with ASD met this criterion. In the TD group, only 1 child met this criterion. Additionally, almost half of the children in the ASD group demonstrated scores <100 on the core language measure.
Speech-Evoked Cortical Responses
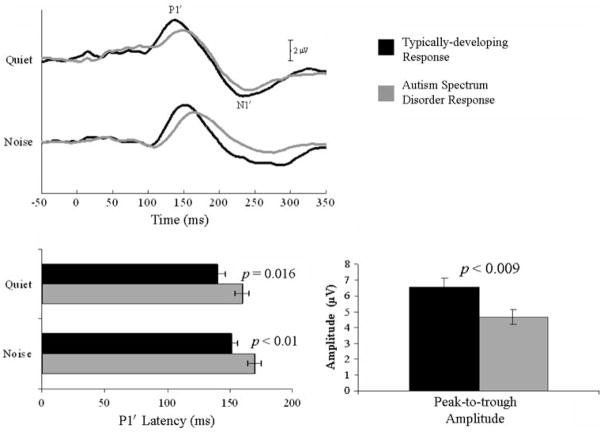
The mixed-design RMANOVA indicated significant between-group main effects on measures of P1′ latency (F(1,25) = 7.37; p = 0.012) and peak-to-trough amplitude (F(1,25) = 4.52; p = 0.044). Follow-up protected independent t-tests (one-tailed) indicated delayed P1′ latencies in both quiet and background noise [t(25) = −2.27; p = 0.016 and t(25) = −2.51; p < 0.001, respectively] and reduced peak-to-trough amplitudes in quiet [t(25) = 2.57; p = 0.009] in children with ASD compared to TD children (Fig. 1). See Table 3 for means and standard deviations of all dependent variables.
Fig. 1.
Grand average cortical responses (quiet (top); background noise (bottom)) of typically-developing (TD) children (black lines) and children with ASD (gray lines). Children with ASD demonstrated significant delays in P1′ latency in both the quiet and background noise conditions (p ≤ 0.017), as well as significant reductions in peak-to-trough amplitudes in the quiet condition
Table 3.
Cortical response measures
| Quiet |
Noise |
|||
|---|---|---|---|---|
| TD mean (SD) | ASD mean (SD) | TD mean (SD) | ASD mean (SD) | |
| P1′ latency (ms)* | 140.14 (20.625) | 159.69 (22.809) | 151.91 (14.193) | 169.91 (20.571) |
| N1′ latency (ms) | 235.41 (21.432) | 244.91 (30.480) | 236.41 (26.693) | 247.41 (32.117) |
| Peak-to-trough duration (ms) | 95.27 (22.202) | 85.22 (19.211) | 84.50 (23.671) | 77.50 (29.051) |
| Peak-to-trough amplitude (mV)* | 6.56 (1.894) | 4.66 (1.899) | 5.22 (2.109) | 4.13 (1.961) |
| Slope (μV/ms) | −0.07 (0.030) | −0.05 (0.020) | −0.07 (0.031) | −0.05 (0.019) |
| Signal-to-noise ratio RMS | 9.13 (4.574) | 10.67 (2.504) | 8.97 (6.147) | 8.23 (3.376) |
| Quiet-to-noise correlation (r-value) | – | – | 0.82 (0.467) | 0.54 (0.690) |
Mean (SD) of cortical response measures in quiet and background noise conditions are reported for both TD children (n = 11) and children with ASD (n = 16). Responses of children with ASD demonstrated significantly prolonged P1′ latencies in quiet and background noise, as well as reduced peak-to-trough amplitudes in quiet compared to responses of TD children (* p ≤ 0.017)
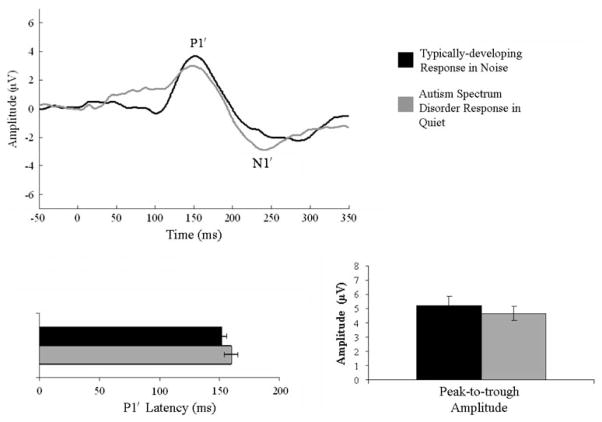
Within group analyses (paired t-tests) indicated that noise adversely affected the TD response, while the ASD response was similar in quiet to noise [t(15) ≤ 1.81; p ≥ 0.045, all comparisons]. Specifically, in background noise, the TD response demonstrated a delayed P1′ latency [t(10) = −2.73; p = 0.011] and a reduced peak-to-trough amplitude [t(10) = 2.48; p = 0.017]. However, peak-to-trough duration became shorter in background noise [t(10) = 2.5; p = 0.015]. As a final comparison, TD responses in noise were compared to ASD responses in quiet and revealed no significant differences in any of the dependent variables [t(25) ≤ 1.00; p ≥ 0.163, all comparisons; Fig. 2].
Fig. 2.
Comparison of the typical response in background noise to the ASD response in quiet. Responses to stimuli in background noise in the TD group showed no significant differences from the ASD response in quiet. These results suggest that the children with ASD encode speech in quiet similarly to the manner in which TD children encode speech in background noise, giving them a disadvantage for speech perception
Correlations with Cognitive and Language Abilities
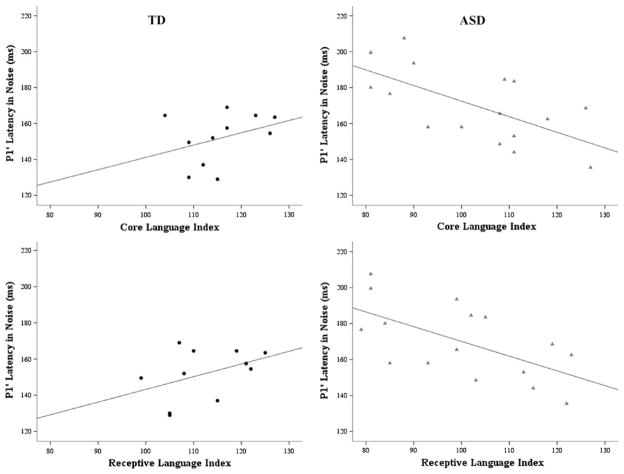
Correlations were computed between neurophysiological measures of P1′ latency in quiet, P1′ latency in background noise, peak-to-trough amplitude in quiet and cognitive and language abilities. Robust relationships between the latency measures existed only in the group of children with ASD. There was a significant relationship between P1′ latency in quiet and verbal mental ability (r = −0.57; p < 0.02). P1′ latency in noise correlated with full scale mental ability (r = −0.65; p = 0.006), verbal mental ability (r = −0.70; p = 0.003) and both core and receptive language ability (r = −0.64; p = 0.01 and r = −0.61; p = 0.01, respectively; Fig. 3). For all of these relationships, earlier response latencies were related to better cognitive and language scores. Peak-to-trough amplitude did not relate to the cognitive and language scores.
Fig. 3.
Significant correlations between cortical responses and language indices. Children with ASD showed significant correlations between P1′ latency in background noise and core and receptive language scores (gray triangles on right panel plots; r ≥ − 0.57, p ≤ 0.02, all comparisons). TD children did not show these relationships (black circles on left panel plots). Similar patterns were identified between P1′ latency in quiet and background noise and mental ability scores for children with ASD (data not shown)
Discussion
Summary of Neurophysiological Results
Children clinically diagnosed as being on the autism spectrum showed both timing and amplitude deficits in cortical processing of speech in quiet as well as timing deficits in background noise. As anticipated, TD children showed P1′ latency increases and peak-to-trough amplitude reductions when encoding speech in background noise. In contrast, children with ASD showed deficits in P1′ latency and peak-to-trough amplitude compared to TD children, but no additive effect of background noise, such that in this study, children with ASD processed speech in both quiet and noise comparably to the manner in which TD children encode speech in noise. Previous studies have investigated and dismissed the effect of video sounds on evoked potentials in the “quiet” condition (Lavoie et al. 2008; McArthur et al. 2003). However, given that children with ASD had quiet responses comparable to TD noise responses, it is possible that the soundtrack had a larger impact on the quiet responses of the children with ASD.
Interestingly, although the ASD response in noise was significantly different from the TD response in noise, the effect of white background noise on the P1′ response was similar between groups, such that regardless of starting latency, the shift in latency from quiet to noise was of the same degree (~10 ms) across both groups. This observation further speaks to the importance of response integrity in quiet conditions because degraded processing in quiet is further severely perpetuated by background noise.
Summary of Correlations
Although this study did not show correlations with age, typically, cortical response latencies become earlier with maturation (Cunningham et al. 2000). Thus, these data support the notion that children with ASD may have a developmental delay (Gage et al. 2003). Interestingly, although delayed latencies are consistent with an immature response, the reduced amplitude in children with ASD defied the maturational pattern. Therefore maturation of the response does not provide a complete explanation of results shown here.
Finally, correlations between response measures and cognitive (full-scale and verbal) and language (core and receptive) abilities were found only in children with ASD. These data are consistent with recent findings of correlations between verbal mental ability and cortical evoked potentials in children with ASD (Salmond et al. 2007).
Relationship to Previous Findings
Converging evidence from evoked potential and magnetic resonance imaging studies implicates abnormal differentiation of cortical areas important for language processing in ASD, which may explain, in part, the results of the current study (review in Volkmar et al. 2004). The prolonged response timing found in the current study may be indicative of aberrant connectivity (Courchesne and Pierce 2005; Just et al. 2004; Minshew and Williams 2007; Wickelgren 2005) such that sound cannot efficiently propagate the auditory pathway, resulting in delayed latencies. Additionally, the observed reduced amplitude in ASD may reflect poor coordination and decreased neural synchrony in response to speech. Further, neural noise in the cortex, associated with increased intra-connectivity and synaptic activity, may impede a robust stimulus-triggered response, but the equivalent signal-to-noise ratios between groups in the current study do not support this idea. Finally, another possibility is that reduced experience with language in ASD prevents normal development of auditory cortex.
Overall, our results are consistent with converging evidence of cortical speech processing deficits in children with ASD (Whitehouse and Bishop 2008), although they show some variations from previously reported studies (Ceponiene et al. 2003; Jansson-Verkasalo et al. 2003; Lepisto et al. 2005, 2006). With the exception of Lepisto and colleagues’ study (2006), reduced amplitudes have been reported in response to speech in quiet. The current study differed in that it examined peak-to-trough amplitude rather than peak-to-baseline amplitudes. Because some children demonstrated N1′ responses that were above baseline, peak-to-trough amplitudes were reported here. Additionally, variations in results may be indicative of the different speech syllables and qualitatively different responses; our stimulus elicited a robust positive peak between 100–200 ms and a negativity occurring between 200–300 ms while others reported positive peaks in the 50–150 ms range and negative peaks in the 150– or 180–300 ms range. Also potentially accounting for differences is that both Ceponiene and colleagues (2003) and Lepisto and colleagues (2005, 2006) used a vowel rather than a CV syllable. Children with autism in the study by Lepisto and colleagues (2005) differed from the controls on measures of performance and verbal mental ability, whereas in this study, the children with ASD did not differ from TD children on measures of mental ability. Finally, some studies focused only on specific subtypes of the ASD, i.e., children with Asperger’s disorder (Jansson-Verkasalo et al. 2003; Lepisto et al. 2006) or autism (Ceponiene et al. 2003; Lepisto et al. 2005). Thus, the heterogeneity of children with ASD (Freitag 2007; Happe et al. 2006; London 2007; Salmond et al. 2007; Tager-Flusberg and Caronna 2007) may account for the varying results. Any of these disparities individually, or in combination, may have contributed to across-study differences.
These results imply a different mechanism of deficit in children with ASD compared to children with other language-based disorders such as developmental dyslexia. In the latter group, children with LP exhibited cortical deficits only in background noise; poor timing in noise adversely affected quiet-to-noise response correlations (Cunningham et al. 2001; King et al. 2002; Warrier et al. 2004; Wible et al. 2002, 2005). Instead, children with ASD showed impairments in cortical encoding of “da” in both quiet and background noise conditions. Thus, children with ASD start out at a disadvantage for speech processing in quiet (significantly delayed P1′ latency and reduced peak-to-trough amplitude) and maintain abnormalities processing speech in background noise (significantly delayed P1′ latency) compared to TD children. Interestingly, children with ASD also show more pervasive auditory subcortical deficits compared to children with other language impairments (Banai et al. 2009; Cunningham et al. 2001; Russo et al. 2009, 2008; Wible et al. 2004).
Implications
This study provides new data on speech-related cortical processing deficits in quiet and implicates atypical cortical processing of speech in background noise in ASD. These data reinforce the functional relationship between cortical speech processing and cognitive and language profiles in children with ASD. Viewed in the context of known atypical cortical organization in ASD and the robustness of cortical plasticity, this approach may aid in the assessment of auditory remediation in ASD.
These data suggest two potential explanations for the correlation between cortical processing and verbal abilities found in the ASD group: (1) children with deficient auditory systems may be unable to develop advanced verbal skills due to overall cognitive deficits (performance-verbal discrepancy scores) or (2) children with weak verbal abilities are unable to fine-tune their auditory systems, thus culminating in poor cortical differentiation for speech and impaired speech sound processing. Future longitudinal studies characterizing speech-evoked cortical processing throughout development in relation to the development of verbal abilities might arbitrate between the possibilities. Both scenarios support early remediation of language and auditory function. Future research should focus on speech-evoked cortical processing in quiet and background noise conditions in both young children and adolescents with ASD.
By including children with a range of diagnosis of ASD made by community clinicians, this study lacked the application of more precise tools for research and academic classification of subjects, such as the ADOS (Lord et al. 2000, 1989) and ADI-R (Le Couteur et al. 1989; Lord et al. 1994). With data from the ADOS and ADI-R unavailable, both our ability to characterize specific deficits in these children with ASD and our ability to identify encoding differences between diagnostic categories (e.g., autism versus Asperger’s disorder) was limited. Therefore, future studies should utilize these tools to better characterize participants and to better be able to draw conclusions about the relationships between diagnoses, language abilities, and cortical processing of speech-like sounds. Additionally, future work should more vigorously investigate the relationship between neurophysiological effects of both pure quiet and a variety of background noise conditions and behavioral tests of speech perception in noise in children with ASD.
Acknowledgments
This research was supported by NIH R01 DC01510. The authors declare that they have no competing financial interests. We would like to thank the children who participated in this study and their families; Trent Nicol, Gabriella Musacchia, and Daniel Abrams for assisting with peak picking; Trent Nicol and Erika Skoe for their contributions to this research, particularly in technical aspects of data processing and software development; Jane Hornickel for her assistance with data collection.
Contributor Information
Nicole Russo, Email: nicole_russo@rush.edu.
Nina Kraus, Email: nkraus@northwestern.edu.
References
- Alcantara JI, Weisblatt EJ, Moore BC, Bolton PF. Speech-in-noise perception in high-functioning individuals with autism or asperger’s syndrome. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2004;45(6):1107–1114. doi: 10.1111/j.1469-7610.2004.t01-1-00303.x.. [DOI] [PubMed] [Google Scholar]
- Banai K, Hornickel JM, Skoe E, Nicol T, Zecker S, Kraus N. Reading and subcortical auditory function. Cerebral Cortex (New York, NY) 2009 doi: 10.1093/cercor/bhp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai K, Nicol T, Zecker SG, Kraus N. Brainstem timing: Implications for cortical processing and literacy. The Journal of Neuroscience. 2005;25(43):9850–9857. doi: 10.1523/JNEUROSCI.2373-05.2005.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DV, Hardiman M, Uwer R, von Suchodoletz W. Maturation of the long-latency auditory ERP: Step function changes at start and end of adolescence. Developmental Science. 2007;10(5):565–575. doi: 10.1111/j.1467-7687.2007.00619.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: A voxel-based morphometry MRI study. NeuroImage. 2004;23(1):364–369. doi: 10.1016/j.neuroimage.2004.06.016.. [DOI] [PubMed] [Google Scholar]
- Boucher J. Language development in autism. International Journal of Pediatric Otorhinolaryngology. 2003;67(Suppl 1):S159–S163. doi: 10.1016/j.ijporl.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Bruneau N, Bonnet-Brilhault F, Gomot M, Adrien JL, Barthelemy C. Cortical auditory processing and communication in children with autism: Electrophysiological/behavioral relations. International Journal of Psychophysiology. 2003;51(1):17–25. doi: 10.1016/S0167-8760(03)00149-1.. [DOI] [PubMed] [Google Scholar]
- Bruneau N, Roux S, Adrien JL, Barthelemy C. Auditory associative cortex dysfunction in children with autism: Evidence from late auditory evoked potentials (N1 wave-T complex) Clinical Neurophysiology. 1999;110(11):1927–1934. doi: 10.1016/S1388-2457(99)00149-2.. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Lepisto T, Shestakova A, Vanhala R, Alku P, Naatanen R, et al. Speech-sound-selective auditory impairment in children with autism: They can perceive but do not attend. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(9):5567–5572. doi: 10.1073/pnas.0835631100.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceponiene R, Shestakova A, Balan P, Alku P, Yiaguchi K, Naatanen R. Children’s auditory event-related potentials index sound complexity and “speechness”. The International Journal of Neuroscience. 2001;109(3–4):245–260. doi: 10.3109/00207450108986536. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: Stability and change in syndrome expression. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48(2):128–138. doi: 10.1111/j.1469-7610.2006.01685.x.. [DOI] [PubMed] [Google Scholar]
- Connolly JF. The influence of stimulus intensity, contra-lateral masking and handedness on the temporal N1 and the T complex components of the auditory N1 wave. Electroencephalography and Clinical Neurophysiology. 1993;86(1):58–68. doi: 10.1016/0013-4694(93)90067-6.. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Lincoln AJ, Yeung-Courchesne R, Elmasian R, Grillon C. Pathophysiologic findings in nonretarded autism and receptive developmental language disorder. Journal of Autism and Developmental Disorders. 1989;19(1):1–17. doi: 10.1007/BF02212714.. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Current Opinion in Neurobiology. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001.. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Nicol T, Zecker SG, Bradlow A, Kraus N. Neurobiologic responses to speech in noise in children with learning problems: Deficits and strategies for improvement. Clinical Neurophysiology. 2001;112(5):758–767. doi: 10.1016/S1388-2457(01)00465-5.. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Nicol T, Zecker S, Kraus N. Speech-evoked neurophysiologic responses in children with learning problems: Development and behavioral correlates of perception. Ear and Hearing. 2000;21(6):554–568. doi: 10.1097/00003446-200012000-00003.. [DOI] [PubMed] [Google Scholar]
- Ferri R, Elia M, Agarwal N, Lanuzza B, Musumeci SA, Pennisi G. The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clinical Neurophysiology. 2003;114(9):1671–1680. doi: 10.1016/S1388-2457(03)00153-6.. [DOI] [PubMed] [Google Scholar]
- Freitag CM. The genetics of autistic disorders and its clinical relevance: A review of the literature. Molecular Psychiatry. 2007;12(1):2–22. doi: 10.1038/sj.mp.4001896.. [DOI] [PubMed] [Google Scholar]
- Gage NM, Siegel B, Callen M, Roberts TP. Cortical sound processing in children with autism disorder: An MEG investigation. NeuroReport. 2003a;14(16):2047–2051. doi: 10.1097/00001756-200311140-00008.. [DOI] [PubMed] [Google Scholar]
- Gage NM, Siegel B, Roberts TP. Cortical auditory system maturational abnormalities in children with autism disorder: An MEG investigation. Brain Research. 2003b;144(2):201–209. doi: 10.1016/S0165-3806(03)00172-X.. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Abbas PJ, Worthington DW. Stimulus calibrations in ABR measurements. In: Jacobson JT, editor. The auditory brainstem response. San Diego: College-Hill Press; 1985. pp. 49–62. [Google Scholar]
- Groen MA, Alku P, Bishop DV. Lateralisation of auditory processing in Down syndrome: A study of T-complex peaks Ta and Tb. Biological Psychology. 2008;79(2):148–157. doi: 10.1016/j.biopsycho.2008.04.003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JW. Handbook of auditory evoked responses. Needham Heights: Allyn and Bacon; 1992. [Google Scholar]
- Happe F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nature Neuroscience. 2006;9(10):1218–1220. doi: 10.1038/nn1770.. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Jou RJ, Keshavan MS, Varma R, Minshew NJ. Increased frontal cortical folding in autism: A preliminary MRI study. Psychiatry Research. 2004;131(3):263–268. doi: 10.1016/j.pscychresns.2004.06.001.. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Muddasani S, Vemulapalli M, Keshavan MS, Minshew NJ. An MRI study of increased cortical thickness in autism. The American Journal of Psychiatry. 2006;163(7):1290–1292. doi: 10.1176/appi.ajp.163.7.1290.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EA, Warrier CM, Nicol TG, Zecker SG, Kraus N. Neural plasticity following auditory training in children with learning problems. Clinical Neurophysiology. 2003;114(4):673–684. doi: 10.1016/S1388-2457(02)00414-5.. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, et al. Abnormal asymmetry in language association cortex in autism. Annals of Neurology. 2002;52(5):588–596. doi: 10.1002/ana.10349.. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Kenet T. Brain abnormalities in language disorders and in autism. Pediatric Clinics of North America. 2007;54(3):563–583. doi: 10.1016/j.pcl.2007.02.007. vii.. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Kennedy DN, Filipek PA, et al. Brain asymmetries in autism and developmental language disorder: A nested whole-brain analysis. Brain. 2005;128(Pt 1):213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Hood L. Clinical applications of the auditory brainstem response. Singular Publishing Group, Inc; San Diego: 1998. [Google Scholar]
- Hoormann J, Falkenstein M, Hohnsbein J. Early attention effects in human auditory-evoked potentials. Psychophysiology. 2000;37:29–42. doi: 10.1017/S0048577200981290. [DOI] [PubMed] [Google Scholar]
- Jacobson JT, editor. The auditory brainstem response. San Diego: College-Hill Press; 1985. [Google Scholar]
- Jansson-Verkasalo E, Ceponiene R, Kielinen M, Suominen K, Jantti V, Linna SL, et al. Deficient auditory processing in children with Asperger syndrome, as indexed by event-related potentials. Neuroscience Letters. 2003;338(3):197–200. doi: 10.1016/S0304-3940(02)01405-2.. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Barry RJ, Anderson JW, Coyle SF. Age-related changes in child and adolescent event-related potential component morphology, amplitude and latency to standard and target stimuli in an auditory oddball task. International Journal of Psychophysiology. 1996;24(3):223–238. doi: 10.1016/S0167-8760(96)00065-7.. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of under-connectivity. Brain. 2004;127(Pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- King C, Warrier CM, Hayes E, Kraus N. Deficits in auditory brainstem pathway encoding of speech sounds in children with learning problems. Neuroscience Letters. 2002;319(2):111–115. doi: 10.1016/S0304-3940(01)02556-3.. [DOI] [PubMed] [Google Scholar]
- Klatt D. Software for cascade/parallel formant synthesizer. The Journal of the Acoustical Society of America. 1980;67:971–975. doi: 10.1121/1.383940. [DOI] [Google Scholar]
- Kraus N, McGee TJ, Zecker SG, Nicol T, Koch DB. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: Behavioral and electrophysiological measures. Developmental Science. 2005;8(1):F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Lavoie BA, Hine JE, Thornton RD. The choice of distracting task can affect the quality of auditory evoked potentials recorded for clinical assessment. International Journal of Audiology. 2008;47(7):439–444. doi: 10.1080/14992020802033109.. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, et al. Autism diagnostic interview: A standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19(3):363–387. doi: 10.1007/BF02212936.. [DOI] [PubMed] [Google Scholar]
- Lepisto T, Kujala T, Vanhala R, Alku P, Huotilainen M, Naatanen R. The discrimination of and orienting to speech and non-speech sounds in children with autism. Brain Research. 2005;1066(1–2):147–157. doi: 10.1016/j.brainres. 2005.10.052. [DOI] [PubMed] [Google Scholar]
- Lepisto T, Silokallio S, Nieminen-von Wendt T, Alku P, Naatanen R, Kujala T. Auditory perception and attention as reflected by the brain event-related potentials in children with asperger syndrome. Clinical Neurophysiology. 2006;117(10):2161–2171. doi: 10.1016/j.clinph.2006.06.709.. [DOI] [PubMed] [Google Scholar]
- Lincoln AJ, Courchesne E, Harms L, Allen M. Sensory modulation of auditory stimuli in children with autism and receptive developmental language disorder: Event-related brain potential evidence. Journal of Autism and Developmental Disorders. 1995;25(5):521–539. doi: 10.1007/BF02178298.. [DOI] [PubMed] [Google Scholar]
- London E. The role of the neurobiologist in redefining the diagnosis of autism. Brain Pathology (Zurich, Switzerland) 2007;17(4):408–411. doi: 10.1111/j.1750-3639.2007.00103.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Richler J. Early diagnosis of children with autism spectrum disorders. In: Charman T, Stone W, editors. Social and communication development in autism spectrum disorders: Early identification, diagnosis, & intervention. New York, NY: Guilford Press; 2006. pp. 35–59. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. doi: 10.1023/A:1005592401947.. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, et al. Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19(2):185–212. doi: 10.1007/BF02211841.. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145.. [DOI] [PubMed] [Google Scholar]
- Martin BA, Sigal A, Kurtzberg D, Stapells DR. The effects of decreased audibility produced by high-pass noise masking on cortical event-related potentials to speech sounds/ba/ and /da/ The Journal of the Acoustical Society of America. 1997;101(3):1585–1599. doi: 10.1121/1.418146.. [DOI] [PubMed] [Google Scholar]
- McArthur GM, Bishop DV, Proudfoot M. Do video sounds interfere with auditory event-related potentials? Behavior Research Methods, Instruments and Computers: a journal of the Psychonomic Society Inc. 2003;35(2):329–333. doi: 10.3758/bf03202561. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism: Cortex, connectivity, and neuronal organization. Archives of Neurology. 2007;64(7):945–950. doi: 10.1001/archneur. 64.7.945.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RA, Behen ME, Rothermel RD, Chugani DC, Muzik O, Mangner TJ, et al. Brain mapping of language and auditory perception in high-functioning autistic adults: A pet study. Journal of Autism and Developmental Disorders. 1999;29(1):19–31. doi: 10.1023/A:1025914515203.. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology. 1987;24(4):375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x.. [DOI] [PubMed] [Google Scholar]
- Oades RD, Walker MK, Geffen LB, Stern LM. Event-related potentials in autistic and healthy children on an auditory choice reaction time task. International Journal of Psychophysiology. 1988;6(1):25–37. doi: 10.1016/0167-8760(88)90032-3.. [DOI] [PubMed] [Google Scholar]
- Ponton C, Eggermont JJ, Khosla D, Kwong B, Don M. Maturation of human central auditory system activity: Separating auditory evoked potentials by dipole source modeling. Clinical Neurophysiology. 2002;113(3):407–420. doi: 10.1016/S1388-2457(01)00733-7.. [DOI] [PubMed] [Google Scholar]
- Rapin I, Dunn M. Update on the language disorders of individuals on the autistic spectrum. Brain and Development. 2003;25(3):166–172. doi: 10.1016/S0387-7604(02)00191-2.. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Bawn SD, Benkers TL, Reite ML, Rogers SJ. Smaller left hemisphere planum temporale in adults with autistic disorder. Neuroscience Letters. 2002;328(3):237–240. doi: 10.1016/S0304-3940(02)00521-9.. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Camou SL, Reite ML, Rogers SJ. Planum temporale volume in children and adolescents with autism. Journal of Autism and Developmental Disorders. 2005;35(4):479–486. doi: 10.1007/s10803-005-5038-7.. [DOI] [PubMed] [Google Scholar]
- Russo N, Nicol T, Trommer B, Zecker S, Kraus N. Brainstem transcription of speech is disrupted in children with autism spectrum disorders. Developmental Science. 2009 doi: 10.1111/j.1467-7687.2008.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo N, Skoe E, Trommer B, Nicol T, Zecker S, Bradlow A, et al. Deficient brainstem encoding of pitch in children with autism spectrum disorders. Clinical Neurophysiology. 2008;119(8):1720–1731. doi: 10.1016/j.clinph.2008.01.108.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalasti S, Lepistö T, Toppila E, Kujala T, Laakso M, Nieminen-von Wendt T, et al. Language abilities of children with asperger syndrome. Journal of Autism and Developmental Disorders. 2008;38(8):1574–1580. doi: 10.1007/s10803-008-0540-3.. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Vargha-Khadem F, Gadian DG, de Haan M, Baldeweg T. Heterogeneity in the patterns of neural abnormality in autistic spectrum disorders: Evidence from ERP and MRI. Cortex. 2007;43(6):686–699. doi: 10.1016/S0010-9452(08)70498-2.. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals. 4. San Antonio, TX: Harcourt Assessment, Inc; 2003. [Google Scholar]
- Seri S, Cerquiglini A, Pisani F, Curatolo P. Autism in tuberous sclerosis: Evoked potential evidence for a deficit in auditory sensory processing. Clinical Neurophysiology. 1999;110(10):1825–1830. doi: 10.1016/S1388-2457(99)00137-6.. [DOI] [PubMed] [Google Scholar]
- Shriberg LD, Paul R, McSweeny JL, Klin AM, Cohen DJ, Volkmar FR. Speech and prosody characteristics of adolescents and adults with high-functioning autism and asperger syndrome. Journal of Speech, Language, and Hearing Research: JSLHR. 2001;44(5):1097–1115. doi: 10.1044/1092-4388(2001/087).. [DOI] [PubMed] [Google Scholar]
- Siegal M, Blades M. Language and auditory processing in autism. Trends in Cognitive Sciences. 2003;7(9):378–380. doi: 10.1016/S1364-6613(03)00194-3.. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Caronna E. Language disorders: Autism and other pervasive developmental disorders. Pediatric Clinics of North America. 2007;54(3):469–481. doi: 10.1016/j.pcl.2007.02.011.vi.. [DOI] [PubMed] [Google Scholar]
- Tsatsanis K. Neuropsychological aspects of autism and related conditions. In: Volkmar F, Paul R, Klin A, Cohen D, editors. Handbook of autism and pervasive developmental disorders. Vol. 1. New York, NY: Wiley & Sons; 2005. pp. 365–381. [Google Scholar]
- Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2004;45(1):135–170. doi: 10.1046/j.0021-9630.2003.00317.x.. [DOI] [PubMed] [Google Scholar]
- Warrier CM, Johnson KL, Hayes EA, Nicol T, Kraus N. Learning impaired children exhibit timing deficits and training-related improvements in auditory cortical responses to speech in noise. Experimental Brain Research. 2004;157(4):431–441. doi: 10.1007/s00221-004-1857-6.. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJO, Bishop D. Do children with autism ‘switch off’ to speech sounds? An investigation using event-related potentials. Developmental Science. 2008;11(4):516–524. doi: 10.1111/j.1467-7687.2008.00697.x.. [DOI] [PubMed] [Google Scholar]
- Whiting KA, Martin BA, Stapells DR. The effects of broadband noise masking on cortical event-related potentials to speech sounds/ba/and/da/ Ear and Hearing. 1998;19(3):218–231. doi: 10.1097/00003446-199806000-00005.. [DOI] [PubMed] [Google Scholar]
- Wible B, Nicol T, Kraus N. Abnormal neural encoding of repeated speech stimuli in noise in children with learning problems. Clinical Neurophysiology. 2002;113(4):485–494. doi: 10.1016/S1388-2457(02)00017-2.. [DOI] [PubMed] [Google Scholar]
- Wible B, Nicol T, Kraus N. Atypical brainstem representation of onset and formant structure of speech sounds in children with language-based learning problems. Biological Psychology. 2004;67:299–317. doi: 10.1016/j.biopsycho.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Wible B, Nicol T, Kraus N. Correlation between brainstem and cortical auditory processes in normal and language-impaired children. Brain. 2005;128(Pt 2):417–423. doi: 10.1093/brain/awh367. [DOI] [PubMed] [Google Scholar]
- Wickelgren I. Neurology. Autistic brains out of synch? Science [New York, NY (Dayton, Ohio)] 2005;308(5730):1856–1858. doi: 10.1126/science.308.5730.1856. [DOI] [PubMed] [Google Scholar]
- Woerner C, Overstreet K. Wechsler abbreviated scale of intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]