Abstract
Mechanical pain contributes to the morbidity associated with inflammation and trauma, but primary sensory neurons that convey the sensation of acute and persistent mechanical pain have not been identified. Dorsal root ganglion (DRG) neurons transmit sensory information to the spinal cord using the excitatory transmitter glutamate1, a process that depends on glutamate transport into synaptic vesicles for regulated exocytotic release. Here we report that a small subset of cells in the DRG expresses the low abundance vesicular glutamate transporter VGLUT3. In the dorsal horn of the spinal cord, these afferents project to lamina I and the innermost layer of lamina II, which has previously been implicated in persistent pain caused by injury2. Since the different VGLUT isoforms generally exhibit a nonredundant pattern of expression3, we used VGLUT3 knock-out (KO) mice to assess the role of VGLUT3+ primary afferents in the behavioral response to somatosensory input. The loss of VGLUT3 specifically impairs mechanical pain sensation and in particular the mechanical hypersensitivity to normally innocuous stimuli that accompanies inflammation, nerve injury and trauma. Direct recording from VGLUT3+ neurons in the DRG further identifies them as a poorly understood population of unmyelinated, low threshold mechanoreceptors (C-LTMRs)4,5. The analysis of VGLUT3 KO mice now indicates a critical role for C-LTMRs in the mechanical hypersensitivity caused by injury.
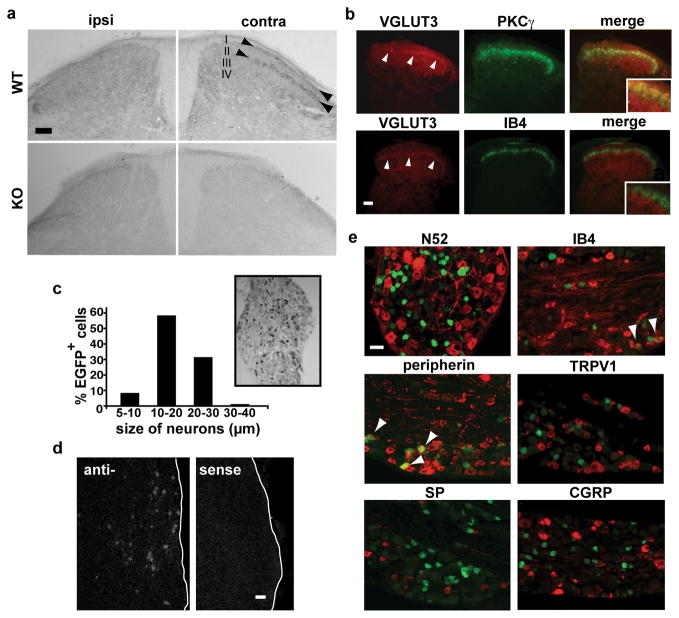
In contrast to the more abundant isoforms VGLUT1 and 2, VGLUT3 is expressed by a small number of isolated neuronal populations6. Consistent with this general pattern, VGLUT1 and 2 occur at high levels throughout dorsal and ventral horns of the spinal cord7,8, whereas VGLUT3 has a much more restricted distribution. In the dorsal horn, distinct bands within superficial laminae I and inner II immunostain specifically for VGLUT3 (Fig. 1a). Unilateral dorsal rhizotomy (L3-5) eliminates VGLUT3 immunoreactivity in the ipsilateral, but not the contralateral dorsal horn, establishing the peripheral origin of these processes. Within inner lamina II, VGLUT3 immunoreactivity overlaps almost exclusively with a band of interneurons expressing the γ isoform of protein kinase C (PKC), and not with afferents that bind the lectin IB4 (Fig. 1b). This discrete projection suggests expression of the transporter by DRG neurons that convey specific sensory modalities.
Figure 1. VGLUT3 is expressed by a unique subset of small- and medium-sized DRG neurons that project to dorsal horn lamina I and the inner part of lamina II.
a, Immunostaining shows VGLUT3 in laminae I and innermost II (arrowheads) of the dorsal horn in WT but not VGLUT3 KO mice. Dorsal rhizotomy abolishes VGLUT3 immunoreactivity in ipsilateral laminae I and II. Calibration bars, 50 μm. b, VGLUT3 immunofluorescence (red) in the dorsal horn overlaps to a large extent with interneurons that express PKCγ (green), but little if at all with the binding to IB4 (green). c, Immunoperoxidase labeling of a DRG section from a VGLUT3 EGFP mouse shows the size and distribution of EGFP+ cells (size bar = 30 μm). d, In situ hybridization for VGLUT3 labels small- and medium-sized neurons in the trigeminal ganglion (TG) of WT mice. Hybridization with a sense probe confers no detectable signal. e, EGFP+ DRG neurons (green) colocalize strongly with peripherin (92%), to a small extent with IB4 (7%) but rarely with N52 and not at all with TRPV1, substance P or CGRP. Arrowheads indicate colocalization. Size bar in d and e, 30 μm.
To identify the DRG neurons expressing VGLUT3, we produced bacterial artificial chromosome (BAC) transgenic mice that express EGFP under the control of VGLUT3 regulatory sequences (VGLUT3 EGFP mice; Supplementary Fig. 1a) since the VGLUTs do not themselves generally localize to cell bodies. Consistent with the faithful expression from most BACs, VGLUT3 EGFP BAC transgenic mice label for EGFP only in isolated cell populations in the brain known to express VGLUT3 (Supplementary Fig. 1b)6. In the spinal cord, EGFP expression resembles that of VGLUT3, with labeled fibers restricted to dorsal horn lamina I and the PKCγ layer of lamina II (Supplementary Fig. 2a), and unilateral L3-L5 dorsal rhizotomy eliminates EGFP immunoreactivity in the ipsilateral dorsal horn (Supplementary Fig. 2b). Since EGFP in the BAC replaced VGLUT3 protein-coding sequences, we also double stained for both EGFP and endogenous VGLUT3, and observed colocalization in the dorsal horn (Supplementary Fig. 3). Few if any EGFP+ cell bodies are observed in the spinal cord of adult mice, consistent with the rare labeling for VGLUT3 by in situ hybridization (data not shown).
Taking advantage of the BAC transgenic mice, we examined the expression of VGLUT3 in adult sensory ganglia. Approximately 11% of trigeminal ganglion (TG) (n=205/1916) and ~10% of L4/L5 DRG (n=237/2260) neurons express EGFP, and these cells are small-medium in size (<30 μm; Fig. 1c), consistent with in situ hybridization for VGLUT3 in TG (Fig. 1d). In addition, ~92% of EGFP+ neurons express peripherin (n=207/226) (Fig. 1e), a marker of cells with unmyelinated axons. Indeed, very few (<1%) EGFP+ cells (n=2/214) express the N52 antigen that labels DRG cells with myelinated axons. In general, peripherin+ cells either bind the lectin IB4 or express neural peptides9, but we find that only ~7% of EGFP+ cells in DRG and TG bind IB4 (n=19/279), and none express the peptides substance P or CGRP (Fig. 1e and data not shown). In addition, EGFP+ cells do not express the primary heat sensor TRPV1 (Fig. 1e). VGLUT3+ neurons thus represent a previously uncharacterized population of unmyelinated neurons that project to laminae I and II inner of the dorsal horn.
The expression of VGLUT3 by unmyelinated primary sensory neurons projecting to superficial layers of the dorsal horn suggested that these cells might convey pain or temperature sensation. To assess their role, we used VGLUT3 KO mice10,11. These animals exhibit sensorineural deafness and rare non-convulsive seizures, but are otherwise normal in terms of motor coordination, vision and mating behavior, consistent with the highly restricted distribution of VGLUT3. Importantly, VGLUT1 and 2 do not colocalize with VGLUT3 in spinal cord terminals (Supplementary Fig. 3), indicating that VGLUT3+ afferents should not transmit any glutamatergic signal to the spinal cord in the absence of VGLUT3.
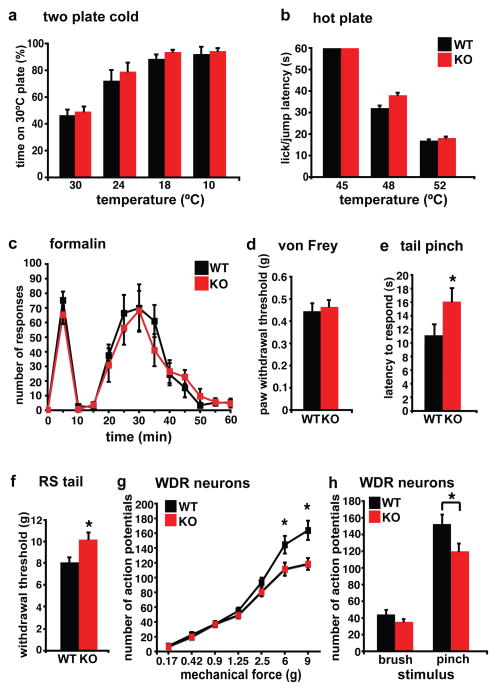
VGLUT3 KO mice do not differ from wild type (WT) littermates in sensitivity to cold or heat (Fig. 2a,b). In addition, we observed no difference in the response to formalin. Since formalin acts through TRPA1 receptors12 that are expressed by a subset of TRPV1+ neurons (Fig. 2c), this result is consistent with the expression of VGLUT3 by sensory neurons distinct from those expressing TRPV1 (Fig. 1e). VGLUT3 KO mice also do not differ from WT littermates in the threshold for withdrawal from stimulation with von Frey hairs of increasing strength, suggesting normal sensitivity to light noxious mechanical stimuli (Fig. 2d). However, the KO mice do show a significantly delayed response to placement of a clip on the tail (Fig. 2e), as well as an increased threshold for tail withdrawal measured in the Randall-Selitto apparatus (Fig. 2f). Thus, in contrast to their normal temperature sensation, formalin sensitivity and von Frey hair threshold, VGLUT3 KO mice exhibit a selective albeit partial defect in the response to intense noxious mechanical stimuli.
Figure 2. VGLUT3 KO mice show a selective defect in acute mechanical pain sensation to intense noxious stimuli.
a, Using a two-plate preference chamber to assess cold sensitivity, VGLUT3 KO and WT mice show the same preference for the 30°C plate over 24°, 18°or 10°C plates (n=6 mice for both genotypes). b, Using a hot plate to assess heat sensitivity, the latency of VGLUT3 KO mice to exhibit nocifensive behaviors is similar to that of WT littermates at both 48°C and 52°C (n=8). c, Both genotypes show a similar, biphasic response to 2% formalin injected into the hindpaw (n≥15). d, Mechanical thresholds measured using von Frey filaments do not differ between genotypes (n≥10). e, Measuring the acute mechanical pain sensitivity to tail clip, VGLUT3 KO mice show a significantly longer latency to respond than WT (16 ± 2 versus 11 ± 1; n≥17), indicating a reduced sensitivity to acute mechanical pain. p < 0.05, Mann-Whitney U test. f, VGLUT3 KO mice show a higher threshold than WT (10.2 ± 0.5 versus 8.1 ± 0.4; n= 9) to tail withdrawal on compression in the Randall-Selitto test. p<0.05, Mann-Whitney U test. g, Single unit extracellular recordings in the dorsal horn of the lumbar spinal cord show the response of WDR neurons to graded punctate mechanical stimulation of the plantar hindpaw receptive field. The genotypes differ specifically in response to 6 and 9 g. *p<0.05 by Fisher’s LSD post hoc test, n=50–53 cells. h, The same group of WDR neurons in VGLUT3 KO mice respond significantly less than those in WT to pinch (*p=0.026; students t-test), but not brush stimuli (p>0.05; student’s t-test) applied to the receptive field for 3 sec. n=10 mice for both genotypes (g,h). Error bars indicate SEM.
To assess further the contribution of VGLUT3+ primary afferents to acute mechanical pain, we recorded from dorsal horn wide dynamic range (WDR) neurons, which respond to innocuous as well as noxious mechanical stimuli13. WDR neurons from both WT and KO mice fire at progressively higher frequencies in response to increasing mechanical force applied to the hindpaw (Fig. 2g). At the highest forces applied (6g and 9g), however, WDR neurons of VGLUT3 KO mice show significantly less firing than those of WT animals. In addition, WDR neurons of KO mice show significantly less response to pinch (a noxious stimulus), but do not differ in their response to brush (an innocuous stimulus) (Fig. 2h). The response of WDR neurons to mechanical stimuli thus depends on VGLUT3 only for the strongest mechanical stimuli, correlating with the specific behavioral defect of KO mice in acute mechanical pain sensation.
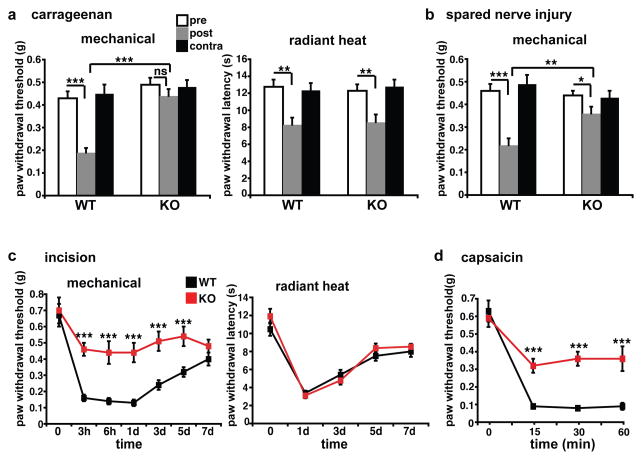
Since VGLUT3 appears important for acute mechanical pain, we also assessed the role of VGLUT3+ primary afferents in persistent hypersensitivity states caused by inflammation, nerve injury and trauma. In a model of chronic, inflammatory pain produced by injection of carrageenan into the hindpaw14, von Frey threshold drops markedly in WT mice, but remains unchanged in the KO (Fig. 3a). However, KO and WT mice show the same increase in sensitivity to radiant heat. VGLUT3 KO mice thus exhibit a selective defect in mechanical hypersensitivity in this model of inflammatory pain. Using the model of spared nerve injury (SNI)15 to assess neuropathic pain, WT animals develop a profound mechanical hypersensitivity ipsilateral to the SNI, whereas VGLUT3 KO mice again show a much smaller reduction in mechanical threshold (Fig. 3b). In the Brennan model of post-surgical pain16, KO mice also exhibit a much smaller reduction in mechanical threshold than WT (Fig. 3c), and the frequency of paw withdrawal to light and intermediate von Frey stimuli is reduced (Supplementary Fig. 4). However, the two genotypes do not differ in thermal hyperalgesia (Fig. 3c). The loss of VGLUT3 thus causes a remarkably modality-specific defect in the mechanical hypersensitivity associated with inflammatory, neuropathic and post-operative pain.
Figure 3. VGLUT3 KO mice show a profound, selective defect in the mechanical hypersensitivity produced by inflammation, nerve injury and trauma.
a, VGLUT3 KO mice and WT littermates were injected with carrageenan in the hindpaw. Tested 24 hours later, both genotypes show a significant increase in sensitivity to radiant heat, but only WT mice show a significant decrease in mechanical threshold. (n≥10 mice) b, In the spared nerve injury model of neuropathic pain, WT mice exhibit a significant decrease in mechanical threshold (52%) three days after sciatic nerve section, but KO animals exhibit only a modest reduction (18%). (n≥9 for both genotypes) *p<0.05, **p<0.01, ***p<0.001 by one-way ANOVA with Tukey’s HSD post-hoc analysis for a and b. c, In WT mice, an incision made in the plantar hindpaw produces a robust decrease in mechanical threshold, but KO mice show only a mild reduction for up to 5 days. Response to radiant heat does not differ between genotypes at all times tested (n=6). d, In WT mice, capsaicin injection into the ankle produces a profound decrease in mechanical threshold measured in the hindpaw at 15, 30 and 60 minutes post-injection, whereas KO mice show only a modest decrease (n=7). ***p<0.001, one-way ANOVA with Newman-Keuls post-hoc analysis for c and d. Error bars indicate SEM.
The importance of VGLUT3+ neurons for persistent mechanical pain produced by tissue injury may reflect their role in the induction of central sensitization, or in expression of the sensitized state, such as enhancing or transmitting the sensation of pain provoked by an innocuous mechanical stimulus. To distinguish between these two possibilities, we used the model of capsaicin-induced secondary mechanical hyperalgesia. Capsaicin directly activates TRPV1+ afferents that convey temperature sensation17, but when injected into the ankle, produces a mechanical hypersensitivity on the adjacent plantar hindpaw18. Since TRPV1+ afferents do not express VGLUT3 (Fig. 1e), and VGLUT3 KO mice show a normal response to the acute injection of capsaicin (Supplementary Fig. 5c), the loss of VGLUT3 should not interfere with induction of the sensitized state, enabling us to assess the role of VGLUT3+ afferents in the detection of innocuous mechanical stimuli as painful. As shown in Figure 3d, the mechanical thresholds of WT animals drop dramatically for at least one hour after capsaicin injection, but the KO mice show only a modest change. Because VGLUT3+ afferents do not contribute to central sensitization in this model of tissue injury, they must be involved in transmitting the input that produces pain.
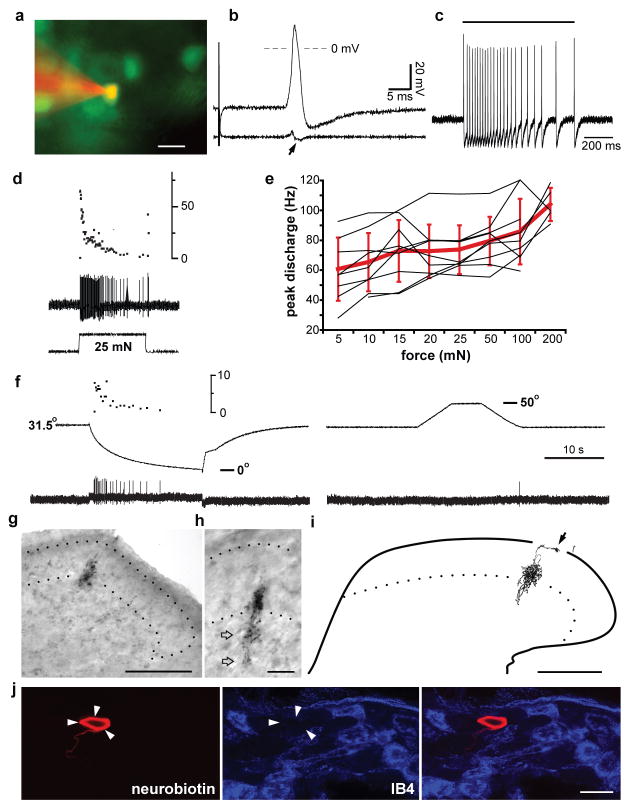
To characterize the properties of VGLUT3+ cells, we recorded intracellularly from EGFP+ DRG neurons of the BAC transgenic mice in an ex vivo preparation with the thoracic cord, DRG, nerves and skin intact19. With the exception of additional staining in the intermediolateral region of the thoracic cord, EGFP (and VGLUT3) expression appears similar at thoracic and lumbar levels (Supplementary Fig. 6). EGFP+ cells exhibit large amplitude overshooting somal spikes, with an inflection on the falling phase, and an average conduction velocity of 0.6 ± 0.1 m/sec (n=28) (Fig. 4b and Supplementary Fig. 7a), consistent with the lack of myelination suggested by their immunoreactivity for peripherin and not N52 (Fig. 1e and Supplementary Fig. 6b). Of 28 cells characterized, all were exquisitely sensitive to the finest von Frey hair available (mechanical threshold ≤ 0.07 mN; Fig. 4c), responded better to slowly than rapidly moving tactile stimuli (Supplementary Fig. 7b), and adapted to stationary stimuli (Fig. 4c,d). Receptive fields were small (≤ 1 mm2) and composed of 1–3 discrete spots. Using a series of controlled forces, we observed a trend to higher instantaneous firing rates for stronger stimuli (Fig. 4e and Supplementary Fig. 7c), with a significantly larger response to 200 mN than 5–25 mN (p<0.01, n=5–9) and to 50 and 100 mN than 5 mN (p<0.05). Thermal sensitivity was assessed in a subset of the neurons, and all of these responded to cold (4°C, n=28) but not hot (52°C, n=3) solution. Controlled temperature ramps (from 31.5° C to −4°) revealed a threshold for cold stimuli of 25 ± 1° C (n=7), whereas ramps from 31.5° to 50° C elicited no response (Fig. 4f). Thus, VGLUT3 expression identifies a class of unmyelinated, low threshold mechanoreceptors (C-LTMRs)4,5. Reconstruction of central projections from seven of these cells injected with Neurobiotin showed small, dense longitudinal columns extending 150–200 μm rostrocaudally in lamina II inner (Fig. 4g–i and Supplementary Fig. 8), with additional minor terminals in laminae III and I, consistent with the immunoreactivity for VGLUT3. Previous work has conflicted on the expression of IB4 by C-LTMRs19,20, and a small number of EGFP+ DRG neurons label with IB4 (Fig. 1e), but none of 25 physiologically identified C-LTMRs bound this lectin (Fig. 4j).
Figure 4. VGLUT3 expression in DRG uniquely identifies C-LTMRs.
a, An EGFP+ cell is stained by Alexa 555 from an intracellular microelectrode (red) while recording from an ex vivo somatosensory preparation. b, Electrical stimulation of the dorsal cutaneous nerve evokes a high amplitude, overshooting action potential. The first derivative (lower trace) reveals an inflected falling phase (arrow) typical for C-fiber spikes. c, Application of a 0.07 mN von Frey filament to the skin receptive field (duration indicated by bar) elicits a burst of spikes. d, Response to 25 mN force applied with a feedback-controlled stimulator (lower trace) shows adaptation in the continued presence of the force (middle trace). Upper trace shows the instantaneous frequency of spikes. e, Peak discharge rate of individual EGFP+ neurons (black lines) to increasing force (5–200 mN) shows a small increase with stronger stimuli (p<0.01 for 5–25 mN versus 200 mN by ANOVA with Bonferroni post hoc correction, n=5–9); red bars indicate mean ± SD. f, Temperature ramps performed with a Peltier device (middle trace) evoke firing in an EGFP+ neuron (lower trace) on cooling (left) but not heating (right), Upper trace (left) shows the instantaneous firing rate. g, Low power photomicrograph of central terminals arborizing in lamina IIi; dotted lines indicate the substantia gelatinosa (SG). h, Photomicrograph of different section at higher power shows arborization extending into lamina III (open arrows). i, Camera lucida reconstruction of arborization across four serial 50 μm sections, showing additional input to lamina I (arrow); the ventral border of SG is indicated by a dotted line, and the border between gray and white matter by a solid line. j, Staining of a DRG section for IB4 (blue, middle) shows that the Neurobiotin-labeled cell (red, left) is IB4-negative. Scale bars: 20 μm (a, h, j); 100 μm (g, i). Physiological calibration: 20 mV and 5 msec (b), 200 msec (c).
The results show that primary sensory neurons expressing VGLUT3 convey two distinct features of mechanical pain sensation. First, behavioral analysis of the KO indicates that VGLUT3+ cells contribute to acute pain in response to intense noxious mechanical stimuli, and recordings from WDR neurons support this role. Although C-LTMRs are very sensitive to low intensity mechanical stimulation, they do show a significantly greater response to high intensity stimuli, and may therefore participate in acute mechanical pain sensation as well. It nonetheless remains possible that other, less abundant VGLUT3+ neurons contribute to the acute sensory phenotype observed in the KO. Since nociceptive inputs from both high-threshold myelinated Aδ and unmyelinated C-fibers terminate in lamina I21, the acute mechanical pain sensation conveyed by VGLUT3+ neurons may involve the projections to lamina I.
Second, VGLUT3+ neurons contribute to the mechanical hypersensitivity that occurs in the setting of tissue or nerve injury. Previous work has described several mechanisms that may account for the hypersensitivity to innocuous stimuli that develops after injury. In models of inflammation, unmyelinated polymodal sensory neurons develop increased sensitivity to mechanical stimuli22. Large myelinated Aβ neurons have also been implicated in chronic pain23: after injury, Aβ neurons increase the excitability of spinal cord neurons, suggesting a role in central sensitization24. In contrast, the selective defect in mechanical hypersensitivity of the VGLUT3 KO, together with the sensitivity of C-LTMRs to light touch and the identification of most VGLUT3+ afferents as C-LTMRs, now indicate that C-LTMRs are required to generate pain in response to innocuous mechanical stimuli. The loss of capsaicin-induced secondary mechanical hyperalgesia in the KO further supports a role for C-LTMRs in expression rather than induction of the sensitized state. Consistent with this role, VGLUT3+ C-LTMRs terminate in the inner layer of lamina II, which does not receive acute nociceptive input25–27, but rather contains interneurons expressing PKCγ that, like VGLUT3, are required for injury-associated mechanical hyperalgesia2. Since the ablation of DRG neurons expressing the tetrodotoxin-insensitive Na+ channel Nav1.8 produces a similar phenotype28, VGLUT3+ neurons may also express this channel.
In the absence of injury, we are unable to detect a behavioral role for VGLUT3 in the transmission of innocuous mechanical information. This likely reflects the presence of large, myelinated fibers that also convey the sensation of light touch. Human psychophysical studies have implicated C-LTMRs specifically in acute pleasant touch29, but this behavior would be difficult to assess in rodents. The expression of mechanical hypersensitivity after injury may thus involve a change in the sensation conveyed by C-LTMRs, from pleasant touch to pain.
Methods Summary
Guinea pig antibodies to VGLUT3 and TRPV1 were generous gifts of H. Hioki and T. Kaneko (VGLUT3) and D. Julius (TRPV1).
Mouse genetics
VGLUT3 KO mice and WT littermates were back-crossed to C57Bl/6 for at least 8 generations. 1–3 month old animals were used for histology, behavior and electrophysiology. The VGLUT3 EGFP BAC transgene was generated by homologous recombination in bacteria as previously described30. The EGFP cDNA and SV40 polyadenylation sequences were amplified by PCR from IRES2-EGFP (BD Biosciences) and inserted into BAC RP2488H21 that contains the mouse vglut3 gene (Supplementary Fig. 1a). Fertilized B6SJL oocytes were injected with purified VGLUT3 EGFP BAC DNA, implanted into pseudopregnant females and brain slices from transgenic offspring screened by immunocytochemistry for transgene expression.
Electrophysiology
Single unit extracellular recordings were made from single WDR neurons in the L4-L5 dorsal horn in response to graded von Frey monofilaments, brush and pinch stimuli, each applied for 3 seconds to the plantar hindpaw receptive field. Experimenter was blind to genotype, and statistical analysis was performed by two-way mixed-model ANOVA with Fisher’s protected least significant difference (LSD) post-hoc test or Student t-test. Intracellular recordings were made from EGFP+ neurons in an ex vivo preparation with dorsal skin, nerve and spinal cord intact19. Action potentials evoked with a suction electrode on the nerve bundle were used to calculate conduction velocity. To determine the response properties of recorded neurons, calibrated von Frey hairs, feedback-controlled mechanical stimulator, aCSF solutions (warm or cold) and feedback-controlled Peltier stimulator were applied to the skin receptive field.
Supplementary Material
Acknowledgments
We thank D. Bautista, D. Julius, and members of the Basbaum and Edwards laboratories for advice and D. Woodbury and C. Cassidy from the Woodbury laboratory for help with experiments. The work was supported by the Johns Hopkins Blaustein Pain Research Fund (YG) and by grants from NARSAD (RPS, RHE) and NIH (RPS, YG, SNR, CJW, AIB and RHE).
Footnotes
Author Information
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
Full methods are available in the supplementary information.
Author Contributions
R.P.S. created the VGLUT3 KO and VGLUT3 EGFP BAC transgenic mice; X.W. and R.P.S. performed behavior and histology experiments; Y.G. performed in vivo spinal cord recordings; and C.J.W. and R.P.S. performed ex vivo recordings. R.H.E., R.P.S. and A.I.B. wrote the manuscript with contributions from C.J.W., Y.G., S.N.R. and X.W.
The authors declare no competing financial interests.
References
- 1.Yoshimura M, Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol. 1990;430:315–35. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–83. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- 3.Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–43. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- 5.Iggo A, Kornhuber HH. A quantitative analysis of non-myelinated cutaneous mechano-receptors. J Physiol. 1968;198:113. [PubMed] [Google Scholar]
- 6.Gras C, et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–51. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todd AJ, et al. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- 8.Li JL, Fujiyama F, Kaneko T, Mizuno N. Expression of vesicular glutamate transporters, VGluT1 and VGluT2, in axon terminals of nociceptive primary afferent fibers in the superficial layers of the medullary and spinal dorsal horns of the rat. J Comp Neurol. 2003;457:236–49. doi: 10.1002/cne.10556. [DOI] [PubMed] [Google Scholar]
- 9.Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–32. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 10.Seal RP, et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–75. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gras C, et al. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nature Neurosci. 2008;11:292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- 12.McNamara CR, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104:13525–30. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung JM, Kenshalo DR, Jr, Gerhart KD, Willis WD. Excitation of primate spinothalamic neurons by cutaneous C-fiber volleys. J Neurophysiol. 1979;42:1354–69. doi: 10.1152/jn.1979.42.5.1354. [DOI] [PubMed] [Google Scholar]
- 14.Kayser V, Guilbaud G. Local and remote modifications of nociceptive sensitivity during carrageenin-induced inflammation in the rat. Pain. 1987;28:99–107. doi: 10.1016/0304-3959(87)91064-5. [DOI] [PubMed] [Google Scholar]
- 15.Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4:465–70. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 16.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 17.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 18.Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 19.Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci. 2006;26:2981–2990. doi: 10.1523/JNEUROSCI.4863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q, et al. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nature Neurosci. 2007;10:946–8. doi: 10.1038/nn1937. [DOI] [PubMed] [Google Scholar]
- 21.Christensen BN, Perl ER. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970;33:293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- 22.Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann NY Acad Sci. 2002;966:343–54. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- 23.Woolf CJ, Doubell TP. The pathophysiology of chronic pain--increased sensitivity to low threshold A beta-fibre inputs. Curr Opin Neurobiol. 1994;4:525–34. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 24.Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–4. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 25.Light AR, Trevino DL, Perl ER. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979;186:151–71. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- 26.Boada MD, Woodbury CJ. Myelinated skin sensory neurons project extensively throughout adult mouse substantia gelatinosa. J Neurosci. 2008;28:2006–14. doi: 10.1523/JNEUROSCI.5609-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–44. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrahamsen B, et al. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–5. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- 29.Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nature Neurosci. 2009;12:547–8. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- 30.Yang XW, Model P, Heintz N. Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nature Biotechnol. 1997;15:859–65. doi: 10.1038/nbt0997-859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.