Abstract
Mesenchymal stem cells (MSCs) are multipotent cells that have the capacity to develop into different mature mesenchymal cell types. They were originally isolated from bone marrow, but MSC-like cells have also been isolated from other tissues. The common feature of all of these tissues is that they all house blood vessels. It is, thus, possible that MSCs are associated with perivascular locations. The objective of this work was to test the hypothesis that MSCs are associated with blood vessels by verifying if MSC frequency positively correlates with blood vessel density. To this end, samples from highly and poorly vascularized adipose tissue sites of two equine donors were collected and processed for histology and cell isolation. MSC frequency in these samples was estimated by means of CFU-F assays, which were performed under MSC conditions. Culture-adherent cells from equine adipose tissue and bone marrow were culture expanded, tested for differentiation into mesenchymal cell types in vitro, and implanted in vivo in porous ceramic vehicles to assess their osteogenic capacity, using human MSCs and brain pericytes as controls. The differentiation assays showed a difference between adipose tissue–derived cells as compared to equine bone marrow MSCs. While differences in CFU-F frequencies between both donors were evident, the CFU-F numbers correlated directly with blood vessel densities (r2 = 0.86). We consider these preliminary data as further evidence linking MSCs to blood vessels.
Introduction
Mesenchymal stem cells (MSCs) are multipotent cells that have the capacity to differentiate into different mature cell types such as osteoblasts, chondrocytes, and adipocytes.1 MSCs also possess trophic and immunomodulatory properties that may be useful in the treatment of a range of clinical conditions.2 These adult progenitor cells have been isolated and culture expanded from bone marrows of different species.3–6 The existence of MSCs in adipose tissue was proposed in 2001 by Zuk et al.,7 and to date there is evidence that MSC-like cells can be isolated from a large variety of tissues and organs.8,9 The widespread distribution of MSCs could be explained if they were functionally associated with blood vessels. This hypothesis is supported by findings from morphological10–13 and immunohistological14,15 observations and from experiments demonstrating the differentiation of pericytes into mesenchymal cell types in vitro.16–18 The absence of MSC-specific markers makes the task of identifying them in vivo difficult, and, consequently, the results mentioned above are yet prone to different interpretations. For example, since MSCs are largely defined by means of in vitro assays, antibodies developed against these cultured cells might recognize molecules spuriously expressed as a consequence of in vitro cultivation and skew immunohistological analyses. In this context, assessing if a causal, quantitative relationship between blood vessel density and MSC number exists could provide further information to verify the hypothesis that MSCs are physically associated with blood vessels in vivo. Indeed, the possible link between MSCs and vasculature has not been explored on a quantitative basis.
Adipose tissue provides a useful source of MSCs because adipocytes and the vascular–stromal fraction can be easily separated from each other due to their differences in buoyant densities. The CFU-F assay, used as a tool to assess MSC frequency in different backgrounds,6,19 indicates that MSC numbers may be high in preparations of adipose tissue–derived cells.20 It is possible, thus, that MSC frequency in adipose tissue is associated with blood vessels. Testing this assumption using tissue samples from humans would require a large sample number to account for intrinsic donor-to-donor variation. Subcutaneous fat in the tail head of horses is highly vascularized (HV) as compared to adipose tissue from the area just lateral to the insertion of the tail (T.T. Sand and R.J. Harman, unpublished observations), allowing for the study of the relationship between vascular density and MSC content in the same individual, thus eliminating donor variation. Hence, to verify if a correlation between MSC frequency and blood vessel density exists, we analyzed HV and poorly vascularized (PV) adipose tissue sites from horses for their MSC content, CFU-F frequency, and blood vessel density. The cellular fractions obtained, which contained the CFU-Fs, were operationally defined as MSCs based on their proliferation and differentiation capabilities. The results indicate that CFU-F number is directly proportional to blood vessel density, providing suggestive quantitative evidence of MSC association with blood vessels.
Materials and Methods
Horse adipose tissue and bone marrow samples
Adipose tissue samples obtained from both the tail head (HV) and an area just lateral to the insertion of the tail (PV) of two horses were supplied by Vet-Stem (Poway, CA). Samples from each site were divided into three subsamples that were individually (a) enzymatically dissociated to render a cell suspension using Vet-Stem technology, (b) fixed in 10% neutral-buffered formalin for standard paraffin processing, and (c) embedded in Tissue-TeK® Optimal Cutting Temperature compound (Sakura, Torrance, CA) and frozen in liquid nitrogen for cryosectioning. Cell suspensions were shipped overnight to Skeletal Research Center from Vet-Stem as is customary in ice and kept at 4°C until used in the experiments on the day they were received. These cells are termed equine adipose tissue–derived cells (eATDCs) here. Bone marrow from each equine donor was separately collected and shipped under the same conditions. One of the horses (Horse 1) was sedentary, and the other (Horse 2) was fit.
Cell culture
For culture expansion, a fraction of horse cell suspensions were plated at approximately 1 × 107 cells per 100 mm dish (Becton Dickinson, Franklin Lakes, NJ) in 7 mL low-glucose Dulbecco's modified Eagle's medium (DMEM-LG; Sigma-Aldrich, St. Louis, MO) with 10% fetal bovine serum (FBS) from selected lots,21 conditions previously described for the culture of human MSCs (hMSCs).3,4 Human bone marrow samples were obtained from three healthy volunteers after informed consent and were processed as described22 to generate hMSC cultures to be used as positive controls. Equine bone marrow was processed in the same manner, and the cells isolated were cultured under the same conditions as for the positive control, hMSCs, as conditions optimized for the culture of equine MSCs were not described at the time this work was devised and conducted. Human brain vascular pericytes (HBVPs) were acquired from ScienCell (San Diego, CA) and were cultured in a proprietary medium according to the supplier's instructions. The purpose in culturing HBVPs under these conditions was to maintain their pericytic phenotype until use in the experiments.
CFU-F assay
The CFU-F assay for eATDCs was performed by plating cell suspensions in six-well dishes with a growth area of 9.6 cm2 per well (Becton Dickinson), in triplicate wells, at four different densities: 10,000 cells per well, 5000 cells per well, 1000 cells per well, and 100 cells per well. This protocol was adapted from that described by Meirelles and Nardi,6 considering that CFU-F frequency in adipose tissue is higher than in bone marrow.20 At 8 days of culture, the dishes were fixed with 95% ethanol for 5 min at room temperature and stained for 30 min with crystal violet. The colonies were scored by microscopic examination using a grid placed under the dish; the criteria for inclusion were morphology and 10 or more cells.
In vitro differentiation assays
For osteogenic differentiation assays, 30,000 cells per well were transferred to six-well dishes in DMEM-LG supplemented with 10% FBS. On the next day, the medium was switched to DMEM-LG supplemented with 10% FBS, 10−7 M dexamethasone, and 50 μM ascorbic acid 2-phosphate. This medium was further augmented with 2 mM β-glycerophosphate on the 10th day of culture.22 Alkaline phosphatase (ALP) and calcium assays were performed essentially as described elsewhere.22 Alizarin Red S stain was also used to visualize calcium-rich extracellular matrix.6 Adipogenic differentiation assay was performed by culturing cells for 10 days in adipogenic induction medium (DMEM-HG [high glucose] supplemented with 10% FBS, 100 μM indomethacin, 0.1 μM dexamethasone, 0.5 mM IBMX, and 10 μg/mL bovine insulin), and for an additional week in adipogenic maintenance medium (DMEM-HG augmented with 10% FBS and 10 μg/mL bovine insulin), as previously described.22 Lipid droplets were visualized by staining with Oil Red O solution.6 Chondrogenic assay was performed using a modification23 of the pellet culture method described by Johnstone et al.24 Briefly, 2.5 × 106 cells per well were transferred to V-bottom, 96-well polypropylene microplates (Phenix, Hayward, CA), centrifuged at 400 g for 10 min, and cultured in a chemically defined medium consisting of LG-DMEM with ITS+ premix (Becton Dickinson), insulin (6.25 μg/mL), transferrin (6.25 μg/mL), selenous acid (6.25 μg/mL), linoleic acid (5.35 μg/mL), and bovine serum albumin (1.25 μg/mL), sodium pyruvate (1 mM), ascorbate 2-phosphate (100 μM), dexamethasone (10−7 M), and TGF-β1 (10 ng/mL, recombinant human; R&D Systems, Minneapolis, MN). Cell pellets were harvested at 1, 2, and 3 weeks, and sectioned and stained with Toluidine Blue O or subjected to glycosaminoglycan (GAG) assay as previously described.25
Ceramic cube assay
Implantation of MSC-loaded porous ceramic cubes into immunocompromised host animals as an in vivo assay for their osteochondrogenic potential has been described previously.26,27 Briefly, blocks of porous ceramic (mean pore size of 200 μm) consisting of 60% tricalcium phosphate and 40% hydroxyapatite (Zimmer, Warsaw, IN) were cut into cubes measuring 3 mm per side. The ceramic cubes were washed with water to remove ceramic dust, dried under a heating lamp, and sterilized in an autoclave. To optimize cell attachment, ceramic cubes were combined with a 100 μg/mL solution of human fibronectin (Becton Dickinson) in Tyrode's salt solution in a 12 × 75–mm sterile, capped polystyrene tube (Becton Dickinson). A 20-gauge needle attached to a 30-mL syringe was inserted through the cap of the tube, and the syringe plunger was retracted to evacuate air from the tube, thus generating a partial vacuum and permitting the fibronectin solution to enter the pores of the cubes with the exiting of air bubbles. Cubes remained in the fibronectin solution for 2 h at room temperature and were then air-dried overnight in a laminar flow hood.
First- or second-passage MSCs were trypsinized and resuspended at a concentration of 5 × 106 cells/mL in serum-free medium, and transferred to a 12 × 75–mm sterile tube. Fibronectin-coated ceramic cubes were added to the tube, and air was withdrawn with a 20-mL syringe as the procedure for fibronectin coating.
Cell-loaded cubes were incubated at 37°C for 2 h and then implanted subcutaneously into pockets created by blunt dissection on the dorsal surface of SCID mice (Charles River Laboratories, Wilmington, MA) anesthetized with 0.15 mL of anesthetic cocktail per 25 g mouse. The cocktail consisted of a mixture of ketamine (8.6 mg/mL), xylazine (1.7 mg/mL), and acepromazine (0.28 mg/mL). The skin incision was closed with wound clips. At defined times postimplantation, the animals were euthanized by injection of Euthasol, 0.2 mL/mouse (Virbac Animal Health, Fort Worth, TX), and the cubes were harvested. This protocol was approved by the Institutional Animal Care and Use Committee. After removal, the ceramic cubes were fixed with 10% neutral-buffered formalin overnight, kept in the fixative solution until subjected to decalcification, and embedded in paraffin. Five-micrometer sections were prepared and stained with Mallory-Heidenhain. Bone formation was quantified subjectively by viewing and scoring every 10th section; section scores ranged from 0 to 4 as previously described.28
Immunostaining
Samples from HV and PV adipose tissue sites from both equine donors were cryosectioned, washed with phosphate-buffered saline containing 0.1% bovine serum albumin (PBS-BSA), and incubated with primary antibodies against α smooth muscle actin, CD13, CD31, CD44, CD133, collagen IV, platelet-derived growth factor receptor, desmin, or von Willebrand factor; antibodies Stro-1, SH2, SH3, SH4, 90F9, or 97E2; or with fluorescein isothiocyanate (FITC)–conjugated Ulex europaeus agglutinin I. All of these are known to react with antigens of human origin. The slides that received primary antibodies were washed with PBS-BSA, incubated with an FITC-conjugated secondary antibody, washed once again, and mounted using glycerol. All incubations were carried out for 1 h at room temperature. The fluorescence was assessed using an upright fluorescence microscope (Leica Microsystems, Bannockburn, IL). Images were acquired with a coupled 1.4 MP digital camera (SPOT-RT; Diagnostic Instruments, Sterling Heights, MI) using SPOT 4.0 software (Diagnostic Instruments). The exposure time was set to 3.5 s. The primary antibody was omitted from some slides to provide a negative control. Cultured cells were subjected to immunostaining using antihigh molecular weight melanoma-associated antigen and 3G5 antibodies using the same procedure.
Vascularity analysis
To assess the vascular density, fat samples from both HV and PV sites were subjected to standard paraffin sectioning and stained with hematoxylin and eosin. Five images per section were taken from three nonconsecutive sections of each adipose tissue sample using a 1.3 MP digital camera (AxioCam MRc; Zeiss, Hallbergmoos, Germany) coupled to an upright microscope (Zeiss). The field of each image was randomly acquired. If a field chosen randomly was located on the border or outside the section, a different field was chosen. Using Adobe Photoshop software, all blood vessels that could be visually recognized in each picture were cut and pasted into a new blank picture to generate a correspondent new image containing the vessels alone. The images containing only the blood vessels were processed using CellProfiler software29 to count the number of pixels that corresponded to the blood vessels in each image. Pixels represent the area occupied by blood vessels in the micrographs of 5-μm-thick sections; hence, the volume or density of vessels is inferred. The set of instructions (pipeline) used with CellProfiler is provided as supplemental material (available online at www.liebertonline.com/ten). The average number of pixels was calculated for each section and used to generate the means for each sample. Student's t-tests were used to compare the results.
Results
In vitro differentiation assays
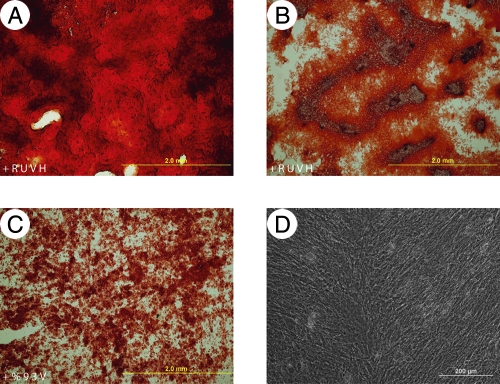
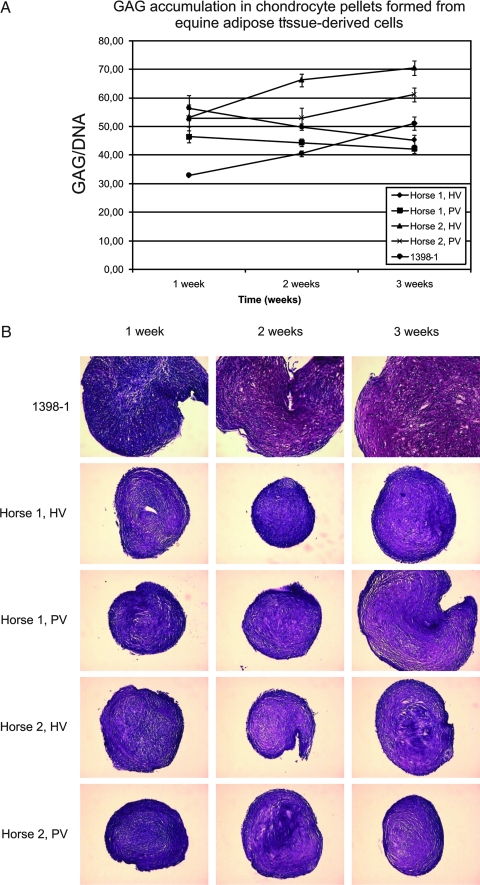
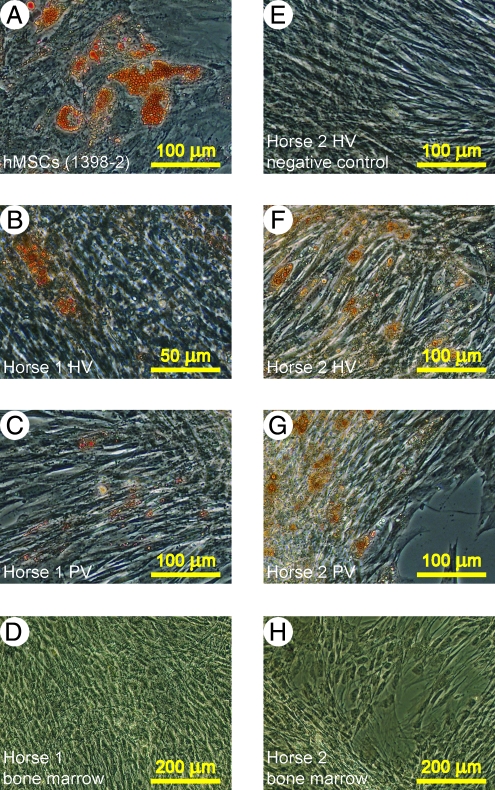
To test eATDCs operationally as MSCs in culture, they were subjected to the same expansion and differentiation conditions employed for hMSCs, with both equine and human bone marrow–derived MSCs as a positive control. HBVPs were likewise included for comparative purposes under MSC conditions. As shown in Tables 1 and 2, when equine bone marrow–derived MSCs (eMSCs) were subjected to osteogenic differentiation, they exhibited elevated ALP and calcium levels compared with their matched controls. Osteogenic differentiation was further established with the Alizarin Red S stain showing calcium-rich extracellular matrix (Fig. 1). The single hMSC population used in the osteogenic differentiation experiment displayed poor in vitro osteogenic differentiation capability; this is unfortunately not representative of our usual experience.3,22 HBVPs, on the other hand, exhibited good in vitro osteogenic properties. Differentiation of eATDCs along the osteogenic pathway could not be determined because the cells detached from the dish before the first assay (ALP) could be done; for emphasis, these conditions were not optimized for horse MSCs. eATDCs subjected to chondrogenic differentiation in vitro were shown to accumulate a sulfated GAG-rich extracellular matrix as evidenced by GAG levels and staining of sections through the pellets with Toluidine Blue O (Fig. 2); eMSCs also exhibit chondrogenic expression under these conditions (not shown). When subjected to adipogenic differentiation conditions, however, eATDCs from Horse 2 accumulated lipid droplets far more efficiently than eATDCs from Horse 1, indicating donor variation under these hMSC-optimized conditions (Fig. 3). eMSCs did not differentiate into adipocytes using these conditions.
Table 1.
ALP Activity (as nmol p-Nitrophenol/mL/min/μg DNA) of eMSCs Subjected to Osteogenic Differentiation as Compared with hMSCs (1400-2) and HBVPs
| |
Osteogenic |
Control |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Horse 1 | 6.8 | 2.6 | 1.4 | 1.0 |
| Horse 2 | 5.5 | 0.6 | 0.8 | 0.5 |
| 1400-2 | 3.4 | 0.2 | 0.8 | 0.1 |
| HBVPs | 11.2 | 2.1 | 1.3 | 0.1 |
One set of triplicates had its ALP activity (nmol p-nitrophenol/mL/min) assayed, and values were normalized using DNA content of a second set of matched triplicates.
Table 2.
Calcium (μg/μg DNA) Content of eMSCs Subjected to Osteogenic Differentiation as Compared with hMSCs (1400-2) and HBVPs
| |
Osteogenic |
Control |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| 1 week | ||||
| Horse 1 | 346.3 | 176.8 | 5.4 | 5.1 |
| Horse 2 | 400.2 | 210.3 | 2.3 | 0.5 |
| 1400-2 | 5.6 | 0.7 | 2.3 | 1.0 |
| HBVPs | 7.2 | 0.9 | 2.3 | 0.8 |
| 2 weeks | ||||
| Horse 1 | 382.4 | 87.3 | 7.7 | 2.6 |
| Horse 2 | 407.5 | 46.3 | 10.1 | 8.2 |
| 1400-2 | 32.9 | 6.0 | 1.9 | 1.0 |
| HBVPs | 288.0 | 100.0 | 1.8 | 0.4 |
One set of triplicates had its calcium content assessed, and values were normalized using DNA content of a second set of matched triplicates.
FIG. 1.
Osteogenic differentiation of equine bone marrow MSCs. Calcium-rich extracellular matrix was stained with Alizarin Red S after 3 weeks under osteogenic differentiation conditions.22 (A, B) Horse 1 and Horse 2 MSCs, respectively. (C) HBVPs. (D) hMSCs (1400-2). Color images available online at www.liebertonline.com/ten.
FIG. 2.
Chondrogenic differentiation of eATDCs. Chondrogenic pellets23 were generated from eATDCs from HV and PV sites from two equine donors (Horse 1 and Horse 2) or from hMSCs (1398-1). (A) GAG accumulation at 1, 2, and 3 weeks. (B) Histological sections from pellets harvested at 1, 2, and 3 weeks stained with Toluidine Blue. Color images available online at www.liebertonline.com/ten.
FIG. 3.
Adipogenic differentiation of eATDCs. eATDCs from HV and PV sites or from bone marrow from two equine donors (Horse 1 and Horse 2) were subjected to adipogenic differentiation22 using hMSCs (1398-2) as a positive control. At the end of the differentiation period, cells were stained using Oil Red O. (A) Human cells. (B) eATDCs from Horse 1's HV site. (C) eATDCs from Horse 1's PV site. (D) Horse 1's bone marrow cells. (E) eATDCs from Horse 2's HV site not subjected to adipogenic differentiation. (F) eATDCs from Horse 2's HV site. (G) eATDCs from Horse 2's PV site. (H) Horse 2's bone marrow cells. No adipocytes could be observed in (E), (D), and (H). Color images available online at www.liebertonline.com/ten.
Ceramic cube assay
To assess eATDCs' osteogenic potential in vivo, they were introduced into porous ceramic cubes and implanted into immunocompromised mice, using both hMSC-loaded or empty cubes as controls. The cubes were harvested at 3 or 6 weeks postimplantation; bone formation was subjectively scored by viewing every 10th serial section of each cube; scores ranged from 0 to 4 according to the amount of bone observed, where 0 = no bone, 1 = up to 25%, 2 = 25% to 50%, 3 = 50% to 75%, and 4 = 75% to 100%.28
Freshly isolated, uncultured eATDCs from both HV and PV from the two equine donors did not produce bone at 3 or 6 weeks postimplantation. The control cubes loaded with hMSCs (preparation number 1397) harvested at 3 or 6 weeks had very little bone, with average scores of 0.17 and 0.77, respectively.
A second set of ceramic cube implants was carried out to test the osteogenic potential of eMSCs as compared to hMSCs (preparation number 1400). In this experiment, eMSCs displayed maximal osteogenic potential as compared to the positive control. The mean subjective scores of cubes loaded with cells from Horse 1, Horse 2, or hMSCs were, respectively, 4, 3.89, and 1.97 at 3 weeks postimplantation, and 3.83, 3.89, and 2.53 at 6 weeks postimplantation.
The in vivo osteogenic potential of cultured HBVPs was also checked by means of the ceramic cube assay. These did not exhibit bone formation at the 3 and 6 weeks time points, whereas hMSCs (preparation number 1400) used as a positive control had average scores of 2.08 and 2.17 (at 3 and 6 weeks, respectively). These results are in contrast with those from the in vitro osteogenic assays, where brain pericytes exhibited high ALP levels and calcified matrix, and hMSC population 1400 exhibited low ALP levels and no matrix mineralization.
CFU-F assay
At 8 days of culture, the cells seeded at the two higher cell densities of the CFU-F assay formed colonies that overlapped, and hence were not suitable for quantitative assessment. Only colonies of wells seeded with 1000 cells were considered for further analyses, because CFU-F numbers in wells seeded with 100 cells were too low. As shown in Table 3, there was a significant difference in the observed CFU-F frequency per 1000 input cells between the two donors; however, no significant difference could be detected between the two adipose tissue sites using this assay. The average number of colonies per 1000 input cells was used to establish a relation between the number of CFU-Fs and the wet weight of the samples, as shown in Table 4. As expected, the mean CFU-F number per mg of adipose tissue from Horse 1 was higher in the HV fat tissue sample, even though such a difference did not reach significance (p ≤ 0.94). Contrary to the expectations, however, the mean CFU-F number per mg of tissue from Horse 2 was higher in the fat tissue sample supposedly PV. Such a difference was also not significant (p ≤ 0.50). It should be stressed here that the total cell yield was higher for the HV sample, but the number of CFU-Fs/1000 cells was similar.
Table 3.
Fibroblastic Colonies Per Well (9.6 cm2) Per Input Cells from HV and PV Adipose Tissue from Two Equine Donors
| Horse 1, PV | Horse 1, HV | Horse 2, PV | Horse 2, HV | |
|---|---|---|---|---|
| 1000 cells/well | ||||
| 11.0 | 17.0 | 58.0 | 48.0 | |
| 8.0 | 11.0 | 44.0 | 54.0 | |
| 21.0 | 11.0 | 54.0 | 42.0 | |
| Average | 13.3 | 13.0 | 52.0 | 48.0 |
| SEM | 5.1 | 2.7 | 5.3 | 4.0 |
| SD | 6.8 | 3.5 | 7.2 | 6.0 |
| 100 cells/well | ||||
| 2.0 | 1.0 | 5.0 | 6.0 | |
| 1.0 | 1.0 | 8.0 | 6.0 | |
| 2.0 | 1.0 | 5.0 | 9.0 | |
| Average | 1.7 | 1.0 | 6.0 | 7.0 |
| SEM | 0.4 | 0.0 | 1.3 | 1.3 |
| SD | 0.6 | 0.0 | 1.7 | 1.7 |
Table 4.
CFU-F Numbers Per mg of Tissue for HV and PV Tissue Samples from Two Equine Donors
| Animal | Sample | Wet weight corresponding to 1000 cells (mg) | CFU-Fs/1000 cells | CFU-Fs/mg | SEM |
|---|---|---|---|---|---|
| Horse 1 | HV | 0.50 | 13.0 | 25.9 | 4.0 |
| PV | 0.92 | 13.3 | 14.6 | 4.3 | |
| Horse 2 | HV | 0.78 | 48.0 | 61.3 | 4.4 |
| PV | 0.76 | 52.0 | 68.2 | 5.5 |
Vascularity analysis
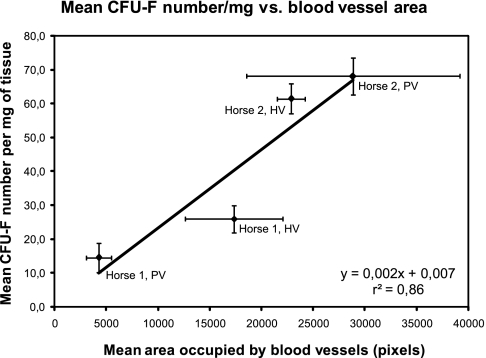
To establish if a relationship existed, the normalized CFU-F numbers were plotted against the density of blood vessels for each tissue sample. Several antibodies directed to human endothelial cells or pericytes were tested for their reactivity with equine antigens in cryosections. Since the human-specific antibodies used did not react with horse fat specimens, the detection of blood vessels by immunofluorescence could not be accomplished, and the vascularity in the fat tissue samples was thus assessed by means of the analysis of stained paraffin-embedded sections. The results indicated that Horse 2's supposedly PV tissue sample was actually more vascularized than the sample obtained from HV fat site (Fig. 4). Finally, the mean CFU-F numbers per mg of fat tissue were plotted as a function of the mean area occupied by the blood vessels in each sample. As a result, a strong positive correlation (r2 = 0.86) between these two variables was observed (Fig. 4).
FIG. 4.
Correlation between CFU-F number/mg of tissue and blood vessel area. The mean number of CFU-Fs of each horse adipose tissue–derived cell sample was normalized using sample wet weights and as a function of the mean area occupied by blood vessels. Points corresponding to PV and HV samples from each equine donor (Horse 1 and Horse 2) are indicated. The correlation coefficient (r2) was calculated using Microsoft Excel. Error bars represent SEM.
Discussion
The exact location of MSCs in vivo is still poorly understood. Evidence suggests that the perivascular niche houses stem cells, and it is possible that all MSCs are associated with blood vessels in vivo. To test this possible association, equine adipose tissue samples from two different anatomic sites were obtained and used as a source of cells for different in vitro and in vivo assays. The samples' relative vascular densities were also analyzed. To further investigate the possible relationship between MSCs and perivascular cells, these MSCs were compared to HBVPs.
To analyze if the expanded cultured-adherent cells derived from equine adipose tissue can be operationally defined as MSCs, eATDCs were subjected to differentiation assays developed for hMSCs. To control for species-dependent differences, eMSCs were subjected to the same assays. An interesting finding is that eATDCs behaved differently as compared to eMSCs. The former exhibited at least some adipogenic capability under the conditions employed, while the latter did not. Even though all eATDCs were seeded at the same cell density, they retracted during the process of adipogenic differentiation and formed clusters, leaving portions of the culture area empty (not shown) and making photographic documentation difficult. This behavior is attributable to the use of conditions that were optimized for inducing adipogenesis in hMSCs, as eATDCs have been shown to robustly differentiate into adipocytes under equine-optimized conditions.30
eMSCs exhibited osteogenic properties in vitro, while eATDCs detached from the dish before the assay could be completed; detachment was a consequence of a progressive increase in the cells' contractility, indicative of conversion to a myofibroblastic phenotype rather than differentiation along the osteogenic lineage. The differences between these two populations were clearly evident in the ceramic cube assay, where eMSCs exhibited very high osteogenic activity as compared with no bone formation from eATDCs. eATDCs do display osteogenic differentiation under other culture conditions,30 and hence their inability to differentiate along the osteoblastic lineage in this study indicates that they require different differentiation conditions compared to eMSCs. This can be extended to explain the apparent poor osteogenic potential of eATDCs as compared to eMSCs in the in vivo ceramic cube assay. On the other hand, both eMSCs and eATDCs displayed chondrogenic differentiation capabilities in vitro. Based on this information, it is reasonable to consider eATDCs operationally as MSCs, although these are clearly distinctive from eMSCs.
The CFU-F assay has been previously used to estimate MSC frequency in bone marrow. The CFU-F assay was used here to verify variations in the apparent MSC frequency according to the vascular density of the adipose tissue samples. Data shown in Tables 3 and 4 indicate that CFU-F numbers greatly differed between the two equine donors. The number of CFU-Fs per 1000 input cells was very similar between the two anatomic sites in each donor (nearly 13 for both sites in Horse 1 and nearly 50 for both sites in Horse 2). When CFU-F numbers were normalized using the samples' wet weights, there were more CFU-Fs in the HV site of Horse 1. Interestingly, there were no differences in CFU-F frequency relative to blood vessel density between Horse 2's samples; this was later found to be a consequence of high vascularity in both adipose tissue sites in Horse 2.
The lack of antibodies recognizing equine blood vessel–associated antigens made the use of a nonconventional method (cutting blood vessels from digital images to count their correspondent number of pixels) necessary to estimate blood vessel density. Although this method is subject to errors, these are minimized as blood vessels are clearly identifiable in adipose tissue sections stained with hematoxylin and eosin. In addition, the vascularity analyses performed on tissue sections obtained from the different sites of the two equine donors were subject to the same experimental error, allowing for the assessment of relative blood vessel densities. The results indicated that there were no significant differences in blood vessel densities in Horse 2's samples; whereas, blood vessel density was indeed higher in Horse 1's HV sample as compared to the PV sample. Indeed, CFU-F numbers normalized using samples' wet weights correlated well with blood vessel density (Fig. 4). These results call for experimentation using a larger number of donors and samples; however, it is noteworthy that this association can be found using this small sample number.
Linear correlation between normalized CFU-F numbers and blood vessel density in the adipose tissue samples studied is indicative of physical association of MSCs with blood vessels. Previous studies have suggested that the pericyte is a possible mesenchymal progenitor in vitro and in vivo.10–13,16–18 To further investigate this possibility, HBVPs commercially obtained were subjected to differentiation assays designed for hMSCs. HBVPs clearly differed from hMSCs, and this difference could be explained by the different conditions used to culture these two cell types (a proprietary low-serum medium for HBVPs vs. DMEM-LG with 10% FBS from selected lots for hMSCs). No bone formation by eATDCs as compared to maximal bone formation by eMSCs in vivo could, likewise, reflect different conditions: eATDCs were not subjected to culture prior to the ceramic cube assay, while eMSCs were isolated and cultured strictly under hMSC conditions. Zannettino et al.31 have recently isolated pericytes from human adipose tissue using the 3G5 antibody. These were shown to form bone in vivo using a ceramic powder composed of hydroxyapatite and tricalcium phosphate as the vehicle, but after culture in medium containing 20% FBS. These results suggest that the differentiation potential of pericytes may be influenced by culture in the presence of high concentrations of serum, and this could explain why fresh eATDCs, or HBVPs cultured under low-serum conditions, did not form bone in vivo in this study. In fact, the similar behavior of eATDCs as compared to HBVPs under these circumstances suggests that both cell types are alike.
Conclusion
The validation of eATDCs as MSCs on an operational basis plus the linear correlation between the number of fibroblastic colonies formed by them and the vascular density of the samples studied provide a link between cultured MSCs and the vasculature. We believe that these data, although preliminary, add quantitative support to other studies that linked MSCs to the perivascular niche by means of qualitative approaches. MSC association with the vasculature validates the use of adipose tissue as a source of mesenchymal progenitors for clinical purposes since its stromal–vascular fraction can be isolated away from most adipocytes, and is likely to provide fresh cells containing higher absolute numbers of MSCs as compared with other cell populations such as bone marrow mononuclear cells.
Supplementary Material
Acknowledgments
We gratefully acknowledge the assistance of Ms. Kitsie J. Penick with the GAG assay, of Dr. J. Michael Sorrell and Ms. Marilyn Baber with immunostainings, and of Ms. Lisa Walsh with paraffin histology. Supported in part by National Institutes of Health (NIH) and by Mr. Dick J. Randall. L. da S.M. received a Traveling Fellowship from The Company of Biologists.
References
- 1.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 2.Caplan A.I. Dennis J.E. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 3.Haynesworth S.E. Goshima J. Goldberg V.M. Caplan A.I. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Mosca J.D. Hendricks J.K. Buyaner D. Davis-Sproul J. Chuang L.C. Majumdar M.K. Chopra R. Barry F. Murphy M. Thiede M.A. Junker U. Rigg R.J. Forestell S.P. Bohnlein E. Storb R. Sandmaier B.M. Mesenchymal stem cells as vehicles for gene delivery. Clin Orthop Relat Res. 2000;379:S71. doi: 10.1097/00003086-200010001-00011. [DOI] [PubMed] [Google Scholar]
- 6.Meirelles L.da S. Nardi N.B. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702. doi: 10.1046/j.1365-2141.2003.04669.x. [DOI] [PubMed] [Google Scholar]
- 7.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J. Benhaim P. Lorenz H.P. Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 8.da Silva Meirelles L. Chagastelles P.C. Nardi N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 9.Krampera M. Marconi S. Pasini A. Galie M. Rigotti G. Mosna F. Tinelli M. Lovato L. Anghileri E. Andreini A. Pizzolo G. Sbarbati A. Bonetti B. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone. 2007;40:382. doi: 10.1016/j.bone.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Richardson R.L. Hausman G.J. Campion D.R. Response of pericytes to thermal lesion in the inguinal fat pad of 10-day-old rats. Acta Anat (Basel) 1982;114:41. doi: 10.1159/000145577. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Flores L. Gutierrez R. Gonzalez P. Varela H. Inducible perivascular cells contribute to the neochondrogenesis in grafted perichondrium. Anat Rec. 1991;229:1. doi: 10.1002/ar.1092290102. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Flores L. Gutierrez R. Lopez-Alonso A. Gonzalez R. Varela H. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res. 1992;275:280. [PubMed] [Google Scholar]
- 13.Brighton C.T. Hunt R.M. Early histologic and ultrastructural changes in microvessels of periosteal callus. J Orthop Trauma. 1997;11:244. doi: 10.1097/00005131-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Bianco P. Riminucci M. Gronthos S. Robey P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 15.Shi S. Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2F003;18:696. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 16.Brighton C.T. Lorich D.G. Kupcha R. Reilly T.M. Jones A.R. Woodbury R.A., 2nd. The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res. 1992;275:287. [PubMed] [Google Scholar]
- 17.Reilly T.M. Seldes R. Luchetti W. Brighton C.T. Similarities in the phenotypic expression of pericytes and bone cells. Clin Orthop Relat Res. 1998;346:95. [PubMed] [Google Scholar]
- 18.Farrington-Rock C. Crofts N.J. Doherty M.J. Ashton B.A. Griffin-Jones C. Canfield A.E. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 19.Wexler S.A. Donaldson C. Denning-Kendall P. Rice C. Bradley B. Hows J.M. Adult bone marrow is a rich source of human mesenchymal “stem” cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 20.Fraser J.K. Wulur I. Alfonso Z. Hedrick M.H. Fat tissue: an under appreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Lennon D.P. Haynesworth S.E. Bruder S.P. Jaiswal N. Caplan A.I. Human and animal mesenchymal progenitor cells from bone marrow: identification of serum for optimal selection and proliferation. In Vitro Cell Dev Biol Anim. 1996;32:602. [Google Scholar]
- 22.Lennon D.P. Caplan A.I. Mesenchymal stem cells for tissue engineering. In: Vunjak-Novakovic G., editor; Freshney R.I., editor. Culture of Cells for Tissue Engineering. Hoboken, NJ: Wiley; 2006. pp. 23–59. [Google Scholar]
- 23.Penick K.J. Solchaga L.A. Welter J.F. High-throughput aggregate culture system to assess the chondrogenic potential of mesenchymal stem cells. Biotechniques. 2005;39:687. doi: 10.2144/000112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnstone B. Hering T.M. Caplan A.I. Goldberg V.M. Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 25.Carrino D.A. Arias J.L. Caplan A.I. A spectrophotometric modification of a sensitive densitometric Safranin O assay for glycosaminoglycans. Biochem Int. 1991;24:485. [PubMed] [Google Scholar]
- 26.Dennis J.E. Haynesworth S.E. Young R.G. Caplan A.I. Osteogenesis in marrow-derived mesenchymal cell porous ceramic composites transplanted subcutaneously: effect of fibronectin and laminin on cell retention and rate of osteogenic expression. Cell Transplant. 1992;1:23. doi: 10.1177/096368979200100106. [DOI] [PubMed] [Google Scholar]
- 27.Dennis J.E. Caplan A.I. Porous ceramic vehicles for rat-marrow-derived (Rattus norvegicus) osteogenic cell delivery: effects of pre-treatment with fibronectin or laminin. J Oral Implantol. 1993;19:106. [PubMed] [Google Scholar]
- 28.Dennis J.E. Konstantakos E.K. Arm D. Caplan A.I. In vivo osteogenesis assay: a rapid method for quantitative analysis. Biomaterials. 1998;19:1323. doi: 10.1016/s0142-9612(97)00170-1. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter A.E. Jones T.R. Lamprecht M.R. Clarke C. Kang I.H. Friman O. Guertin D.A. Chang J.H. Lindquist R.A. Moffat J. Golland P. Sabatini D.M. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidal M.A. Kilroy G.E. Lopez M.J. Johnson J.R. Moore R.M. Gimble J.M. Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet Surg. 2007;36:613. doi: 10.1111/j.1532-950X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 31.Zannettino A.C. Paton S. Arthur A. Khor F. Itescu S. Gimble J.M. Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.