Abstract
This study investigated the delivery of bone morphogenetic protein (BMP)4-secreting muscle-derived stem cells (MDSC-B4) capable of inducing bone formation in mice using collagen gel (CG), fibrin sealant (FS), and gelatin sponge carriers. After implanting these various cell-loaded scaffolds intramuscularly or into critical-size skull defects, we measured the extent of heterotopic ossification and calvarial defect healing over a 6-week period via radiographic, radiomorphometric, histological, and micro-computed tomography analyses. As expected, in the absence of MDSC-B4, there was no ectopic ossification and only minimal calvarial regeneration using each type of scaffold. Although CG and gelatin sponges loaded with BMP4-secreting cells produced the most ectopic bone, FS constructs produced bone with comparably less mineralization. In the mouse calvaria, we observed MDSC-B4-loaded scaffolds able to promote bone defect healing to a variable degree, but there were differences between these implants in the volume, shape, and morphology of regenerated bone. MDSC-B4 delivery in a gelatin sponge produced hypertrophic bone, whereas delivery in a CG and FS healed the defect with bone that closely resembled the quantity and configuration of native calvarium. In summary, hydrogels are suitable carriers for osteocompetent MDSCs in promoting bone regeneration, especially at craniofacial injury sites.
Introduction
Supplemental bone grafting is often required to heal critical-size bone defects after skeletal injury in orthopedic surgery, neurosurgery, and dentistry. Traditionally, the most common source of harvested tissue includes bone auto- and allograft, but these harvests are limited in supply and fraught with donor site morbidities, and there are concerns about disease transmission and immune rejection when using allografts. Consequently, intensive efforts on developing alternative approaches include fabricating osteogenic, osteoinductive, osteoconductive, and osteointegrative bone graft substitutes. Current bone engineering strategies mainly focus on transplanting cells embedded within supportive matrices and biomolecules, effectively creating a “tissue engineered construct” that has shown some success in repairing and regenerating bone tissue capable of restoring pathologically altered structures.1,2 Some have described this approach as consisting of an interactive triad of viable osteocompetent cells, soluble osteoinductive signals, and osteoconductive matrices or scaffolds.1,3
Skeletal muscle contains stem cells with the ability to differentiate into osteoblasts under the influence of proper inductive factors that have driven other progenitor cells toward the osteogenic lineage. Muscle-derived stem cells (MDSCs) stimulated or genetically engineered to express bone morphogenetic protein (BMP)2 or BMP4 have been shown to undergo osteogenic differentiation in vitro, form ectopic bone in vivo, and heal bone defects of the skull and long bones.4–7 A 5-mm diameter defect in the adult mouse calvaria is unable to heal spontaneously and has been recognized as a valid model and a robust bed for tissue engineered bone–regeneration strategies.8
With an abundance of delivery systems now made available to tissue engineers, selecting the appropriate biomaterial for bone engineering is critical for a successful outcome. In vivo, the ideal biomaterial must successfully deliver exogenously derived osteogenic factors and/or osteoprogenitor cells into the bone defect, all while evading host rejection before bone formation. Additionally, the biomaterial must preserve the bioactivity of each transported signaling factor, release inductive molecules at a pharmacologically desired rate, and ultimately provide a microenvironment that permits donor cell proliferation and differentiation. Part of providing for this microenvironment includes maintaining the potential space, rather than occupying it with biomaterial, so that native osteogenic cells and blood vessels can colonize the defect and proceed toward normal bone healing. Finally, the ideal delivery vehicle is completely biodegradable or integrates well with the host's bone.9–11 In addition to these in vivo criteria, various in vitro features exist for the ideal delivery system, including a biomaterial that can be easily loaded with osteogenic growth factors10 and multipotent stem cells,12,13 as well as carry genetically modified cells.2
Presently, the most commonly used delivery vehicles are inorganic bone graft substitutes, natural polymers, and synthetic polymeric matrices, in an isolated fashion or as composites of each other. A biomaterial that various investigators, including our group, have widely used to study bone regeneration is the naturally derived polymer, porcine skin gelatin sponge, called Gelfoam (Pharmacia & Upjohn, Kalamazoo, MI), originally designed for hemostasis in the field of general surgery.5,7,14–17 We have since shown that MDSCs can be delivered in Gelfoam to induce ectopic ossification and successfully heal bony defects.4–7,14–16 Reports on similar collagen sponges indicate that, when loaded with recombinant human BMP2, this biomaterial enhances bone formation18 and produces regenerated bone that is comparable in size to regenerated bone obtained using autografts.19 As a result, many now considered absorbable collagen sponges to be a criterion standard scaffold for bone engineering.20 In part because of this, the utility of other scaffolds in combination with MDSCs for inducing bone formation and healing bone defects has not been widely investigated. To this end, our laboratory has begun to demonstrate that retrovirally transduced muscle-derived cells can proliferate in collagen gels (CG) and repair articular cartilage defects,21 whereas BMP4-secreting MDSCs embedded in fibrin glue can acquire a chondrocyte-like phenotype after implantation into an osteochondral defect.22 These results prompted us to investigate the utility of CG and fibrin sealant (FS) as delivery devices for BMP4-secreting MDSCs as a strategy for bone engineering.
In this report, we present experiments involving MDSCs isolated from post-natal murine skeletal muscle, which are transduced with a retroviral vector encoding BMP4 or LacZ. We compare the efficacy of each cell construct after delivery into syngeneic mice through three different scaffolds, including two types of hydrogels (bovine type I CG and FS), as well as a solid-phase gelatin sponge, Gelfoam. We assessed the efficacy of each cell–scaffold construct by measuring the amount of bone formation in the mouse hindlimb or in a critical-size parietal bone defect via radiographic, radiomorphometric, histologic, and micro-computed tomography examination over a 6-week period.
Materials and Methods
Cell isolation and retroviral transduction
We isolated MDSCs using a modified preplate technique, as previously described,23 and transduced these cells with a retroviral vector24 to express LacZ and BMP4 (MDSC-B4). For the control we selected MDSCs transduced with a retroviral vector–expressing LacZ reporter gene (MDSC-Lac) that has been known to be incapable of inducing bone formation. Using non-treated cells as the negative control would not exclude the potential confounding effects related to retroviral transduction. We also used a BMP4 bioassay to quantify the secretion of functional BMP4 after cell transduction.24
Scaffold preparation
Twelve hours before transplanting into the muscle pockets of syngeneic mice, we harvested 2.5 × 105 MDSC-B4 and MDSC-Lac and separately loaded each into a 7-×7-×2-mm squares of Gelfoam. We then incubated the Gelfoam at 37°C in proliferation medium (10% fetal bovine serum, 10% horne serum, 1% penicillin/streptomycin, Dulbecco's modified Eagle medium) until just before surgical implantation. We repeated this procedure with a CG (32.5 mg/mL, NeuColl, Inc., Campbell, CA) at a cell-to-gel ratio of 1:1, for a total volume of 100 μL at the time of transplantation, as well as with a Tisseel FS (Baxter, Mississauga, ON) that was prepared according to manufacturer's instruction during the surgery. Similarly, we prepared scaffolds for transplantation into calvarial defects using 5.0 × 105 MDSC-B4 or MDSC-Lac and reducing the total volume of gels to 50 μL.
Intramuscular transplantation
In accordance with the Animal Research and Care Committee of the Children's Hospital of Pittsburgh (protocol #28-04), each surgical procedure was performed in a sterile fashion under general inhalation anesthesia. We divided 30 normal (C57BL/6J) mice into three groups according to the type of scaffold assigned to each group. Following bilateral posteriomedial skin incisions along the thighs, we created muscle pockets in each hind limb. We inserted scaffolds containing MDSC-B4 into the right limbs and control constructs consisting of MDSC-Lac into the left limbs. After surgery, animals were permitted unrestricted movement within their cages.
Transplantation into calvarial defect
We divided 64 normal (C57BL/6J) 12-week-old male mice into four groups. A control group consisted of untreated mice with a calvarial defect void of any cells or scaffolds. The other three groups consisted of mice receiving MDSC-B4 or MDSC-Lac loaded on Gelfoam, mixed with a CG, or mixed with a FS, as previously described. We created critical-size parietal bone defects using a 5-mm-diameter trephine burr (Fine Science Tools, Inc., Foster City, CA). After recovery from anesthesia, animals were permitted unrestricted movement within the cage. All mice were euthanized 6 weeks post-operatively.
Radiographic evaluation
Using a Faxitron specimen radiography system (MX-20, Faxitron X-ray Co, Wheeling, IL), we monitored for the presence of ectopic bone at 3 and 6 weeks post-intramuscular implantation and calvarial defect healing at 6 weeks post-operatively. Each skull specimen was harvested and fixed in 10% neutral buffered formalin for 24 h and subsequently transferred into 70% ethyl alcohol. After removing soft tissues, including brain substance, from each skull, we radiographed each specimen using diagnostic x-ray film (X-OMAT V, Kodak, Rochester, NY). Radiographs were scanned at 1200 dpi using the Microtek ScanMaker 9800XL (Microtek, Carson, CA). Additionally, we measured ectopic bone area and density along each hind limb, as depicted radiographically, using Northern Eclipse software, version 6.0 (Empix Imaging, Cheektowaga, NY). We then calculated the area of regenerated calvarial bone by subtracting the area of each skull defect remaining at 6 weeks from the original defect area measured at the time of surgery. We then compared mean bone regeneration area of the various treatment groups.
Histological evaluation and detecting ectopic bone mass
We harvested thigh muscles containing ectopic bone at 3 and 6 weeks after intramuscular implantation to evaluate the histology of these tissues. We froze each harvest in 2-methylbutane pre-cooled in liquid nitrogen in preparation for cryostat- sectioning of each tissue; each section was performed at a thickness of 7 μm. We then prepared each slide with von Kossa stain to reveal mineralized matrix and quantified the ectopic bone mass according to dry weight for the remaining specimens harvested after 6 weeks. From these latter tissues, we carefully detached the muscles and soft tissues affixed to each bone nodule, air-dried each nodule for 1 h at room temperature, and weighed each nodule using an analytical balance (AG 204, Mettler-Toledo, Inc., Columbus, OH).
Micro-computed tomography analysis
We assessed calvarial bone healing at 6 weeks after scaffold implantation using micro-computed tomography (μCT 40, Scanco Medical, Switzerland). We scanned at least three samples from each scaffold and MDSC-B4 treatment group that exhibited substantial healing on X-rays. Each skull was scanned in its entirety at an isotropic resolution of 30 μm over an average scan time of 70 min per sample. From these scans, we generated three-dimensional renderings by thresholding to segment each 16-bit gray-scale image. We also calculated the regenerated bone volume (mm3) and regenerated bone mineral density (mg hydroxyapatite (HA)/cm3) within the original defect for each treatment group using Scanco evaluation software, Scanco Medical, Switzerland. We also determined the density of the normal calvarial bone outside the zone of the initial defect to compare the bone mineral densities of the regenerated and native bone for each group of mice that received different scaffolds.
Statistical evaluation
We performed statistical analyses using a one-way analysis of variance multiple comparison and Student t test. Means and standard deviations for bone volume and bone density measurements were compared using the Kruskal-Wallis and Mann-Whitney U test. For all statistical tests, p < 0.05 was considered to be statistically significant.
Results
Ectopic ossification in muscle
Within 3 weeks of the intramuscular implantation of MDSC-B4, there was radiographic evidence of heterotopic ossification along the experimental hind limbs, whereas this was not the case for control limbs implanted with any type of scaffold containing MDSC-Lac (data not presented). We confirmed the deposition of mineralized extracellular matrix using histology from tissue harvested along the sites of MDSC-B4 implantation. Based on results from radiomorphometry, there was a larger area of bone in the Gelfoam and CG implant groups than in the FS implant group. Although this difference in the area of ectopic bone was not statistically significant when comparing the Gelfoam and CG groups, the FS group had a significantly smaller area than the CG group (data not presented).
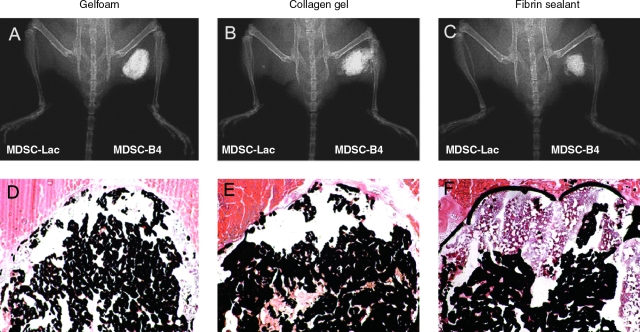
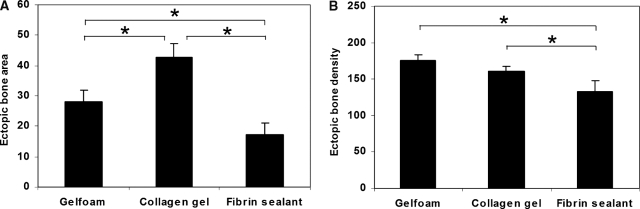
By the end of the sixth week after the hind limb implantation of MDSC-B4-seeded scaffolds, the radiographic evidence of heterotopic ossification was even more pronounced (Fig. 1A–C). Whereas Gelfoam and CG implants demonstrated robust bone formation according to von Kossa staining, the FS implants displayed signs of impeded matrix mineralization (Fig. 1D–F). Based on our radiomorphometry analysis, we were able to detect a difference between the Gelfoam and CG implants, the former of which had a significantly larger area of ectopic bone. As expected from our histological analyses, the ossification area in the FS implant group was significantly smaller than in the other two groups (Fig. 2A). Although there was no statistical difference between the Gelfoam and CG implant groups in radiographic bone density, mice from each of these groups developed bone that was significantly denser than in the FS group (Fig. 2B).
FIG. 1.
Ectopic bone formation at 6 weeks after intramuscular transplantation. (A–C) Radiographic examination revealed the presence of ectopic bone at implantation sites of bone morphogenetic protein 4–secreting muscle-derived stem cells (MDSC-B4) but not at implantation sites of MDSCs transduced with a retroviral vector–expressing LacZ reporter gene (MDSC-Lac) in each scaffold group. (D–F) Deposition of mineralized matrix within the thigh muscles receiving MDSC-B4 implants detected using von Kossa histological staining. Original magnification: ×40. Color images available online at www.liebertonline.com/ten.
FIG. 2.
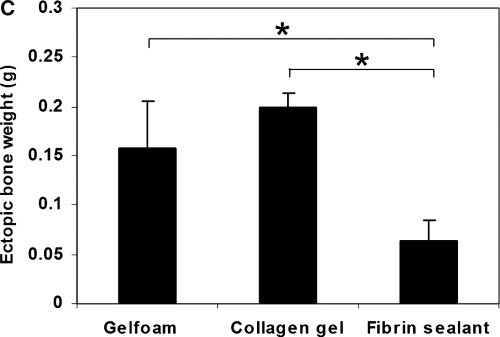
Radiomorphometric and bone mass analysis of heterotopic bone at 6 weeks. (A) Bone area, (B) bone density, (C) bone weight. Data represent means ± standard deviations; n = 3; *p < 0.05.
Neither radiographic nor histological examination at this time showed formation of heterotopic bone in the left hind limbs implanted with MDSC-Lac-seeded scaffolds.
According to quantitative analysis of ectopic bone based on dry weight, the Gelfoam and CG implants significantly outperformed the FS implants in terms of ectopic bone production after the delivery of MDSC-B4, with mean nodule weights of 0.19 ± 0.07 g, 0.2 ± 0.01 g, and 0.06 ± 0.02 g, respectively (Fig. 2C).
Calvarial defect healing
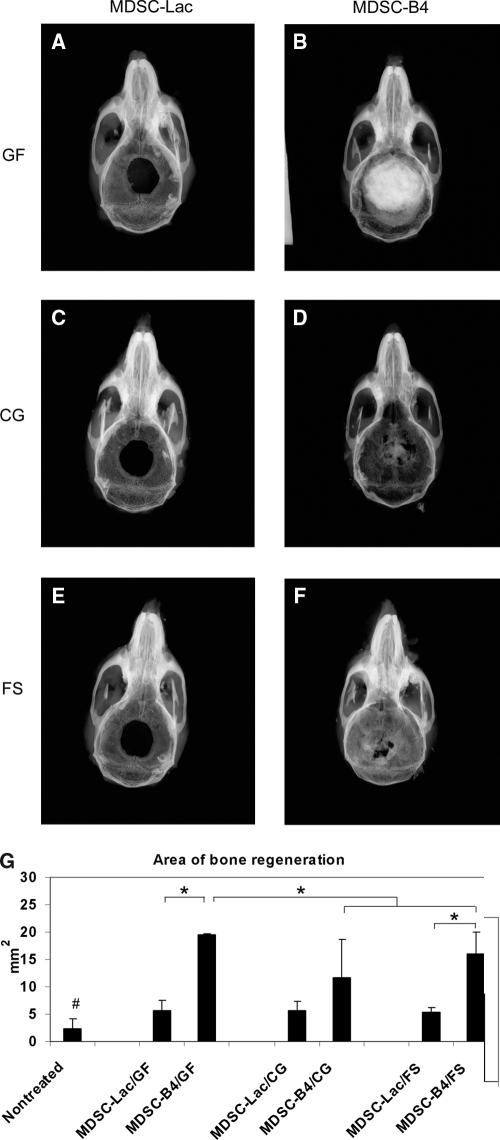
Depending on the type of cells and scaffold transplanted, there were variable degrees of healing along the parietal bone defect detected using radiographic and radiomorphometric evaluation during the sixth post-operative week. Overall, the untreated mice had only a small area of regenerated bone (2.35 ± 1.78 mm2, n = 10). By contrast, mice treated with MDSC-Lac healed slightly better, with almost identical regenerated bone surface area in the Gelfoam (5.72 ± 1.74 mm2, n = 10), CG (5.7 ± 1.69 mm2, n = 10), and FS ((5.31 ± 0.91 mm2, n = 8) groups (Fig. 3A, C, E, G). Mice treated with MDSC-B4-seeded scaffolds exhibited greater calvarial ossification than those treated with MDSC-Lac constructs. Regarding regenerated bone, the Gelfoam and FS scaffolds displayed significant gains, with full defect closure and the largest mean area of regenerated bone (19.58 ± 0.11 mm2, n = 7) occurring in the Gelfoam group (Fig. 3B, G). In comparison, eight of the 10 animals treated with MDSC-B4-seeded FS implants showed complete or nearly complete defect healing, whereas the other two had patent defects with only trace evidence of ossification (mean regenerated bone area 16.03 ± 4.03 mm2, n = 10) (Fig. 3F, G). In the eight mice treated with MDSC-B4-seeded CG implants, healing was complete in four, and there was only minimal osteogenesis in the other four (mean regenerated bone area 11.7 ± 7.04 mm2, n = 8) (Fig. 3D, G). Although the area of regenerated bone with MDSC-B4 was significantly larger in the Gelfoam implant group than in mice receiving CG and FS scaffolds, the difference between these two latter groups was insignificant.
FIG. 3.
Calvarial defect healing 6 weeks post-implantation. (A–F) Representative radiographs of bone healing in different treatment groups. (G) Radiomorphometric evaluation of the regenerated bone area in the various treatment groups. Data represent means ± standard deviations; *p < 0.05; #p < 0.05 vs muscle-derived stem cells transduced with a retroviral vector–expressing LacZ reporter gene treatment groups. GF, Gelfoam; CG, collagen gel; FS, fibrin sealant.
μ-CT analysis of regenerated bone
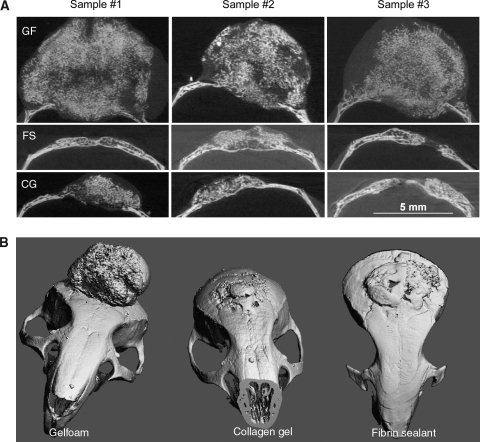
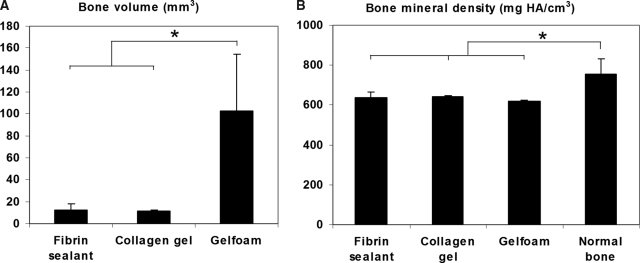
From the mice with radiographic evidence of complete healing after treatment with MDSC-B4, we selected at least three animals from each scaffold group to perform μCT analysis of the regenerated bone (n = 3 in CG, n = 4 in Gelfoam, and n = 5 in FS) This analysis consisted of two- and three-dimensional reconstruction imaging of the repaired defects, which revealed substantial differences in the volume and morphology of regenerated bone with each type of scaffold (Fig. 4A, B). There was extensive, protuberant, and apparently hypertrophic regenerated bone in the Gelfoam implant group, whereas defects treated with CG or FS implants were filled with allometric amounts of regenerated bone, each with a normal shape and compact structure. The differences in regenerated bone volume between the different delivery device groups were considerable, with close to 10 times more bone along the heavily ossified Gelfoam (102.85 ± 51.4 mm3) than on CG (11.57 ± 0.6 mm3) or FS (12.02 ± 6.2 mm3) implantation sites (Fig. 5A). In spite of this, the differences in bone mineral density between these three groups were not statistically significant (618.19 ± 5.6 mg HA/cm3 in Gelfoam, 643.52 ± 1.4 mg HA/cm3 in CG, and 637.9 ± 26.6 mg HA/cm3 in FS). When comparing these densities with those of the adjacent native tissue, those of regenerated bone were significantly lower than normal bone (754.02 ± 79.8 mg HA/cm3) (Fig. 5B).
FIG. 4.
Reconstruction of mice calvaria using micro-computed tomography. (A) Two- dimensional reconstruction of three MDSC-B4-treated samples in each scaffold group, (B) three-dimensional reconstruction of representative sample in each scaffold group. (GF, Gelfoam; FS, fibrin sealant; CG, collagen gel).
FIG. 5.
Micro-computed tomography analysis of the regenerated calvaria. (A) Bone volume, (B) bone mineral density. Data represent means ± standard deviations; n = 5 in fibrin sealant, n = 3 in collagen gel; n = 4 in Gelfoam; *p < 0.05 for bone volume and p < 0.001 for bone density.
Discussion
Ex vivo gene-based therapies involve the addition of genetic material to cells in vitro and the subsequent transplantation of the genetically altered cells. The use of ex vivo gene therapy necessitates the selection of the optimal transgene, the choice of an appropriate cell population, and the selection of an appropriate scaffold to deliver cells to the site of desired activity. The delivery of growth factors of the BMP family has often been employed in similar studies, because these factors can alter the differentiation pathways of progenitor cells toward the osteogenic pathways.25–27 Investigators have used many different cell populations in ex vivo gene-based therapies designed to promote bone healing, including bone marrow stromal cells,28 adipose tissue–derived cells,29 fibrous tissue–derived cells,30 and skeletal muscle–derived cells.4–7
Biomaterials developed from natural polymers have captured the interest of tissue engineers because of their biocompatibility, ease of remodeling, and superior cell adhesiveness, all of which are important for regenerating bone successfully.11 A variety of naturally derived polymeric materials, including collagen, fibrin, gelatin, agarose, hyaluronate, and alginate, have been recently investigated in combination with various growth factors and cell lines to enhance osteogenesis.31–33 The relatively novel approach has garnered particular interest in biomaterials that transform from liquid to more-solid phases after implantation. This morphological property provides delivery devices with the advantage of being able to carry osteoinductive molecules or cells while being administered through less-invasive means such as local injection or arthroscopy, each of which eliminates larger surgical exposure for implantation. Although the mechanical strength of hydrogel-based biomaterials has come into question when considering their application for skeletal regeneration, these delivery devices nonetheless provide a matrix for accelerated tissue formation that in turn will provide desirable mechanical integrity.34
Of all the biomaterials derived from naturally occurring polymers for tissue engineering, collagen-based delivery devices are the most popular because of their ubiquitous presence in tissues, low immunogenicity, and complete resorption. In vitro and in vivo studies have shown that these types of vehicles can deliver bioactive agents with controlled and sustained release.10 In our study, we used a highly purified bovine type I CG that is currently in clinical use and has a greater density than other conventional hydrogels.35 This gel is particularly appealing because it can be easily manipulated at room temperature, at which it is in its semi-viscous phase, whereas upon exposure to a temperature of 37°C, the phase becomes solid. Although most CG have reportedly been able to enhance osteogenesis,36–38 it is important to note that this particular gel exhibited an inherent inhibitory effect on cell proliferation, matrix production, and bone mineralization39 and thus was proposed to prevent bone formation along cranial suture sites.40 Contrary to these reports, however, we observed robust mineralization with the largest amount of ectopic bone in mice treated with these gels after being loaded with MDSC-B4. We further observed near-complete closure of the calvarial defects treated with this delivery construct, albeit with a 50% incidence of extensive bone regeneration.
We believe that a delay in the collagen phase transformation from a liquid to a solid immediately after implantation can explain the variable degrees of defect closure after the implantation of MDCS-B4-loaded CG. Such a delay would have caused the liquid delivery device to leak out along the defect borders. We would like to propose two explanations for why this may have occurred. One possibility is that there may have been slight but biologically significant differences in ambient temperature that we were unable to control for at the time of surgical implantation, potentially resulting in a longer time lapse for phase changes to occur in situ from a gel to a solid biomaterial. The other possibility is that the cell-to-gel ratio of 1:1 that we used in these experiments may not be optimal; reducing the quantity of cells and using a more-concentrated CG may increase the viscosity of our construct, potentially preventing material leakage and cell dissemination. We have based the latter explanation on our preliminary in vitro experiments, in which we tested the effect of gel density on MDSC proliferation and revealed that ratios of 1:2 and 1:3 resulted in larger cell counts within the gels incubated in proliferation medium for 8 days (unpublished data).
Fibrin, another frequently used delivery vehicle, is a natural scaffold that forms during wound healing and is important for hemostasis. Fibrin has been widely used in tissue engineering over the past 3 decades to deliver cells and bioactive substances. Regarding bone engineering, a reported advantage that fibrin has over several other biomaterials is that it is a physiologic delivery system for bone morphogenetic protein,41 beta fibroblastic growth factor, and vascular endothelial growth factor,42 as well as platelets containing angiogenic, mitogenic, and osteogenic growth factors,43 because fibrin is normally packaged with these substances. Additionally, several studies report that fibrin glue is highly conducive to the proliferation and differentiation of osteogenic cells33,44–46 and may thereby provide a highly suitable vehicle for the clinical application of bone regeneration. In spite of these favorable reports supporting the use of fibrin-based delivery devices, some investigators have also reported difficulty with bone formation after the subcutaneous implantation of fibrin gel mixed with bone marrow stromal cells32 and have subsequently proposed that fibrin may physically impair osteogenesis.43 This is consistent with our experimental results, in which there was delayed ossification and a reduction in ectopic bone formation after the implantation of MDSC-B4-loaded fibrin gels into the hind limbs of our experimental mice. This indicates that the aforementioned construct is less conducive than CG and Gelfoam to extra-skeletal ossification. In regards to healing calvarial defects, however, this cell-fibrin gel composite was successful, with 80% of the MDSC-B4-treated mice exhibiting near-complete defect closure within 6 weeks of transplantation. We observed a significant difference in the extent of bone healing between MDSC-B4 and the control MDSC-Lac in the FS and Gelfoam treatment groups that corresponded to a 202% and 242% greater mean area of regenerated bone, respectively. In fact, the FS group had a larger albeit statistically insignificant mean area of regenerated bone in comparison to the mice receiving CG implants.
As proposed for collagen implants, we believe that the concentration of the components used to fabricate FS can affect cell behavior within the gels. The existing commercial fibrin products consist of two human plasma–derived components: a highly concentrated fibrinogen complex (FC) composed primarily of fibrinogen and fibronectin along with catalytic amounts of factor XIII and plasminogen and a high-potency thrombin. Prior studies demonstrated that the behavior of human dermal fibroblasts and human mesenchymal stem cells in fibrin gels in vitro depends on the FC:thrombin concentration ratio. Specifically, fibroblast proliferation and migration are optimal in formulations containing a fibrinogen concentration ranging between 17 and 25 mg/mL, as well as a thrombin concentration of 167 to 250 U/mL.47 Formulations containing a low FC concentration supported the growth of human mesenchymal stem cells, whereas high FC concentrations offered a potential for their osteogenic differentiation.33 The Tisseel FS that we used in our experiments contained 75 to 115 mg/mL of fibrinogen and 400 to 600 U/mL of thrombin. The results from this study as well as those from our prior experience with repairing osteochondral defects22 strongly indicate that this FS formulation is conducive to MDSC proliferation. From this data, we believe that it is important to further examine other formulations of FS to determine which concentrations of ingredients would be optimal for MDSC proliferation and differentiation, as well as for the secretion of growth factors.
Among the major challenges in bone tissue engineering, regulating the amount and form of regenerated tissue is of paramount importance. We noticed a formidable difference in the shape and morphology of the regenerated bone within calvarial defects when comparing the results from each unique scaffold. In particular, we identified hypertrophic bone in the Gelfoam–MDSC-B4 group, with a predominance of outward bony protrusion and only a slight amount of bony intracranial intrusion. By contrast, in the CG and FS groups receiving the same type of cells, the regenerated bone closely resembled native calvarium, with a normal configuration that appeared diploic and had a relatively level surface. Furthermore, the volume of regenerated calvarial bone was nearly 10 times larger for the Gelfoam than for the CG and FS, as demonstrated using μCT analysis.
In conclusion, we demonstrate that the selection of delivery vehicles can affect bone formation and healing. Although a direct correlation between ectopic and orthotopic ossification remains to be shown for the same cell-delivery vehicle, our findings indicate that a more clinically relevant method for testing cell–scaffold constructs is to implant them directly along the site of the bone injury as opposed to studying the outcomes of implanting these constructs subcutaneously or intramuscularly. In our experiments, the CG and FS provided suitable scaffolds for MDSC-mediated bone engineering; this was particularly true of osteogenesis along the sites of bone injury. Injectable hydrogels provide an alternative method for bone grafting that can be of great clinical import when the supply of more-conventional bone grafts, such as allo- or autografts, is limited. This is also an excellent bone substitute for certain applications that require limited bone repair of delicate anatomical structures, such as the cranium, where mechanical stiffness is less important. Finally, the results from our study can pave the way for developing novel tissue-engineering techniques, ultimately assisting surgeons in the reconstruction of skeletal defects.
Acknowledgments
We would like to thank Dr. Amr Moursi, College of Dentistry, New York University, for providing the CG, and Dr. Rasesh Kapadia, Scanco USA, Wayne, PA, for assistance with the μ-CT analysis. This work was supported by funding from the Henry J. Mankin Endowed Chair for Orthopaedic Research at the University of Pittsburgh, the William F. and Jean W. Donaldson Chair at Children's Hospital of Pittsburgh, the Hirtzel Foundation, and grants awarded to Dr. Johnny Huard from the National Institutes of Health (R01 DE13420) and Department of Defense (W81XWH-05-1-0334).
Disclosure Statement
Dr. Johnny Huard acts as a consultant for Cook MyoSite, Inc., a company that has licensed technology developed by his laboratory. The University of Pittsburgh is managing the potential for conflict presented by this relationship in accordance with its policies and procedures.
References
- 1.Sittinger M. Hutmacher DW. Risbud MV. Current strategies for cell delivery in cartilage and bone regeneration. Curr Opin Biotechnol. 2004;15:411. doi: 10.1016/j.copbio.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Hutmacher DW. Garcia AJ. Scaffold-based bone engineering by using genetically modified cells. Gene. 2005;347:1. doi: 10.1016/j.gene.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 3.Ramoshebi LN. Matsaba TN. Teare J. Renton L. Patton J. Ripamonti U. Tissue engineering: TGF- beta superfamily members and delivery systems in bone regeneration. Expert Rev Mol Med. 2002;2002:1. doi: 10.1017/S1462399402004969. [DOI] [PubMed] [Google Scholar]
- 4.Lee JY. Qu-Petersen Z. Cao B. Kimura S. Jankowski R. Cummins J. Usas A. Gates C. Robbins P. Wernig A. Huard J. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright V. Peng H. Usas A. Young B. Gearhart B. Cummins J. Huard J. BMP4-expressing muscle- derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther. 2002;6:169. doi: 10.1006/mthe.2002.0654. [DOI] [PubMed] [Google Scholar]
- 6.Peng H. Wright V. Usas A. Gearhart B. Shen HC. Cummins J. Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen HC. Peng H. Usas A. Gearhart B. Fu FH. Huard J. Structural and functional healing of critical-size segmental bone defects by transduced muscle-derived cells expressing BMP4. J Gene Med. 2004;6:984. doi: 10.1002/jgm.588. [DOI] [PubMed] [Google Scholar]
- 8.Aalami OO. Nacamuli RP. Lenton KA. Cowan CM. Fang TD. Fong KD. Shi YY. Song HM. Sahar DE. Longaker MT. Applications of a mouse model of calvarial healing: differences in regenerative abilities of juveniles and adults. Plast Reconstr Surg. 2004;114:713. doi: 10.1097/01.prs.0000131016.12754.30. [DOI] [PubMed] [Google Scholar]
- 9.Seeherman H. The influence of delivery vehicles and their properties on the repair of segmental defects and fractures with osteogenic factors. J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 2):S79. ;. [PubMed] [Google Scholar]
- 10.Seeherman H. Wozney J. Li R. Bone morphogenetic protein delivery systems. Spine. 2002;27(16 Suppl 1):S16. doi: 10.1097/00007632-200208151-00005. [DOI] [PubMed] [Google Scholar]
- 11.Leach JK. Mooney DJ. Bone engineering by controlled delivery of osteoinductive molecules and cells. Expert Opin Biol Ther. 2004;4:1015. doi: 10.1517/14712598.4.7.1015. [DOI] [PubMed] [Google Scholar]
- 12.Hartman EH. Vehof JW. Spauwen PH. Jansen JA. Ectopic bone formation in rats: the importance of the carrier. Biomaterials. 2005;26:1829. doi: 10.1016/j.biomaterials.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi O. Katsube Y. Hirose M. Ohgushi H. Ito H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int. 2008;82:238. doi: 10.1007/s00223-008-9112-y. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY. Musgrave D. Pelinkovic D. Fukushima K. Cummins J. Usas A. Robbins P. Fu FH. Huard J. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical- sized bone defects in mice. J Bone Joint Surg Am. 2001;83-A:1032. doi: 10.2106/00004623-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Peng H. Usas A. Gearhart B. Young B. Olshanski A. Huard J. Development of a self-inactivating tet-on retroviral vector expressing bone morphogenetic protein 4 to achieve regulated bone formation. Mol Ther. 2004;9:885. doi: 10.1016/j.ymthe.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Peng H. Usas A. Hannallah D. Olshanski A. Cooper GM. Huard J. Noggin improves bone healing elicited by muscle stem cells expressing inducible BMP4. Mol Ther. 2005;12:239. doi: 10.1016/j.ymthe.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Geiger M. Li RH. Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Bouxsein ML. Turek TJ. Blake CA. D'Augusta D. Li X. Stevens M. Seeherman HJ. Wozney JM. Recombinant human bone morphogenetic protein-2 accelerates healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am. 2001;83-A:1219. doi: 10.2106/00004623-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Hollinger JO. Schmitt JM. Buck DC. Shannon R. Joh SP. Zegzula HD. Wozney J. Recombinant human bone morphogenetic protein-2 and collagen for bone regeneration. J Biomed Mater Res. 1998;43:356. doi: 10.1002/(sici)1097-4636(199824)43:4<356::aid-jbm3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Ruhe PQ. Kroese-Deutman HC. Wolke JG. Spauwen PH. Jansen JA. Bone inductive properties of rhBMP-2 loaded porous calcium phosphate cement implants in cranial defects in rabbits. Biomaterials. 2004;25:2123. doi: 10.1016/j.biomaterials.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Adachi N. Sato K. Usas A. Fu FH. Ochi M. Han CW. Niyibizi C. Huard J. Muscle derived, cell based ex vivo gene therapy for treatment of full thickness articular cartilage defects. J Rheumatol. 2002;29:1920. [PubMed] [Google Scholar]
- 22.Kuroda R. Usas A. Kubo S. Corsi K. Peng H. Rose T. Cummins J. Fu FH. Huard J. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54:433. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 23.Qu-Petersen Z. Deasy B. Jankowski R. Ikezawa M. Cummins J. Pruchnic R. Mytinger J. Cao B. Gates C. Wernig A. Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng H. Chen ST. Wergedal JE. Polo JM. Yee JK. Lau KH. Baylink DJ. Development of an MFG-based retroviral vector system for secretion of high levels of functionally active human BMP4. Mol Ther. 2001;4:95. doi: 10.1006/mthe.2001.0423. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y. Cheung KM. Kung HF. Leong JC. Lu WW. Luk KD. In vivo new bone formation by direct transfer of adenoviral-mediated bone morphogenetic protein-4 gene. Biochem Biophys Res Commun. 2002;298:121. doi: 10.1016/s0006-291x(02)02394-x. [DOI] [PubMed] [Google Scholar]
- 26.Chen D. Zhao M. Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 27.Gafni Y. Turgeman G. Liebergal M. Pelled G. Gazit Z. Gazit D. Stem cells as vehicles for orthopedic gene therapy. Gene Ther. 2004;11:417. doi: 10.1038/sj.gt.3302197. [DOI] [PubMed] [Google Scholar]
- 28.Derubeis AR. Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32:160. doi: 10.1023/b:abme.0000007800.89194.95. [DOI] [PubMed] [Google Scholar]
- 29.Zuk PA. Zhu M. Mizuno H. Huang J. Futrell JW. Katz AJ. Benhaim P. Lorenz HP. Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 30.Rutherford RB. Moalli M. Franceschi RT. Wang D. Gu K. Krebsbach PH. Bone morphogenetic protein-transduced human fibroblasts convert to osteoblasts and form bone in vivo. Tissue Eng. 2002;8:441. doi: 10.1089/107632702760184709. [DOI] [PubMed] [Google Scholar]
- 31.Windhagen H. Witte F. Thorey F. Hurschler C. Wirth CJ. [Injectable carrier system for growth factor application in minimally invasive stimulation of bone healing] Orthopade. 2004;33:1378. doi: 10.1007/s00132-004-0736-y. [DOI] [PubMed] [Google Scholar]
- 32.Xu XL. Lou J. Tang T. Ng KW. Zhang J. Yu C. Dai K. Evaluation of different scaffolds for BMP-2 genetic orthopedic tissue engineering. J Biomed Mater Res B Appl Biomater. 2005 doi: 10.1002/jbm.b.30299. [DOI] [PubMed] [Google Scholar]
- 33.Bensaid W. Triffitt JT. Blanchat C. Oudina K. Sedel L. Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 34.Elisseeff J. Puleo C. Yang F. Sharma B. Advances in skeletal tissue engineering with hydrogels. Orthod Craniofac Res. 2005;8:150. doi: 10.1111/j.1601-6343.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 35.Pachence JM. Collagen-based devices for soft tissue repair. J Biomed Mater Res. 1996;33:35. doi: 10.1002/(SICI)1097-4636(199621)33:1<35::AID-JBM6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.Friess W. Collagen—biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45:113. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 37.Reddi AH. Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000;6:351. doi: 10.1089/107632700418074. [DOI] [PubMed] [Google Scholar]
- 38.Lee CH. Singla A. Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221:1. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 39.Moursi AM. Winnard PL. Fryer D. Mooney MP. Delivery of transforming growth factor-beta2- perturbing antibody in a collagen vehicle inhibits cranial suture fusion in calvarial organ culture. Cleft Palate Craniofac J. 2003;40:225. doi: 10.1597/1545-1569_2003_040_0225_dotgfa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 40.Premaraj S. Mundy BL. Morgan D. Winnard PL. Mooney MP. Moursi AM. Sustained delivery of bioactive cytokine using a dense collagen gel vehicle collagen gel delivery of bioactive cytokine. Arch Oral Biol. 2006;51:325. doi: 10.1016/j.archoralbio.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Kawamura M. Urist MR. Human fibrin is a physiologic delivery system for bone morphogenetic protein. Clin Orthop Relat Res. 1988:302. [PubMed] [Google Scholar]
- 42.Wong C. Inman E. Spaethe R. Helgerson S. Fibrin-based biomaterials to deliver human growth factors. Thromb Haemost. 2003;89:573. [PubMed] [Google Scholar]
- 43.Hokugo A. Ozeki M. Kawakami O. Sugimoto K. Mushimoto K. Morita S. Tabata Y. Augmented bone regeneration activity of platelet-rich plasma by biodegradable gelatin hydrogel. Tissue Eng. 2005;11:1224. doi: 10.1089/ten.2005.11.1224. [DOI] [PubMed] [Google Scholar]
- 44.Isogai N. Landis WJ. Mori R. Gotoh Y. Gerstenfeld LC. Upton J. Vacanti JP. Experimental use of fibrin glue to induce site-directed osteogenesis from cultured periosteal cells. Plast Reconstr Surg. 2000;105:953. doi: 10.1097/00006534-200003000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Yamada Y. Boo JS. Ozawa R. Nagasaka T. Okazaki Y. Hata K. Ueda M. Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J Craniomaxillofac Surg. 2003;31:27. doi: 10.1016/s1010-5182(02)00143-9. [DOI] [PubMed] [Google Scholar]
- 46.Catelas I. Sese N. Wu BM. Dunn JC. Helgerson S. Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006;12:2385. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 47.Cox S. Cole M. Tawil B. Behavior of human dermal fibroblasts in three-dimensional fibrin clots: dependence on fibrinogen and thrombin concentration. Tissue Eng. 2004;10:942. doi: 10.1089/1076327041348392. [DOI] [PubMed] [Google Scholar]