Abstract
The possibility of using multipotent adult bone marrow–derived mesenchymal stem cells (MSCs) for tissue-engineering applications hinges on the ability to predictably control their differentiation. Previously, we showed the osteogenic potential of adult bone marrow–derived MSCs cultured on thin films of poly(lactide-co-glycolide) (PLGA) depends in part on the identity of extracellular matrix (ECM) ligands initially deposited onto the material from serum in the culture medium. Here we have addressed the hypothesis that remodeling of the PLGA surface via the de novo synthesis of ECM proteins by the MSCs may also play an important role in governing their osteogenic differentiation. Supporting this hypothesis, increasing amounts of fibronectin and type-I collagen were synthesized and deposited onto thin-film PLGA substrates, whereas vitronectin levels diminished over a 28-day time course. Integrin expression profiles changed accordingly, with higher levels of α2β1 and α5β1 than αvβ3 at three different time points. The mitogen-activated protein kinase (MAPK) and phosphatidyl inositol-3-kinase (PI3K) pathways were also activated in MSCs cultured on these substrates, and their inhibition significantly inhibited osteogenic differentiation as assessed according to alkaline phosphatase activity and mineral deposition. These data indicate that initial ECM deposition, subsequent matrix remodeling, and corresponding integrin expression profiles influence osteogenesis in MSCs cultured on PLGA in part by engaging MAPK and PI3K signaling pathways. Understanding the mechanisms by which stem cells respond to different polymers will be critical in their eventual therapeutic use.
Introduction
The classic paradigm for tissue engineering involves seeding an appropriate cell source on or within a scaffold that facilitates cell growth, organization, and differentiation into functional tissues. The prototypical scaffolds in this paradigm are often composed of biodegradable polymers, which can be tailored to degrade at a rate coincident with new tissue development1 and readily support cell attachment. However, the mechanism of cell adhesion to most synthetic polymers is indirect and relies on the passive deposition of extracellular matrix (ECM) proteins,2,3 a process that is difficult to control and predict. As a result, such traditional polymeric scaffolds may lack the specificity of protein-resistant substrates covalently modified with specific ECM ligands4 and therefore may fail to provide important instructive signals in a stable fashion. Nevertheless, because materials such as poly(lactide-co-glycolide) (PLGA) are FDA-approved, easily processed, and relatively inexpensive, they continue to be widely used in the tissue-engineering field. It is therefore critical to understand the mechanisms by which these scaffolding materials may govern cell function and the consequences of cell-mediated remodeling events on the stability of the cell–material interface and thus the performance of these polymer scaffolds.
Equally as important as the scaffold in the classic tissue-engineering paradigm are the cells. Stem cells in particular represent an attractive cell source for regenerative medicine based on their potential to regenerate a wide range of tissues. A great deal of excitement is associated with the use of embryonic stem cells because of their pluripotency, but many adult stem cells, including bone marrow–derived mesenchymal stem cells (MSCs), provide viable alternatives in some cases and do not raise the same sociopolitical debates.5 MSCs are particularly attractive in the context of bone tissue engineering and other reconstructive applications, given that autologous cells may be used for eventual therapy.6 Prior studies using marrow stromal cells have also demonstrated their capacity to differentiate into multiple lineages,7 including bone,8,9 cartilage,10 fat,11,12 tendon,13 muscle,14,15 and even nervous tissue.16,17 Other studies have shown their ability to act in a paracrine fashion when delivered with other cell types to support more-complex morphogenetic events, such as capillary morphogenesis.18,19 Given the wide interest in their therapeutic potential, an important hurdle to expanding their use in clinical applications is to predictably control their differentiation in vitro and in vivo. This may be accomplished by better understanding the signaling pathways that govern their fate decisions.
The roles of various soluble factors in promoting the osteogenic differentiation of MSCs have been extensively studied. Dexamethasone, bone morphogenetic protein-2 (BMP-2), β-glycerophosphate, ascorbic acid, and transforming growth factor-β (TGF-β) have all been identified as key soluble factors involved in regulating osteogenesis.7,8 Media containing these factors stimulate activation of signal transduction pathways to upregulate osteogenic genes, including type I collagen (Col I), osteocalcin (OCN), osteopontin (OPN), and alkaline phosphatase (ALP), and the eventual deposition of calcium phosphate mineral.20 Although the ECM's role in osteogenesis is not well characterized, prior studies using committed osteoblasts have shown a role for integrins, a family of heterodimeric cell adhesion receptors that provide a biophysical bridge and a biochemical link between cells and their ECM21,22 in osteogenesis.23–28 Whether the ECM (or substrate analogs of the ECM) instructs MSC differentiation via similar integrin-dependent mechanisms remains unclear.
In prior work using two common synthetic polymeric substrates as platforms for cell culture, we documented that the passive adsorption of two key matrix proteins, vitronectin (VN) and Col I, primarily mediates the adhesion of MSCs.2 A subsequent study showed that MSCs on VN- or Col I–coated substrates differentially activated the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase (PI3K) signal transduction pathways.29 However, the synthesis and secretion of new ECM proteins by the cells accompanies prolonged culture of MSCs on polymer scaffolds, and it is unclear what effect this may have on stem cell fate. Here we have used MSCs and PLGA substrates to more precisely address how cell-mediated remodeling of the substrate influences osteogenesis. Our findings reveal that the de novo synthesis and deposition of ECM proteins by the MSCs alters the chemical identity of the polymeric substrate, stimulates changes in integrin expression profiles, and thereby alters the MAPK and PI3K signaling pathways to influence osteogenesis.
Materials and Methods
Materials
Purified Col I, VN, and fibronectin (FN) were purchased from Cohesion (Palo Alto, CA), Chemicon International (Temecula, CA), and BD Transduction Laboratories (Lexington, KY), respectively. Monoclonal antibodies to phosphorylated p44/42 MAPK (also called extracellular signal–regulated kinase, ERK1/2), Akt-1 and β-actin were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Antibodies to ERK-1 and FN were purchased from BD Transduction Laboratories. A rabbit polyclonal anti-pser473-Akt antibody was purchased from Biosource International, Inc. (Camarillo, CA). Mouse monoclonal anti-α2β1, anti-α5β1, and anti-αVβ3 integrin antibodies were obtained from Chemicon International (Temecula, CA). Horseradish peroxidase–conjugated secondary anti-mouse and anti-rabbit antibodies were all purchased from Jackson Immunoresearch Laboratories, Inc. (Baltimore, PA). PD98059, which inhibits MEK-1 in the MAPK pathway, and LY294002, which inhibits the PI3K pathway, were purchased from Cell Signaling Technology, Inc. and used at concentrations of 50 μM and 20 μM, respectively. Kaleidoscope pre-stained and low-range pre-stained sodium dodecyl sulfate polyacrylamide gel electrophoresis standards were obtained from Bio-Rad Laboratories, Inc. (Hercules, CA). Anti-OPN antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All other routine tissue culture supplies and reagents were purchased from Fisher Scientific or Invitrogen (Carlsbad, CA).
Routine cell culture
Cryopreserved human bone marrow–derived MSCs were purchased from Lonza (Walkersville, MD) at passage 2. The manufacturer tests these cells for purity using flow cytometry and for their ability to differentiate into osteogenic, chondrogenic, and adipogenic lineages. The cells are positive for the cell surface markers CD105, CD166, CD29 (integrin β1), and CD44 and negative for CD14, CD34 and CD45. In our experiments, the MSCs were routinely cultured and expanded in a non-differentiating growth medium consisting of Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 4 mM of L-glutamine, 100 units/mL of penicillin and 0.1 mg/mL of streptomycin. Cells were grown in a 5% CO2 atmosphere at 37°C, and the medium was renewed every 2 to 3 days. Before confluence, cells were detached using trypsin- ethylenediaminetetraacetic acid (Invitrogen) and passaged 1:3 into fresh culture flasks. All experiments were conducted using cells below passage 8.
Preparation of polymeric films for cell cultures
Polymer films for two-dimensional cell culture were fabricated by spin-coating a 1% solution of 85:15 PLGA (Sigma-Aldrich, St. Louis, MO) dissolved in tetrahydrofuran onto silanized slides (Fisher Scientific) as previously described.2 After coating, slides were placed in a fume hood to permit residual organic solvents to evaporate, washed extensively, and subsequently sterilized using ultraviolet light before seeding with cells. Cells were seeded in standard growth medium at a pre-defined density (2.5 × 104 cells/cm2) onto these PLGA-coated slides and cultured overnight to permit attachment and spreading. The medium was then changed to growth medium with or without osteogenic supplements (OS medium; 0.1 μm dexamethasone, 10 mM β-glycerophosphate, and 50 μg/mL ascorbic acid). For the coating experiments with purified ECM proteins, PLGA-coated substrates were incubated overnight with Col I (100 μg/mL), FN (5 μg/mL), or VN (2 μg/mL), followed by washing with PBS before culturing cells. (Although these coating solutions were of different concentrations, they represent the values recommended by the manufacturers for each ECM ligand; all three exceed the theoretical 1 μg/cm2 saturation density of ECM ligands on 1-cm2 24-well dishes.30
Flow cytometric analysis of integrin expression
The cell surface expression of α2β1, α5β1, and αVβ3 integrins was quantified using quantitative flow cytometry as previously described.29 Briefly, MSCs (2.5 × 104 cells/cm2) were cultured on 1% PLGA-coated thin films in normal growth medium for 24 h. After that, the medium was replaced with OS medium, and cells were cultured for a total of 7 days and 14 days, with medium changes every other day. MSCs were harvested from their culture environment, counted, and resuspended in PBS plus 0.1% bovine serum albumin (BSA). Cell suspensions were incubated with anti-α2β1, anti-α5β1, or anti-αvβ3 antibodies (diluted 1:200) and rotated at 4°C for 45 min. Cells were collected via centrifugation and then washed three to four times with PBS. Bound primary antibodies were then detected by incubating cells with a fluorescein isothiocyanate–conjugated anti-mouse secondary antibody (diluted 1:100) and rotated at 4°C for another 45 min. After additional centrifugation and washing steps to remove unbound antibodies, MSCs were fixed in PBS plus 1% paraformaldehyde and washed one to two more times. Non-specific background staining was determined by staining cells with secondary antibody only in parallel (negative control). Stained cells were then analyzed using a BD FACSCalibur instrument (Becton Dickinson, San Jose, CA). A minimum of 10,000 cells was counted in each sample. All experimental data were analyzed offline using FlowJo software (Tree Star, Inc., Ashland, OR), with the mean fluorescent intensity used to determine the relative expression of each integrin heterodimer.
Determination of relative messenger RNA transcript levels using reverse transcriptase polymerase chain reaction
Reverse transcriptase polymerase chain reaction (RT-PCR) was used to monitor osteoblastic gene expression of MSCs cultured in normal growth medium or OS medium at 1, 7, 14, and 28 days. The messenger RNA (mRNA) levels of Col I (α1 chain), FN, runt-related transcription factor 2 (Runx2), bone sialoprotein (BSP), and OCN were examined and normalized to the levels of glyceraldehyde-3-phosphate dehydrogenase, an internal standard used for relative quantitative purposes. Briefly, at each time point, total RNA was isolated using the SV Total RNA Isolation System kit (Promega Corporation, Madison, WI) per the manufacturer's protocol. Samples were treated with 25 units of DNase I for 25 min at room temperature to remove residual genomic DNA. The concentration of the purified RNA was quantified using Quant-iT RiboGreen RNA assay kit (Molecular Probes, Eugene, OR), per instructions. Total RNA (220-240 ng) was used to synthesize complementary DNA (cDNA) templates using the ImProm-II Reverse Transcription System (Promega Corporation) according to the manufacturer's protocol. The cDNA (2 μL) for each sample was amplified using PCR for 30 cycles in a reaction volume of 50 μL containing 200 nM of each primer, 0.3 mM deoxynucleoside triphosphate (dNTP) (Promega Corporation), 2.5 mM magnesium chloride (MgCl2), and 1 U of Taq DNA polymerase (Denville Scientific, Inc., South Plainfield, NJ). The primer sequences, amplicon size, and annealing temperatures are listed for each analyzed gene in Table 1. Amplified products were separated on 2% agarose gels and stained with ethidium bromide. Intensities of bands were determined according to quantitative densitometry using NIH Image J software.
Table 1.
Primers and Conditions for Reverse Transcriptase Polymerase Chain Reaction
| Gene | Primers (F = forward; R = reverse) | Accession Number | Amplicon Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| Type I collagen | F: 5′-CTGGTGATGCTGGTCCTGTT-3′ | NM_000088 | 620 | 65 |
| R: 5′-CATGTAGGCCACGCTGTTCT-3′ | ||||
| Fibronectin | F: 5′-AGGAGACCACATGAGACT-3′ | X02761 | 355 | 52 |
| R: 5′-TGCCTCTCACACTTCCACT-3′ | ||||
| Runt-related transcription factor 2/core binding protein A-1 | F: 5′-GGAATGCCTCTGCTGTTATG-3′ | NM_001024630 | 581 | 65 |
| R: 5′-TGCCTGGCTCTTCTTACTGA-3′ | ||||
| Bone sialoprotein | F: 5′-CTCAATCTGTGCCACTCACT-3′ | NM_004967 | 355 | 62 |
| R: 5′-TCCTCCTCCTCTTCTGAACT-3′ | ||||
| Osteocalcin | F: 5′-CACCGAGACACCATGAGAGC-3′ | NM_199173 | 270 | 65 |
| R: 5′-GTGGTCAGCCAACTCGTCAC-3′ | ||||
| Glyceraldehyde-3-phosphate dehydrogenase | F: 5′-AAGGTCGGAGTCAACGGATT-3′ | NM_002046 | 443 | 65 |
| R: 5′-CAGGAGGCATTGCTGATGAT-3′ |
Electrophoresis and Western blot analyses
The expression of three different ECM proteins present on the PLGA thin films in the presence of the MSCs was assessed using electrophoresis and Western blot analysis. MSCs were seeded at a density of 2.5 × 104 cells/cm2 on PLGA thin films in normal growth medium or OS medium. At each of four time points (1, 7, 14, and 28 days), cultures were lysed in modified radioimmunoprecipitation assay lysis buffer (150 mM sodium chloride, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris-hydrochloric acid pH 7.5, 1% Nonidet P-40 including 1 μg/mL aprotinin, 1 μg/mL leupeptin, 10 μg/mL phenylmethylsulfonyl fluoride plus 10 μM sodium orthovanadate), yielding intracellular and extracellular proteins. The lysates were cleared using centrifugation at 13,000 rpm for 10 min at 4°C. After a bicinchoninic acid protein assay (Pierce Biotechnology, Inc., Rockford, IL), equal protein amounts were separated using gel electrophoresis and transferred to a polyvinylidene fluoride membrane. After blocking with 5% BSA-PBS for 1 h, membranes were incubated for 2 h at room temperature with anti-FN (1:250) or anti-VN (1:200) antibodies. After washing with PBS plus 0.1% Tween-20, membranes were incubated with horse radish peroxidase–conjugated secondary antibody for 1 h. Finally, membranes were washed again and the bands detected on film using enhanced chemiluminescence. Similar Western blotting techniques were performed to analyze signal transduction events using the following primary antibodies at the indicated dilutions: anti-p44/42-MAPK (1:2000), anti-ERK-1 (1:1000), anti-pser473-Akt (1:1000), anti-Akt-1 (1:1000), anti-OPN (1:200) and anti-β-actin (1:1000).
Immunofluorescence staining experiments
ECM proteins synthesized and deposited by the MSCs onto PLGA thin films were also assessed qualitatively using immunofluorescence detection of type-I collagen, FN, and VN. Briefly, MSCs (2.5 × 104 cells/cm2) were cultured on 1% PLGA-coated silanized slides in growth medium at 37°C with medium changes every other day. After the desired incubation period, slides were washed twice with PBS and subsequently fixed with 4% formaldehyde in PBS. Samples were incubated for 1 to 2 hours with primary antibodies against Col I (Chemicon International, Temecula, CA), VN (Santa Cruz Biotechnology, Santa Cruz, CA) or FN (BD Transduction Laboratories, Lexington, KY) diluted 1:200 in PBS. After washing with PBS, the slides were incubated with corresponding tetramethyl rhodamine iso-thiocyanate–conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) for an additional hour. After some final washing with PBS to remove unbound antibodies, fluorescently labeled samples were detected using a Nikon E800 fluorescence microscope equipped with a digital camera (Optronix, Goleta, CA).
Osteogenic differentiation assays
The osteogenic differentiation of MSCs on PLGA thin films was monitored using an ALP activity assay and the von Kossa method to stain for deposited calcium phosphate mineral, as done previously.29 For both assays, MSCs (2.5 × 104 cells/cm2) were seeded on 35-mm PLGA-coated dishes in serum-containing medium for 24 h. The culture medium was replaced with OS medium and changed every other day. For experiments involving the pharmacologic inhibitors (PD98059 and LY294002), fresh inhibitor was added at each medium change. For the ALP assay, MSCs were lysed after 7 and 14 days in passive lysis buffer (Promega Corporation) for 15 min at room temperature. Lysates were scraped and collected from the PLGA-coated surface and then incubated with 50 mM of p-nitrophenylphosphate in assay buffer (containing 100 mM glycine, 1 mM MgCl2, pH 10.5) at 37°C for 25 minutes. The reaction was stopped with 500 μL 0.1 N sodium hydroxide and the absorbance read at 405 nm. The specific ALP activity was determined using the extinction coefficient for p-nitrophenylphosphate (1.85 × 104/M per cm) and was then expressed in units of ALP activity per mg of protein.
For von Kossa staining, a commercially available kit (American MasterTech Scientific Inc, Lodi, CA) was used to stain calcium phosphate mineral deposited by MSCs cultured on PLGA thin films in OS medium in the presence or absence of PD98059 (50 μM) or LY294002 (20 μM). As in the ALP assays, medium with fresh inhibitors was provided every other day. After 14 days, cells were washed twice with warmed PBS pH 7.4, followed by fixation using 4% formaldehyde in PBS at 25°C for 40 min. Samples were then extensively rinsed with double distilled water and incubated in the presence of 5% silver nitrate, followed by exposure to ultraviolet light for 40 min. After extensive rinsing with water, the cells were treated with 5% sodium thiosulphate for 3 min, rinsed, and then incubated in Nuclear Fast Red stain for 5 min before a final rinse with water. Staining results were visualized on a Nikon E800 (Nikon, Melville, NY) microscope using a 10× objective and the images compiled with Picture Frame software (Optronics, Goleta, CA).
Statistical analysis
All statistical analyses were performed using InStat 2.01 for Macintosh (GraphPad Software, La Jolla, CA). Data are reported as means ± standard deviations, unless otherwise noted. In cases where statistical comparisons were made between three or more groups of data, a one-way analysis of variance was performed, followed by a Student-Newman-Keuls post-test comparison between two data sets at a time. In cases where only two sets of data were compared, a Student unpaired t-test was performed. P-values less than 0.05 denote statistical significance.
Results
ECM synthesis and remodeling by MSCs cultured on PLGA substrates
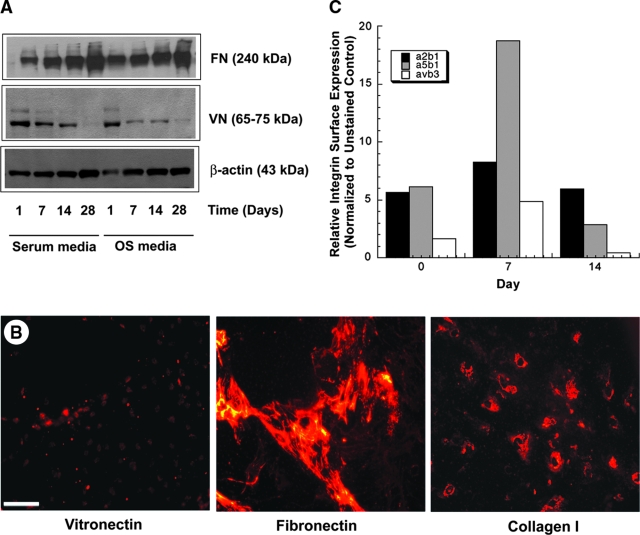
In a prior study, we demonstrated that FN, Col I, and VN are the predominant ECM ligands that deposit from serum to mediate the attachment of MSCs to PLGA substrates.2 However, the stability of these deposited ligands, and in particular their remodeling by the MSCs, was not clear in our initial study. Therefore, here these same proteins were detected using two different methods in MSC cultures grown on PLGA films in regular growth medium or OS medium for up to 28 days (Fig. 1). Western blotting of lysates from cell cultures revealed high levels of FN and VN in both culture media on the PLGA surfaces after just 1 day, consistent with our earlier report.2 Over the time course studied, FN levels gradually increased, whereas VN levels gradually decreased irrespective of the presence of the osteogenic supplements (Fig. 1A). Representative immunofluorescent images of extracellular VN, FN, and Col I from cells cultured in OS medium at an intermediate time point (day 7) support these Western blot data (Fig. 1B). These fluorescent micrographs show low levels of VN relative to FN and Col I and suggest that the newly deposited ECM proteins retain a pericellular localization.
FIG. 1.
Extracellular matrix (ECM) and integrin expression in mesenchymal stem cells (MSCs) cultured on poly(lactide-co-glycolide) (PLGA) substrates. (A) MSCs were cultured on non-tissue culture dishes coated with 85:15 PLGA in the presence of normal growth medium (serum medium) or medium containing osteoinductive supplements (OS medium) for 1, 7, 14, or 28 days. At each time point, cell lysates were analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blot for levels of fibronectin (FN) and vitronectin (VN). Results shown are from a representative blot, similar to those obtained from multiple experiments. (B) MSCs cultured on thin PLGA films in OS medium were also fixed and fluorescently stained for extracellular type I ollagen, VN, or FN. Images shown are representative of one of the intermediate time points (7 days). Scale bar represents 10 μm. (C) Quantitative flow cytometry was used to assess the relative expression of the α2β1, α5β1, and αvβ3 integrins in MSCs, with mean fluorescent intensities used to determine the relative expression of each integrin.
Based on these dynamics of ECM remodeling, next we assessed whether the integrin expression profiles of MSCs would correspondingly change when cultured on PLGA substrates for up to 2 weeks. Flow cytometric analysis of the cells before seeding on the PLGA surfaces revealed that the expression levels of α2β1 and α5β1 integrins were significantly higher than that of αVβ3 (Fig. 1C). As MSCs were cultured on the PLGA substrate in the presence of osteogenic supplements, the expression levels of α2β1 and α5β1 rose in the first 7 days, consistent with increased synthesis and deposition of Col I and FN, the canonical ligands for these two receptors, respectively. The levels of αVβ3 also rose during the first week. To the contrary, after 2 weeks, the levels of αVβ3 were nearly undetectable, consistent with the low levels of the VN ligand for this receptor. The high levels of α5β1 also dropped off substantially from day 7 to 14, whereas the levels of α2β1 returned to baseline, thus remaining relatively stable over the 2-week time course.
Expression patterns of ECM and osteogenic genes as revealed by RT-PCR
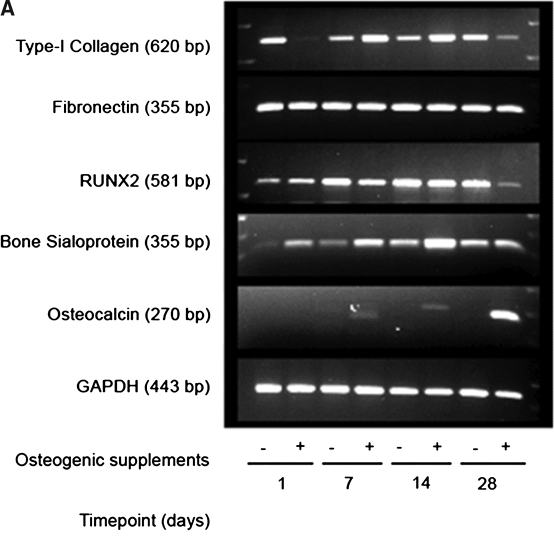
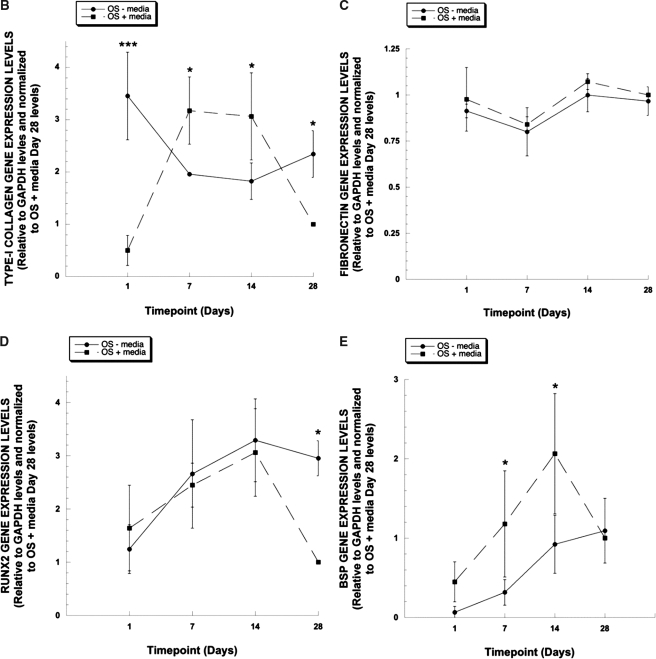
To confirm the results observed at the protein level, the expression patterns of Col I and FN at the mRNA levels were also assessed using RT-PCR, as were the patterns of several known osteogenic markers (Fig. 2). Semi-quantitative analysis of these patterns showed that Col I mRNA expression levels were higher in MSCs cultured in OS medium after 7 and 14 days. On the other hand, Col I mRNA levels were higher in normal growth medium at day 1 and day 28, showing an inverse relationship to the results in the OS medium (Fig. 2B).
FIG. 2.
Extracellular matrix (ECM) and osteogenic gene expression levels in mesenchymal stem cells (MSCs) cultured on poly(lactide-co-glycolide) (PLGA). (A) MSCs were cultured on spin-coated PLGA substrates for 1, 7, 14 and 28 days in normal growth medium or osteoinductive medium (OS medium). Type I collagen, fibronectin, runt-related transcription factor 2 (Runx2), bone sialoprotein, and osteocalcin gene expression levels were assessed by RT-PCR at each timepoint. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) messenger RNA expression was also examined as an internal control. Representative images of ethidium bromide–stained agarose gels are shown. (B–E) After scanning densitometry, type I collagen (B), fibronectin (C), Runx2 (D), and bone sialoprotein (E) gene expression levels were normalized to corresponding GAPDH expression levels, and the ratios were compared to OS medium day 28 condition. Statistical comparisons were made at each time point between the two different media conditions (*p < 0.05, ***p < 0.001).
FN mRNA levels remained relatively unchanged over 28 days in both culture media (Fig. 2C), consistent with the observations using Western blotting at the protein level (Fig. 1). The expression of Runx2 (also called core binding protein A-1 (CBFA-1)), a potent transcription factor involved in the regulation of several osteoinductive genes,31–34 showed increased expression with time in culture over the first 14 days (Fig. 2D), indicative that the MSCs are on a path toward a more-mature osteoblastic fate. By day 28, the levels of Runx2 expression were significantly lower in MSCs cultured in OS medium than in regular growth medium. The gene expression of BSP, a marker that is expressed during the intermediate phase of osteoblastic maturation,31,35 similarly increased with time in culture and was significantly higher in the MSCs in OS medium at 7 and 14 days (Fig. 2E). Finally, the expression of OCN, a late-stage marker of mature osteoblasts,31,35 was highly expressed after 28 days in OS medium (Fig. 2A). OCN expression was not detectable in cultures in regular growth medium.
Coating PLGA with purified ECM proteins induced higher ALP activities than uncoated surfaces
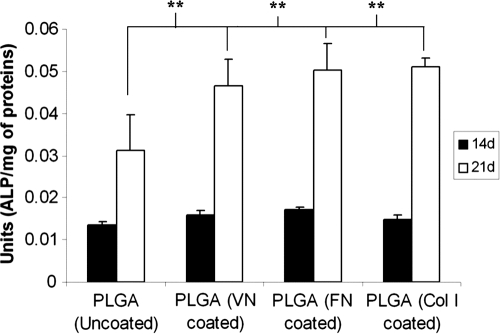
Next, a simple experiment was performed in which the PLGA thin films were coated overnight with solutions of Col I, FN, or VN. After 14 days, MSCs cultured on these ECM-coated PLGA substrates displayed little differences in ALP activity with respect to the different coatings and in comparison with the uncoated substrates (Fig. 3). On the other hand, after 21 days of culture, substrates coated with any one of the three ECM ligands supported significantly higher levels of ALP activity than uncoated substrates.
FIG. 3.
Coating poly(lactide-co-glycolide) (PLGA) substrates with purified extracellular matrix (ECM) proteins alters alkaline phosphatase (ALP) activity. Non-tissue culture dishes spin-coated with a 1% solution of 85:15 PLGA were incubated with a purified ECM protein solution [type I collagen (100 μg/mL), fibronectin (5 μg/mL), or vitronectin (2 μg/mL)] or kept uncoated overnight at 4°C. Mesenchymal stem cells were cultured on these substrates, and ALP activity was assessed after 14 and 21 days of culture. The ALP values for all of the ECM-coated PLGA substrates were significantly higher than the uncoated control surface (***p < 0.001).
MAPK and PI3K signaling pathways are activated in MSCs cultured on PLGA
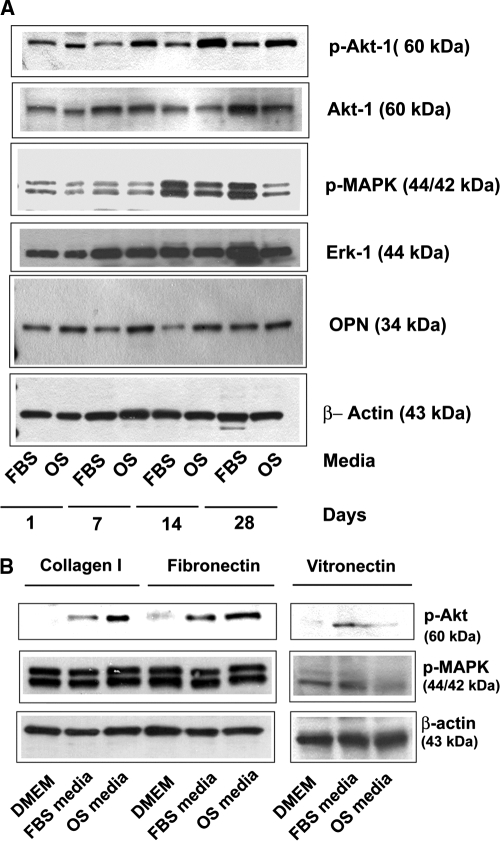
To investigate the downstream signaling pathways that may be involved in the regulation of osteogenesis by MSCs cultured on PLGA substrates, we focused on the MAPK and PI3K signaling pathways, motivated by our earlier studies implicating these pathways.29 MSCs were cultured on PLGA thin films for up to 28 days in normal growth medium or OS medium. Qualitative Western blotting of cell lysates revealed that MSCs cultured on PLGA substrates activated MAPK and PI3K pathways (Fig. 4A). In the case of the PI3K pathway, Akt-1 phosphorylation was higher in MSCs grown in OS medium than in those grown in normal medium. The expression of OPN, another early marker of osteoblastic differentiation, was also higher in MSCs cultured in OS medium than in normal growth medium at all four time points. On the other hand, in the case of the MAPK pathway, activation of ERK1/2 was notably higher in growth medium than in OS medium.
FIG. 4.
Mitogen-activated protein kinase (MAPK) and phosphatidyl inositol-3-kinase (PI3K) pathways are activated in mesenchymal stem cells (MSCs) cultured on poly(lactide-co-glycolide) (PLGA). (A) MSCs were cultured on spin-coated PLGA substrates for 1, 7, 14, and 28 days in normal growth medium (labeled FBS) or osteoinductive medium (labeled OS). Cell lysates at each time point were analyzed using electrophoresis and Western blot by probing for phosphorylated MAPK, phosphorylated Akt, osteopontin, and β-actin. Membranes were stripped and reprobed for the total levels of extracellular signal–regulated kinase and Akt-1. Western blots representative of multiple experiments are shown. (B) MSCs were cultured on substrates coated with fibronectin, type I collagen, and vitronectin in basal medium lacking serum (labeled DMEM), normal growth medium (labeled FBS), or osteoinductive medium (labeled OS). Shown are representative Western blots from lysates generated after 7 days.
We next assessed the activation of the MAPK and PI3K pathways in MSCs cultured on purified ECM proteins to ascertain whether there might be a link between these signaling pathways and the observed ECM synthesis and remodeling described earlier (Fig. 1, 2). To accomplish this, MSCs were seeded in medium without FBS (DMEM), containing FBS, or containing FBS and osteogenic supplements (OS medium) on substrates coated with Col I, FN, or VN. Activation of the MAPK and PI3K pathways were assessed using Western blotting of cell lysates generated after 7 days in culture (Fig. 4B). The results show that MSCs grown on Col I or FN substrates readily activated these two pathways, particularly in medium containing osteogenic supplements. However, cells cultured on VN displayed low levels of PI3K and MAPK signaling in all media. These data correlate with the adsorbed proteins and the signaling events on the PLGA substrates, with high levels of Col I and FN present on the polymer surfaces capable of supporting, or perhaps driving, activation of MAPK and PI3K signaling.
MAPK and PI3K pathways are both required for MSCs to undergo osteogenesis on PLGA
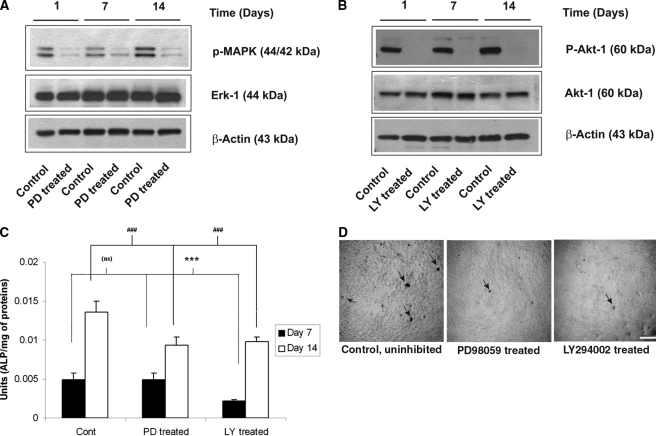
Finally, to examine whether the observed activities of the MAPK and PI3K pathways are actively involved in MSCs undergoing osteogenesis on PLGA, rather than passively correlated, we performed two different end-point assays of osteogenic differentiation in the presence of a MAPK inhibitor (PD98059) or a PI3K inhibitor (LY294002). First, we confirmed the inhibitory nature of these compounds in these particular cells by demonstrating that MSCs cultured in the presence of either of these two inhibitors display lower MAPK (Fig. 5A) and Akt-1 (Fig. 5B) phosphorylation levels, as expected. Then, we assessed the contributions of these two pathways on the osteogenic differentiation of MSCs cultured on PLGA substrates via a quantitative ALP activity assay and a qualitative von Kossa stain of mineral deposits. Inhibition of the PI3K pathway resulted in significantly lower levels of ALP activity than in untreated controls at the 7- and 14-day time points (Fig. 5C). Inhibition of the MAPK pathway resulted in significantly lower ALP activity (relative to untreated controls) after 14 days of culture (Fig. 5C). Similarly, treatment of MSCs cultured on PLGA substrates with either of these compounds for 14 days qualitatively reduced the levels of mineralization as detected using von Kossa staining (Fig. 5D).
FIG. 5.
Mitogen-activated protein kinase (MAPK) and phosphatidyl inositol-3-kinase (PI3K) pathways are required for the osteogenic differentiation of mesenchymal stem cells (MSCs) on poly(lactide-co-glycolide) (PLGA) substrates. (A) MSCs were cultured on spin-coated PLGA substrates in OS medium with or without the MAPK pathway inhibitor PD98059 (50 μM) for 1, 7, or 14 days, with the inhibitor replenished at every medium change. Cell lysates analyzed using Western blot for p-MAPK confirmed the pharmacologic inhibition of the MAPK pathway via PD98059. (B) Inhibition of the PI3K pathway via LY294002 (20 μM) generated similar inhibition of that pathway. (C) MSCs cultured on PLGA substrates with or without PD98059 or LY294002 (20 μM) in OS medium show significant reductions in alkaline phosphatase activity. (D) Pharmacologic inhibition of these pathways also induced qualitative reductions in mineral deposition at 14 days, as revealed by von Kossa staining (mineral deposits denoted by black arrows).
Discussion
Stem cells offer enormous therapeutic potential for regenerative medicine, and it is increasingly important to understand their interactions with natural and synthetic biomaterials used as artificial stem cell niches in vitro or as scaffold carriers for cell delivery in vivo. We have used PLGA thin films as prototypical synthetic polymer substrates and studied their interactions with MSCs, previously showing that the deposition of ECM ligands from serum onto these substrates plays a critical role in the regulation of osteogenesis.2 Here we monitored the dynamics of these substrates over a 28-day culture period, demonstrating that the MSCs remodel the polymer surface to present increasing amounts of FN and Col I and decreasing amounts of VN. The cell surface of the MSCs changes accordingly, with higher levels of the receptors for FN and Col I (α5β1 and α2β1 integrins, respectively) than those for VN (αVβ3 integrin).
Consistent with the idea that the dynamic cell–material interface influences the phenotype of MSCs, our gene expression analysis via RT-PCR suggests that MSCs cultured on PLGA substrates in the presence of osteoinductive medium increasingly express the gene for Col I during the first 14 days of culture (Fig. 2). Greater gene expression of the osteogenic transcription factor Runx2/CBFA-1 and of BSP accompanies this. After 28 days, however, the gene expression patterns suggest that the MSCs are proceeding toward a more-mature osteoblast phenotype characterized by reductions in the levels of mRNA transcripts for Col I, BSP, and Runx2, consistent with reports that activity and/or levels of Runx2 decrease as cells become more mature in their osteoblastic lineage.31,32 Further support for a more maturing osteoblastic phenotype are the increasing levels of OCN mRNA (Fig. 2A) and OPN detected via Western blot (Fig. 4).
MSCs cultured in the absence of the soluble osteoinductive agents were also found to express some of the genes required for the onset of osteogenesis, including Runx2 and BSP (Fig. 2), although the lack of Col I synthesis coupled with significantly lower levels of BSP suggests the delayed onset of a more-mature osteogenic phenotype. Further supporting this argument of a delay, not necessarily a block, of differentiation are our data showing that significantly higher Runx2 expression levels are maintained in medium lacking the osteoinductive supplements at day 28, along with an increasing trend in levels of BSP and collagen throughout the time course. These findings confirm that MSCs are primed for osteogenic differentiation, as might be expected given their bone marrow origins, but their differentiation is dependent on the presence of soluble factors known to stimulate bone formation. The new contribution provided by our data lies in the finding that the dynamic identity of the cell culture substrate is also a critical determinant of the osteogenic process.
Mechanistically, cell adhesion to Col I, FN, and VN has been shown to induce the activity of the MAPK cascade in many cell systems36,37 and is also critical to sustain growth factor–induced activation of ERK1/2, the most widely studied of the MAPK enzymes.38–40 Furthermore, MAPK signaling in response to soluble agonists and ECM ligands appears to play a critical role in the osteogenic differentiation of pre-osteoblastic cells by activating the Runx2/CBFA-1 transcriptional activator.41,42 Our group and others have also shown that different ECM ligands differentially regulate this pathway in MSCs,29,43,44 and our results here show that activation of this pathway in MSCs cultured on PLGA substrates is sustained for up to 28 days (Fig. 4A). The sustained activity of this pathway is consistent with cells cultured on purified FN and Col I but not VN (Fig. 4B). MAPK activity was higher after 21 days for MSCs cultured in normal growth medium than in OS medium which suggests that the osteoinductive supplements may slightly attenuate the highest activation of the MAPK pathway induced by mitogens present in serum. Nevertheless, pharmacologic inhibition of the MAPK pathway shows that its activity is clearly required for ALP activity (Fig. 5C) and mineral deposition (Fig. 5D). The pro-survival PI3K pathway, assessed according to the phosphorylation of Akt, was also activated in MSCs cultured on PLGA substrates (Fig. 4A) and is also essential for osteogenic differentiation (Fig. 5). We previously reported that MSCs cultured on purified VN display lower levels of MAPK and PI3K activity than do those on FN and Col I but are still capable of undergoing osteogenesis albeit via different mechanisms and different kinetics.29 Our data here suggest that the MSCs on PLGA behave in much the same fashion as when they are cultured on purified FN or Col I.29
Based on our findings, a reasonable hypothesis is that MSC adhesion via α2β1 or α5β1 integrins, which typically bind Col I and FN, respectively, is preferred and perhaps even required for osteogenic differentiation, whereas adhesion via αvβ3 is not. This would suggest that simply coating polymeric scaffolds with ECM proteins that enforce cell adhesion via these β1 integrins would be sufficient to support the osteogenic differentiation of MSCs, a simple solution partially supported by the data presented in Fig. 3. However, it is well known that multiple integrins can bind to a single ECM protein (i.e., α5β1 and αvβ3 can both bind to FN) and that multiple ECM proteins can bind a single integrin (i.e., αvβ3 can bind to FN, Col I, and VN).45 Therefore, it may not be just a simple matter of providing a uniform coating to engage a distinct receptor, but instead to identify the surface properties that control the presentation of integrin-specific epitopes within these coatings. For example, an earlier study from Garcia and colleagues used self-assembled monolayers of alkanethiols on gold as model substrates presenting well-defined surface chemistries to show that different chemical functionalities modulate the structure of deposited FN to alter integrin binding.46 Whether this or similar approaches can be extended to control the protein deposition and remodeling process on a biomaterial scaffold material suitable for use in cell transplantation (e.g., PLGA) remains to be seen. On the other hand, becuase we and others have shown that Col I, FN, and VN (which may or may not engage specific integrins) can all support the osteogenic differentiation of MSCs, this suggests that integrin homogeneity, rather than specificity per se, may be capable of conveying a more-permissive osteogenic environment to MSCs. There are many more questions to be addressed regarding the chemical role of the cell–material interface with respect to the regulation of stem cells. Furthermore, differences introduced by changes in ECM dimensionality (i.e., two versus three dimensions)47 and mechanical properties48 are also likely to influence integrins and their associated signaling pathways. It will therefore be critical to consider how all of these ECM-derived inputs are integrated to yield a specific cell-fate decision.
In conclusion, the data presented here suggest that MSCs grown on PLGA alter their microenvironment via FN and Col I synthesis, alter their integrin expression profiles, and alter downstream signaling pathways that are required for osteogenic differentiation. Future work will focus on developing a more-detailed and quantitative understanding of how the surface of polymeric substrates evolves with time in culture and the consequences of that evolution in terms of signal transduction events. Such data will facilitate the development of appropriate cue-signal-response models to mathematically describe how MSCs respond to multiple morphogenetic cues in their environment. Such predictive models will in turn enable a more-rational approach to our efforts to design biomaterials for bone regeneration applications.
Acknowledgments
Support for this work was provided by the National Institutes of Health (1R03DE016117 and 1R01HL085339). We gratefully acknowledge the technical contributions of Aswathi Sreedharan for assistance with cell culture and the University of California at Irvine Center for Immunology for assistance with flow cytometry.
References
- 1.Kim B.S. Mooney D.J. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16:224. doi: 10.1016/s0167-7799(98)01191-3. [DOI] [PubMed] [Google Scholar]
- 2.Chastain S.R. Kundu A.K. Dhar S. Calvert J.W. Putnam A.J. Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ECM ligands and controls their osteogenic differentiation. J Biomed Mater Res A. 2006;78:73. doi: 10.1002/jbm.a.30686. [DOI] [PubMed] [Google Scholar]
- 3.Murphy W.L. Hsiong S. Richardson T.P. Simmons C.A. Mooney D.J. Effects of a bone-like mineral film on phenotype of adult human mesenchymal stem cells in vitro. Biomaterials. 2005;26:303. doi: 10.1016/j.biomaterials.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 4.Drury J.L. Mooney D.J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 5.Caplan A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell. 2007:Physiol. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 6.Caplan A.I. Bruder S.P. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Ter Brugge P.J. Jansen J.A. In vitro osteogenic differentiation of rat bone marrow cells subcultured with and without dexamethasone. Tissue Eng. 2002;8:321. doi: 10.1089/107632702753725076. [DOI] [PubMed] [Google Scholar]
- 9.Warren S.M. Nacamuli R.K. Song H.M. Longaker M.T. Tissue-engineered bone using mesenchymal stem cells and a biodegradable scaffold. J Craniofac Surg. 2004;15:34. doi: 10.1097/00001665-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone B. Hering T.M. Caplan A.I. Goldberg V.M. Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 11.McBeath R. Pirone D.M. Nelson C.M. Bhadriraju K. Chen C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 12.Sordella R. Jiang W. Chen G.C. Curto M. Settleman J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 13.Young R.G. Butler D.L. Weber W. Caplan A.I. Gordon S.L. Fink D.J. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari G. Cusella-De Angelis G. Coletta M. Paolucci E. Stornaiuolo A. Cossu G. Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 15.LaBarge M.A. Blau H.M. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 16.Hermann A. Gastl R. Liebau S. Popa M.O. Fiedler J. Boehm B.O. Maisel M. Lerche H. Schwarz J. Brenner R. Storch A. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117:4411. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Ramos J. Song S. Cardozo-Pelaez F. Hazzi C. Stedeford T. Willing A. Freeman T.B. Saporta S. Janssen W. Patel N. Cooper D.R. Sanberg P.R. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 18.Al-Khaldi A. Al-Sabti H. Galipeau J. Lachapelle K. Therapeutic angiogenesis using autologous bone marrow stromal cells: improved blood flow in a chronic limb ischemia model. Ann Thorac Surg. 2003;75:204. doi: 10.1016/s0003-4975(02)04291-1. [DOI] [PubMed] [Google Scholar]
- 19.Ghajar C.M. Blevins K.S. Hughes C.C.W. George S.C. Putnam A.J. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12:2875. doi: 10.1089/ten.2006.12.2875. [DOI] [PubMed] [Google Scholar]
- 20.Aubin J.E. Advances in the osteoblast lineage. Biochem Cell Biol. 1998;76:899. [PubMed] [Google Scholar]
- 21.Giancotti F.G. Ruoslahti E. Integrin signaling. Science. 1999;285:1028. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 22.Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho R.S. Kostenuik P.J. Salih E. Bumann A. Gerstenfeld L.C. Selective adhesion of osteoblastic cells to different integrin ligands induces osteopontin gene expression. Matrix Biol. 2003;22:241. doi: 10.1016/s0945-053x(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 24.Cheng S.L. Lai C.F. Blystone S.D. Avioli L.V. Bone mineralization and osteoblast differentiation are negatively modulated by integrin alpha(v)beta3. J Bone Miner Res. 2001;16:277. doi: 10.1359/jbmr.2001.16.2.277. [DOI] [PubMed] [Google Scholar]
- 25.El-Amin S.F. Attawia M. Lu H.H. Shah A.K. Chang R. Hickok N.J. Tuan R.S. Laurencin C.T. Integrin expression by human osteoblasts cultured on degradable polymeric materials applicable for tissue engineered bone. J Orthop Res. 2002;20:20. doi: 10.1016/S0736-0266(01)00062-6. [DOI] [PubMed] [Google Scholar]
- 26.El-Amin S.F. Lu H.H. Khan Y. Burems J. Mitchell J. Tuan R.S. Laurencin C.T. Extracellular matrix production by human osteoblasts cultured on biodegradable polymers applicable for tissue engineering. Biomaterials. 2003;24:1213. doi: 10.1016/s0142-9612(02)00451-9. [DOI] [PubMed] [Google Scholar]
- 27.Schneider G.B. Zaharias R. Stanford C. Osteoblast integrin adhesion and signaling regulate mineralization. J Dent Res. 2001;80:1540. doi: 10.1177/00220345010800061201. [DOI] [PubMed] [Google Scholar]
- 28.Stephansson S.N. Byers B.A. Garcia A.J. Enhanced expression of the osteoblastic phenotype on substrates that modulate fibronectin conformation and integrin receptor binding. Biomaterials. 2002;23:2527. doi: 10.1016/s0142-9612(01)00387-8. [DOI] [PubMed] [Google Scholar]
- 29.Kundu A.K. Putnam A.J. Vitronectin and collagen I differentially regulate osteogenesis in mesenchymal stem cells. Biochem Biophys Res Commun. 2006;347:347. doi: 10.1016/j.bbrc.2006.06.110. [DOI] [PubMed] [Google Scholar]
- 30.Mooney D.J. Langer R. Ingber D.E. Cytoskeletal filament assembly and the control of cell spreading and function by extracellular matrix. J Cell Sci. 1995;108 (Pt 6):2311. doi: 10.1242/jcs.108.6.2311. [DOI] [PubMed] [Google Scholar]
- 31.Ducy P. Cbfa1: a molecular switch in osteoblast biology. Dev Dyn. 2000;219:461. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1074>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 32.Karsenty G. Role of Cbfa1 in osteoblast differentiation and function. Semin Cell Dev Biol. 2000;11:343. doi: 10.1006/scdb.2000.0188. [DOI] [PubMed] [Google Scholar]
- 33.Franceschi R.T. The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit Rev Oral Biol Med. 1999;10:40. doi: 10.1177/10454411990100010201. [DOI] [PubMed] [Google Scholar]
- 34.Raouf A. Seth A. Ets transcription factors and targets in osteogenesis. Oncogene. 2000;19:6455. doi: 10.1038/sj.onc.1204037. [DOI] [PubMed] [Google Scholar]
- 35.Aubin J.E. Liu F. Malaval L. Gupta A.K. Osteoblast and chondroblast differentiation. Bone. 1995;17:77S. doi: 10.1016/8756-3282(95)00183-e. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q. Kinch M.S. Lin T.H. Burridge K. Juliano R.L. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602. [PubMed] [Google Scholar]
- 37.Zhu X. Assoian R.K. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howe A.K. Aplin A.E. Juliano R.L. Anchorage-dependent ERK signaling—mechanisms and consequences. Curr Opin Genet Dev. 2002;12:30. doi: 10.1016/s0959-437x(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 39.Renshaw M.W. Ren X.D. Schwartz M.A. Growth factor activation of MAP kinase requires cell adhesion. Embo J. 1997;16:5592. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roovers K. Davey G. Zhu X. Bottazzi M.E. Assoian R.K. Alpha5beta1 integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth factor-treated cells. Mol Biol Cell. 1999;10:3197. doi: 10.1091/mbc.10.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franceschi R.T. Xiao G. Regulation of the osteoblast-specific transcription factor, Runx2: responsiveness to multiple signal transduction pathways. J Cell Biochem. 2003;88:446. doi: 10.1002/jcb.10369. [DOI] [PubMed] [Google Scholar]
- 42.Xiao G. Jiang D. Thomas P. Benson M.D. Guan K. Karsenty G. Franceschi R.T. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275:4453. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- 43.Klees R.F. Salasznyk R.M. Kingsley K. Williams W.A. Boskey A. Plopper G.E. Laminin-5 induces osteogenic gene expression in human mesenchymal stem cells through an ERK-dependent pathway. Mol Biol Cell. 2005;16:881. doi: 10.1091/mbc.E04-08-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salasznyk R.M. Klees R.F. Hughlock M.K. Plopper G.E. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Commun Adhes. 2004;11:137. doi: 10.1080/15419060500242836. [DOI] [PubMed] [Google Scholar]
- 45.Miranti C.K. Brugge J.S. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 46.Keselowsky B.G. Collard D.M. Garcia A.J. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci U S A. 2005;102:5953. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cukierman E. Pankov R. Yamada K.M. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14:633. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 48.Khatiwala C.B. Peyton S.R. Putnam A.J. Intrinsic mechanical properties of the extracellular matrix affect the behavior of pre-osteoblastic MC3T3-E1 cells. Am J Physiol Cell Physiol. 2006;290:C1640. doi: 10.1152/ajpcell.00455.2005. [DOI] [PubMed] [Google Scholar]