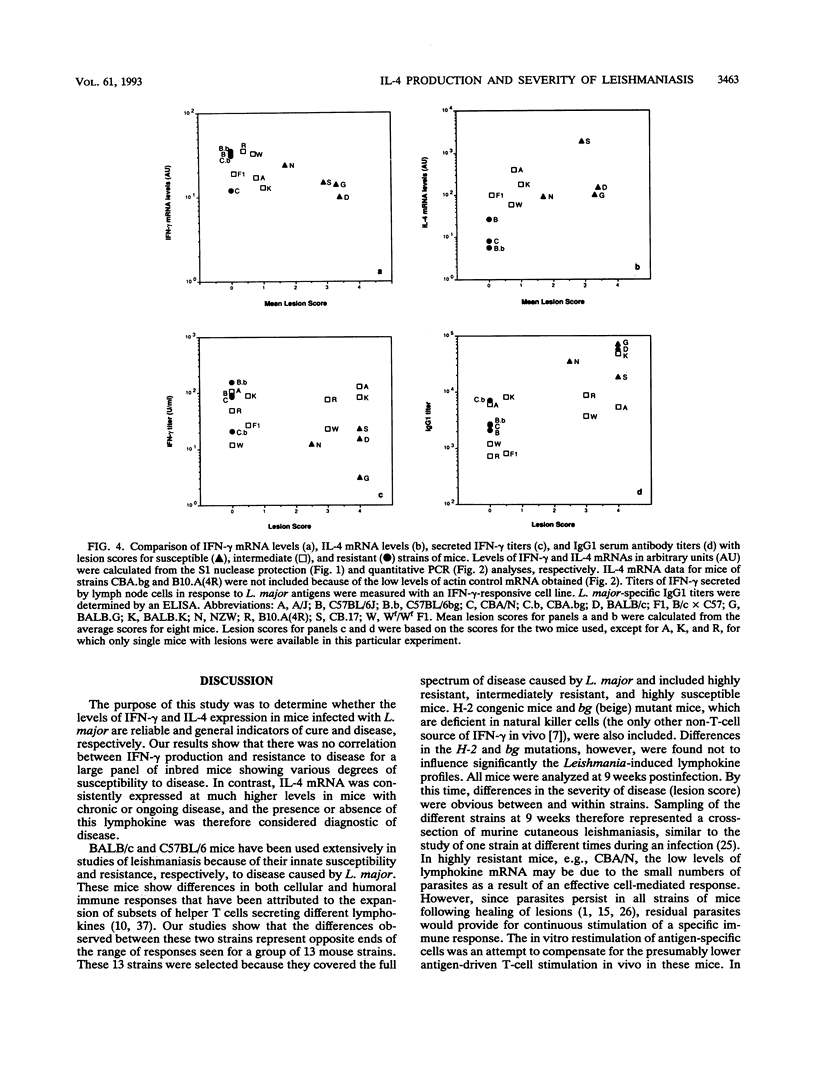
Abstract
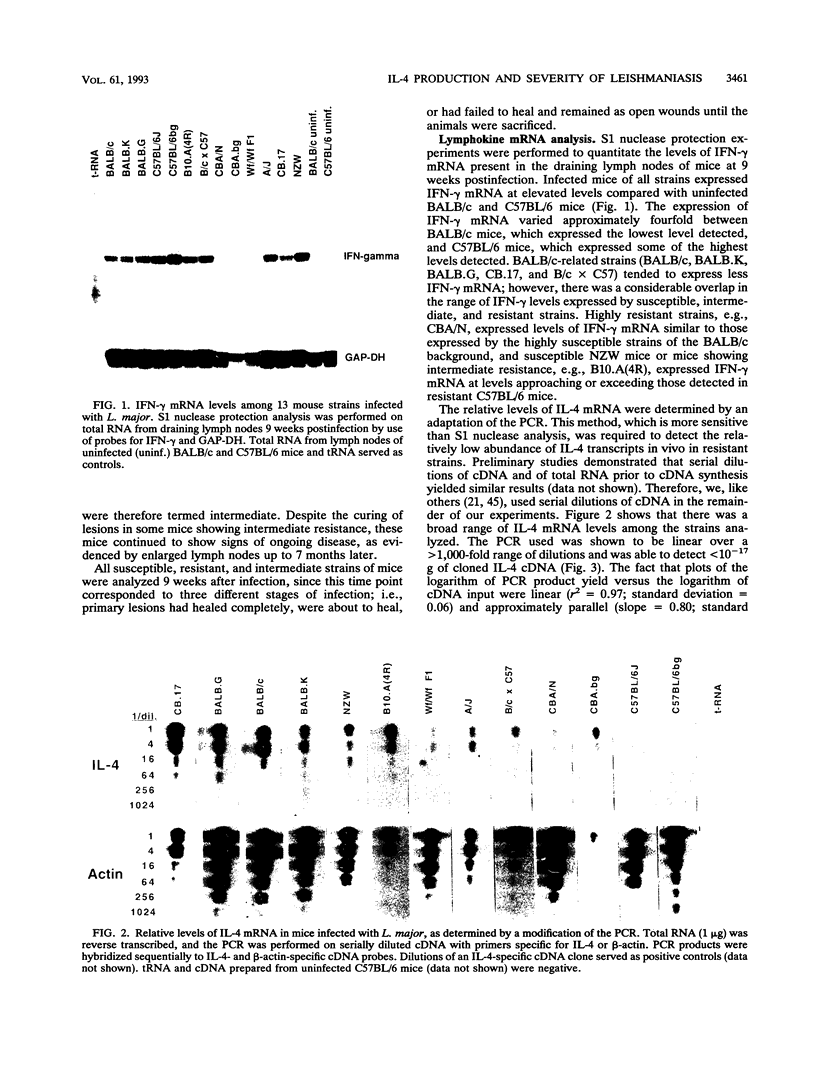
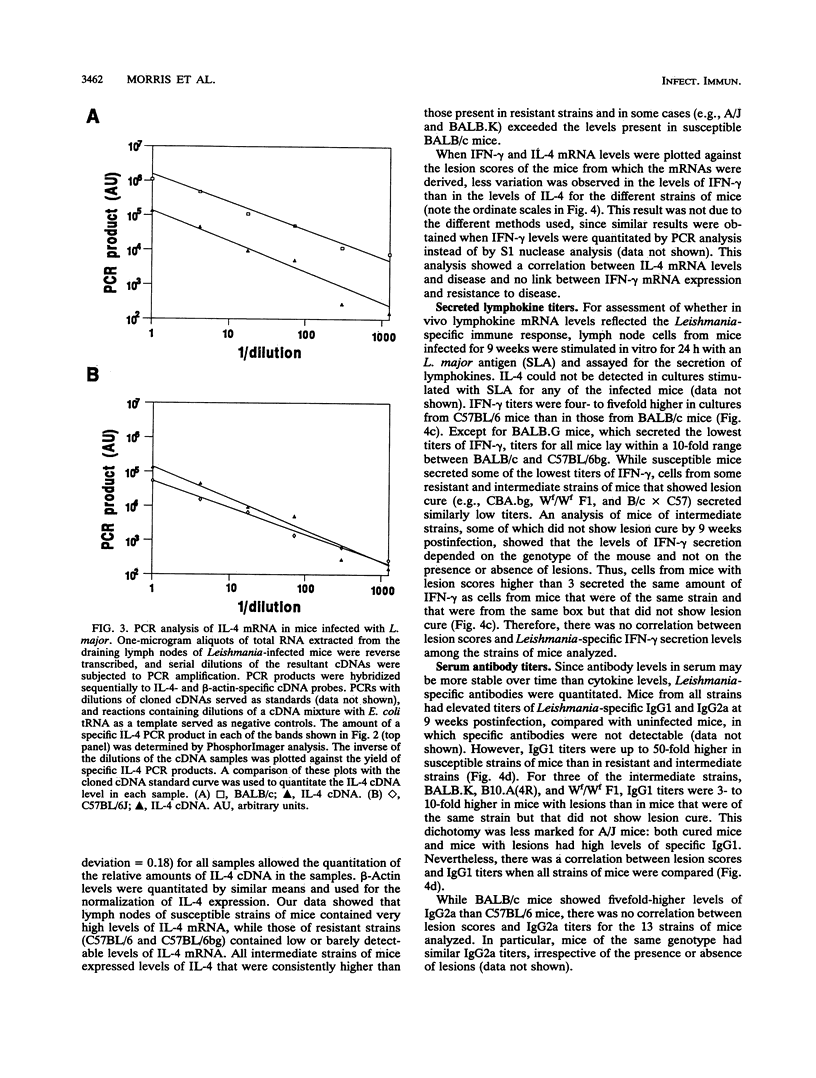
For murine cutaneous leishmaniasis, data to date suggest a correlation between the presence of gamma interferon (IFN-gamma) and resistance in C57BL/6 mice and the presence of interleukin-4 (IL-4) and disease in BALB/c mice. In this study, 13 inbred strains of mice covering the range of susceptibility to disease were infected with Leishmania major to determine whether the subsequent expression of IFN-gamma or IL-4 is a reliable indicator of cure or progressive disease. The presence of IL-4 and IFN-gamma mRNAs in the draining lymph nodes was examined 9 weeks after infection, when differences in disease severity became obvious. There were large differences in the levels of IL-4 mRNA among the different strains, whereas IFN-gamma mRNA was detected at similar levels in all strains. The levels of IL-4 mRNA correlated with lesion score, with susceptible and intermediate strains containing up to 100-fold more than any of the resistant strains. Differences in the levels of IFN-gamma mRNA were within only a fourfold range, with significant overlap among susceptible, intermediate, and resistant strains. Similarly, the levels of IFN-gamma secreted in vitro by lymph node cells from infected mice in response to L. major antigens were within a 10-fold range for most strains, and there was no correlation with lesion score. Analysis of Leishmania-specific antibody levels revealed a correlation between immunoglobulin G1 (IgG1) titers and lesion score, consistent with the role of IL-4 as a switch factor for IgG1. In contrast, there was no correlation between IgG2a titers and lesion score, supporting the notion that IFN-gamma synthesis (which promotes IgG2a production) is not correlated with disease state. These data suggest that along the spectrum of murine cutaneous leishmaniasis, IL-4 is a reliable indicator of disease, but IFN-gamma is not prognostic for resistance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebischer T., Moody S. F., Handman E. Persistence of virulent Leishmania major in murine cutaneous leishmaniasis: a possible hazard for the host. Infect Immun. 1993 Jan;61(1):220–226. doi: 10.1128/iai.61.1.220-226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral-Netto M., Barral A., Brownell C. E., Skeiky Y. A., Ellingsworth L. R., Twardzik D. R., Reed S. G. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science. 1992 Jul 24;257(5069):545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Van Der Meide P. H., Slayter M. V., Nacy C. A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989 Jul 1;143(1):266–274. [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T. A., Wells S. J., Spithill T. W., Pettitt J. M., Humphris D. C., Mukkada A. J. Leishmania major and L. donovani: a method for rapid purification of amastigotes. Exp Parasitol. 1990 Oct;71(3):343–345. doi: 10.1016/0014-4894(90)90039-f. [DOI] [PubMed] [Google Scholar]
- Handa K., Suzuki R., Matsui H., Shimizu Y., Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). II. IL 2-induced interferon gamma production. J Immunol. 1983 Feb;130(2):988–992. [PubMed] [Google Scholar]
- Handman E., Ceredig R., Mitchell G. F. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust J Exp Biol Med Sci. 1979 Feb;57(1):9–29. doi: 10.1038/icb.1979.2. [DOI] [PubMed] [Google Scholar]
- Handman E., Hocking R. E., Mitchell G. F., Spithill T. W. Isolation and characterization of infective and non-infective clones of Leishmania tropica. Mol Biochem Parasitol. 1983 Feb;7(2):111–126. doi: 10.1016/0166-6851(83)90039-7. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Mutha S. S., Locksley R. M. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., McDevitt H. O. Tumour necrosis factor-alpha in murine autoimmune 'lupus' nephritis. Nature. 1988 Jan 28;331(6154):356–358. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kelso A., Metcalf D. Clonal heterogeneity in colony stimulating factor production by murine T lymphocytes. J Cell Physiol. 1985 Apr;123(1):101–110. doi: 10.1002/jcp.1041230115. [DOI] [PubMed] [Google Scholar]
- Leclerc C., Modabber F., Deriaud E., Cheddid L. Systemic infection of Leishmania tropica (major) in various strains of mice. Trans R Soc Trop Med Hyg. 1981;75(6):851–854. doi: 10.1016/0035-9203(81)90430-2. [DOI] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Li Y., Lelchuk R., Chan W. L., Ziltener H. Macrophage activation by interferon-gamma from host-protective T cells is inhibited by interleukin (IL)3 and IL4 produced by disease-promoting T cells in leishmaniasis. Eur J Immunol. 1989 Jul;19(7):1227–1232. doi: 10.1002/eji.1830190712. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E., Troutt A. B., Kelso A. Co-engagement of CD3 with LFA-1 or ICAM-1 adhesion molecules enhances the frequency of activation of single murine CD4+ and CD8+ T cells and induces synthesis of IL-3 and IFN-gamma but not IL-4 or IL-6. Int Immunol. 1992 Apr;4(4):475–485. doi: 10.1093/intimm/4.4.475. [DOI] [PubMed] [Google Scholar]
- Meller-Melloul C., Farnarier C., Dunan S., Faugere B., Franck J., Mary C., Bongrand P., Quilici M., Kaplanski S. Evidence of subjects sensitized to Leishmania infantum on the French Mediterranean coast: differences in gamma interferon production between this population and visceral leishmaniasis patients. Parasite Immunol. 1991 Sep;13(5):531–536. doi: 10.1111/j.1365-3024.1991.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F. Murine cutaneous leishmaniasis: resistance in reconstituted nude mice and several F1 hybrids infected with Leishmania tropica major. J Immunogenet. 1983 Oct;10(5):395–412. doi: 10.1111/j.1744-313x.1983.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Mohler K. M., Butler L. D. Differential production of IL-2 and IL-4 mRNA in vivo after primary sensitization. J Immunol. 1990 Sep 15;145(6):1734–1739. [PubMed] [Google Scholar]
- Moll H., Mitchell G. F., McConville M. J., Handman E. Evidence of T-cell recognition in mice of a purified lipophosphoglycan from Leishmania major. Infect Immun. 1989 Nov;57(11):3349–3356. doi: 10.1128/iai.57.11.3349-3356.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll H., Röllinghoff M. Resistance to murine cutaneous leishmaniasis is mediated by TH1 cells, but disease-promoting CD4+ cells are different from TH2 cells. Eur J Immunol. 1990 Sep;20(9):2067–2074. doi: 10.1002/eji.1830200927. [DOI] [PubMed] [Google Scholar]
- Morris L., Troutt A. B., Handman E., Kelso A. Changes in the precursor frequencies of IL-4 and IFN-gamma secreting CD4+ cells correlate with resolution of lesions in murine cutaneous leishmaniasis. J Immunol. 1992 Oct 15;149(8):2715–2721. [PubMed] [Google Scholar]
- Murray H. W., Stern J. J., Welte K., Rubin B. Y., Carriero S. M., Nathan C. F. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J Immunol. 1987 Apr 1;138(7):2290–2297. [PubMed] [Google Scholar]
- Müller I., Garcia-Sanz J. A., Titus R., Behin R., Louis J. Analysis of the cellular parameters of the immune responses contributing to resistance and susceptibility of mice to infection with the intracellular parasite, Leishmania major. Immunol Rev. 1989 Dec;112:95–113. doi: 10.1111/j.1600-065x.1989.tb00554.x. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Passwell J. H., Shor R., Trau H., Shoham J., Jaffe C. L. Antigen-stimulated lymphokines from patients with cutaneous leishmaniasis induce monocyte killing of Leishmania major intracellular amastigotes. J Immunol. 1987 Dec 15;139(12):4208–4212. [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada E., Trujillo D., Castellanos P. L., Convit J. Gamma interferon production induced by antigens in patients with leprosy and American cutaneous leishmaniasis. Am J Trop Med Hyg. 1987 Nov;37(3):520–524. doi: 10.4269/ajtmh.1987.37.520. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Brenner C. A., Schultz R., Mark D., Werb Z. Developmental expression of PDGF, TGF-alpha, and TGF-beta genes in preimplantation mouse embryos. Science. 1988 Sep 30;241(4874):1823–1825. doi: 10.1126/science.3175624. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Wang A., Mark D., Werb Z. Novel method for studying mRNA phenotypes in single or small numbers of cells. J Cell Biochem. 1989 Jan;39(1):1–11. doi: 10.1002/jcb.240390102. [DOI] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Locksley R. M., Tubbs C., Raff H. V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-gamma in response to Leishmania antigens in vitro. J Immunol. 1986 Jan;136(2):655–661. [PubMed] [Google Scholar]
- Scott P., Pearce E., Cheever A. W., Coffman R. L., Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989 Dec;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Scott P. The role of TH1 and TH2 cells in experimental cutaneous leishmaniasis. Exp Parasitol. 1989 Apr;68(3):369–372. doi: 10.1016/0014-4894(89)90120-3. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Kelso A., Louis J. A. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clin Exp Immunol. 1984 Jan;55(1):157–165. [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989 Dec 1;170(6):2097–2104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutt A. B., Kelso A. Enumeration of lymphokine mRNA-containing cells in vivo in a murine graft-versus-host reaction using the PCR. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5276–5280. doi: 10.1073/pnas.89.12.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutt A. B., Lee F. Tissue distribution of murine hemopoietic growth factor mRNA production. J Cell Physiol. 1989 Jan;138(1):38–44. doi: 10.1002/jcp.1041380107. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]