Abstract
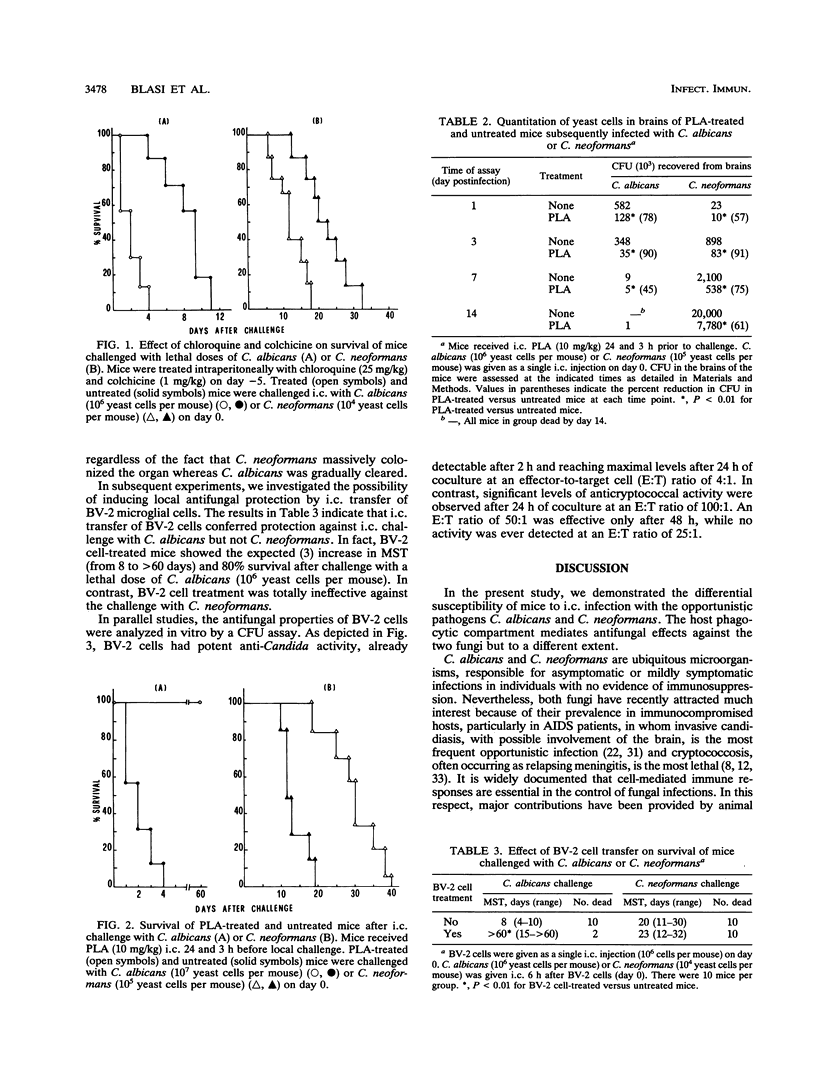
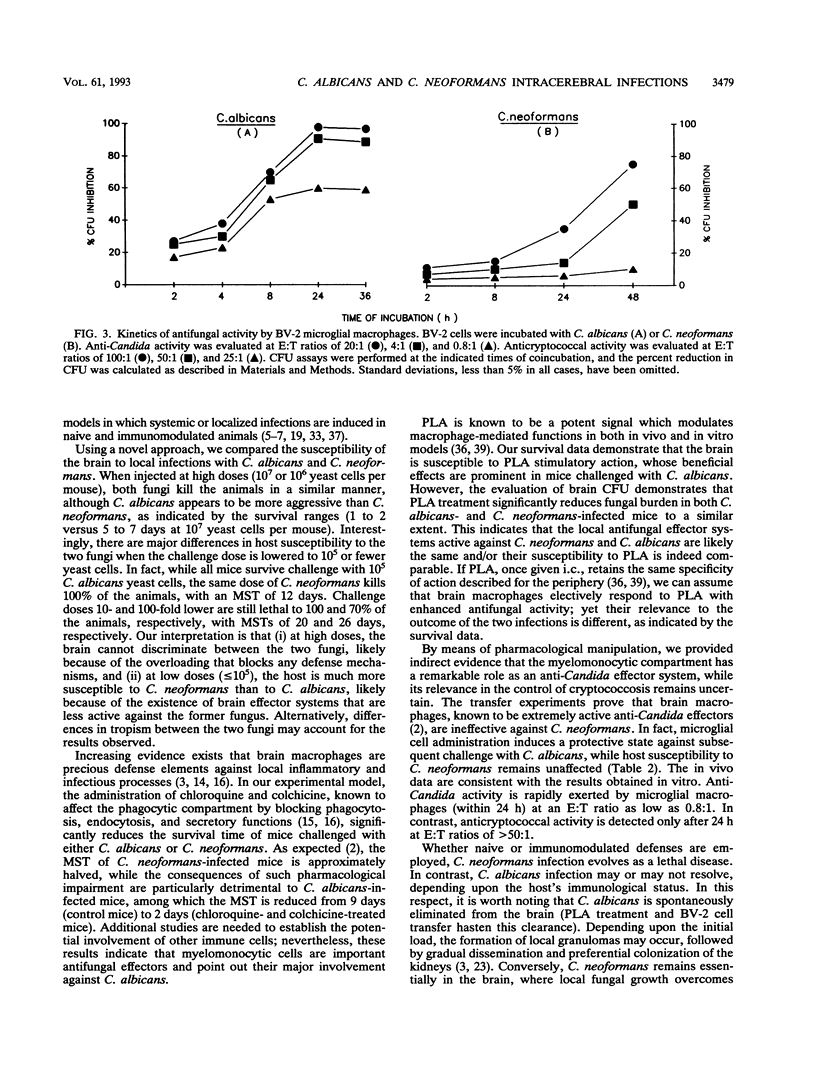
To investigate the immune defense mechanisms employed against fungi in the brain, mice were experimentally infected by intracerebral inoculation of Candida albicans or Cryptococcus neoformans. Parameters such as median survival time and numbers of yeast cells in the brains were assessed for naive and immunomodulated mice. We found that no mice survived either C. albicans or C. neoformans challenge at doses of > or = 10(6) yeast cells per mouse. However, when the inoculum size was decreased (< or = 10(5) yeast cells per mouse), C. albicans was no longer lethal (100% survival), whereas 100 and 70% of the mice still succumbed to challenge doses of 10(4) and 10(3) C. neoformans yeast cells, respectively. Pharmacological manipulation and transfer experiments revealed that the myelomonocytic compartment had a minor role against C. neoformans but was deeply involved in the control of intracerebral C. albicans infection. By counting the number of yeast cells in the brains of naive and immunomodulated animals, we established that, unlike C. albicans, C. neoformans remained essentially in the brain, where massive colonization and damage occurred whether naive or immunomodulated defense mechanisms were employed by the host. Overall, these data suggest that the differential role of the myelomonocytic compartment, together with the diverse tropisms of the two fungi, can explain the different development and outcome of intracerebral C. albicans and C. neoformans infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasi E., Barluzzi R., Bocchini V., Mazzolla R., Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990 May;27(2-3):229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Blasi E., Barluzzi R., Mazzolla R., Mosci P., Bistoni F. Experimental model of intracerebral infection with Cryptococcus neoformans: roles of phagocytes and opsonization. Infect Immun. 1992 Sep;60(9):3682–3688. doi: 10.1128/iai.60.9.3682-3688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E., Mazzolla R., Barluzzi R., Mosci P., Bartoli A., Bistoni F. Intracerebral transfer of an in vitro established microglial cell line: local induction of a protective state against lethal challenge with Candida albicans. J Neuroimmunol. 1991 Jun;32(3):249–257. doi: 10.1016/0165-5728(91)90195-d. [DOI] [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect Immun. 1990 Apr;58(4):1093–1100. doi: 10.1128/iai.58.4.1093-1100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E., Romani L., Vecchiarelli A., Puccetti P., Bistoni F. Role of L3T4+ lymphocytes in protective immunity to systemic Candida albicans infection in mice. Infect Immun. 1989 Nov;57(11):3581–3587. doi: 10.1128/iai.57.11.3581-3587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E., Romani L., Vecchiarelli A., Puccetti P., Bistoni F. T cell subsets and IFN-gamma production in resistance to systemic candidosis in immunized mice. J Immunol. 1990 Jun 1;144(11):4333–4339. [PubMed] [Google Scholar]
- Chandler F. W. Pathology of the mycoses in patients with the acquired immunodeficiency syndrome (AIDS). Curr Top Med Mycol. 1985;1:1–23. doi: 10.1007/978-1-4613-9547-8_1. [DOI] [PubMed] [Google Scholar]
- Collins H. L., Bancroft G. J. Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of tumor necrosis factor-alpha and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur J Immunol. 1992 Jun;22(6):1447–1454. doi: 10.1002/eji.1830220617. [DOI] [PubMed] [Google Scholar]
- Collins H. L., Bancroft G. J. Encapsulation of Cryptococcus neoformans impairs antigen-specific T-cell responses. Infect Immun. 1991 Nov;59(11):3883–3888. doi: 10.1128/iai.59.11.3883-3888.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Serbousek D., Blanchard D. K. Release of tumor necrosis factor by human polymorphonuclear leukocytes. Blood. 1990 Oct 1;76(7):1405–1409. [PubMed] [Google Scholar]
- Eng R. H., Bishburg E., Smith S. M., Kapila R. Cryptococcal infections in patients with acquired immune deficiency syndrome. Am J Med. 1986 Jul;81(1):19–23. doi: 10.1016/0002-9343(86)90176-2. [DOI] [PubMed] [Google Scholar]
- Flesch I. E., Schwamberger G., Kaufmann S. H. Fungicidal activity of IFN-gamma-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989 May 1;142(9):3219–3224. [PubMed] [Google Scholar]
- Giulian D. Ameboid microglia as effectors of inflammation in the central nervous system. J Neurosci Res. 1987;18(1):155-71, 132-3. doi: 10.1002/jnr.490180123. [DOI] [PubMed] [Google Scholar]
- Giulian D., Chen J., Ingeman J. E., George J. K., Noponen M. The role of mononuclear phagocytes in wound healing after traumatic injury to adult mammalian brain. J Neurosci. 1989 Dec;9(12):4416–4429. doi: 10.1523/JNEUROSCI.09-12-04416.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Correlation of natural killer cell activity and clearance of Cryptococcus neoformans from mice after adoptive transfer of splenic nylon wool-nonadherent cells. Infect Immun. 1986 Feb;51(2):547–555. doi: 10.1128/iai.51.2.547-555.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Nabavi N., Sonleitner F., Murphy J. W. Murine natural killer cells are fungicidal to Cryptococcus neoformans. Infect Immun. 1991 May;59(5):1747–1754. doi: 10.1128/iai.59.5.1747-1754.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle G. B., Yates J. L., Lipscomb M. F. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect Immun. 1991 Apr;59(4):1423–1433. doi: 10.1128/iai.59.4.1423-1433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980 Oct;30(1):5–11. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A., Hickey W. F., Morris J. H., Loscalzo J. Candidal infection in the central nervous system. Am J Med. 1984 Jan;76(1):101–108. doi: 10.1016/0002-9343(84)90757-5. [DOI] [PubMed] [Google Scholar]
- Mazzolla R., Barluzzi R., Romani L., Mosci P., Bistoni F. Anti-Candida resistance in the mouse brain and effect of intracerebral administration of interleukin 1. J Gen Microbiol. 1991 Aug;137(8):1799–1804. doi: 10.1099/00221287-137-8-1799. [DOI] [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun. 1991 Jan;59(1):24–28. doi: 10.1128/iai.59.1.24-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G., Storkus W. J., Dawson J. R. Human natural killer cells do not inhibit growth of Cryptococcus neoformans in the absence of antibody. Infect Immun. 1990 Mar;58(3):639–645. doi: 10.1128/iai.58.3.639-645.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Hidore M. R., Nabavi N. Binding interactions of murine natural killer cells with the fungal target Cryptococcus neoformans. Infect Immun. 1991 Apr;59(4):1476–1488. doi: 10.1128/iai.59.4.1476-1488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W. Immunity to fungi. Curr Opin Immunol. 1989;2(3):360–367. doi: 10.1016/0952-7915(89)90142-8. [DOI] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Pendlebury W. W., Perl D. P., Munoz D. G. Multiple microabscesses in the central nervous system: a clinicopathologic study. J Neuropathol Exp Neurol. 1989 May;48(3):290–300. doi: 10.1097/00005072-198905000-00006. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Hobbs M. M., Granger D. L., Durack D. T. Cerebrospinal fluid macrophage response to experimental cryptococcal meningitis: relationship between in vivo and in vitro measurements of cytotoxicity. Infect Immun. 1988 Apr;56(4):849–854. doi: 10.1128/iai.56.4.849-854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Immunity to Candida albicans. Microbiol Rev. 1980 Dec;44(4):660–682. doi: 10.1128/mr.44.4.660-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L., Fioretti M. C., Bianchi R., Nardelli B., Bonmassar E. Intracerebral adoptive immunotherapy of a murine lymphoma antigenically altered by drug treatment in vivo. J Natl Cancer Inst. 1982 May;68(5):817–822. [PubMed] [Google Scholar]
- Ruffmann R., Welker R. D., Saito T., Chirigos M. A., Varesio L. In vivo activation of macrophages but not natural killer cells by picolinic acid (PLA). J Immunopharmacol. 1984;6(4):291–304. doi: 10.3109/08923978409028605. [DOI] [PubMed] [Google Scholar]
- Salkowski C. A., Balish E. Pathogenesis of Cryptococcus neoformans in congenitally immunodeficient beige athymic mice. Infect Immun. 1990 Oct;58(10):3300–3306. doi: 10.1128/iai.58.10.3300-3306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinshamn S., Waage A. Tumor necrosis factor and interleukin-6 in Candida albicans infection in normal and granulocytopenic mice. Infect Immun. 1992 Oct;60(10):4003–4008. doi: 10.1128/iai.60.10.4003-4008.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varesio L., Clayton M., Blasi E., Ruffman R., Radzioch D. Picolinic acid, a catabolite of tryptophan, as the second signal in the activation of IFN-gamma-primed macrophages. J Immunol. 1990 Dec 15;145(12):4265–4271. [PubMed] [Google Scholar]
- Vecchiarelli A., Mazzolla R., Farinelli S., Cassone A., Bistoni F. Immunomodulation by Candida albicans: crucial role of organ colonization and chronic infection with an attenuated agerminative strain of C. albicans for establishment of anti-infectious protection. J Gen Microbiol. 1988 Sep;134(9):2583–2592. doi: 10.1099/00221287-134-9-2583. [DOI] [PubMed] [Google Scholar]


